Abstract
β–thalassemia intermedia syndromes (BTI) cause hemolytic anemia, ineffective erythropoiesis, and widespread complications. Higher fetal globin expression within genotypes reduces globin imbalance and ameliorates anemia. Sodium 2,2 dimethylbutyrate (HQK-1001), an orally bioavailable short-chain fatty acid derivative, induces γ-globin expression experimentally and is well-tolerated in normal subjects. Accordingly, a randomized, blinded, placebo-controlled, Phase I/II trial was performed in 21 adult BTI patients (14 with HbE/β0 thalassemia and 7 with β+/β0 thalassemia intermedia, to determine effective doses for fetal globin induction, safety, and tolerability. HQK-1001 or placebo were administered once daily for 8 weeks at four dose levels (10, 20, 30, or 40 mg/kg/day), and subjects were monitored for laboratory and clinical events. Pharmacokinetic profiles demonstrated a t1/2 of 10–12 hours. Adverse events with HQK-1001 treatment were not significantly different from placebo treatment. Median HbF increased with the 20 mg/kg treatment doses above baseline levels by 6.6% and 0.44 g/dL (p <0.01) in 8/9 subjects; total hemoglobin (Hgb) increased by a mean of 1.1 gm/dL in 4/9 subjects. These findings identify a safe oral therapeutic which induces fetal globin in BTI. Further investigation of HQK-1001 with longer dosing to definitively evaluate its hematologic potential appears warranted.
Keywords: Beta thalassaemia intermedia, fetal hemoglobin, clinical trial, pharmacokinetics
β-thalassemia syndromes are among mankind’s most common hereditary monogenic diseases and are WHO-designated as a global health burden (Weatherall, 2010). β-thalassemias are caused by more than 200 molecular mutations which reduce synthesis of the β-globin chains of hemoglobin A (α2βA2), resulting in excess, unmatched α-chains in developing erythroblasts, which precipitate and damage red blood cell membranes, causing intramedullary hemolysis, anemia, and extramedullary hematopoeisis (Cappellini et al, 2004; Schrier, 1997). β-thalassemia syndromes are classified in severity based on steady-state hemoglobin levels and transfusion dependency. β-thalassemia major, and β–thalassemia intermedia (BTI) are both caused by inheritance of two β-globin gene mutations (Gallo et al, 1979; Galanello et al, 2011). BTI causes moderate anemia in childhood that often progresses to transfusion-dependency in older age, iron loading and unique complications related to expanded erythropoiesis and hemolysis (Gallo et al, 1979; Capellini et al, 2004; Taher et al, 2010; Perrine, 2005; Uda et al, 2008; Galanello et al, 2011).
It has been well-established from genetic studies that higher fetal hemoglobin (HbF) expression within the same genotypes reduces anemia in β-thalassemia (Capellini et al, 2004; Taher et al, 2010; Gallo et al, 1979; Uda et al, 2008; Galanello et al, 2011; Perrine, 2005; Steinberg et al, 2001). Increased synthesis of fetal globin chains (γ–globin) reduces the globin chain imbalance, ineffective erythropoiesis, and hemolysis (Gallo et al, 1979; Galanello et al, 2011; Schrier, 1997). Within genotypes, the differential synthetic levels of fetal globin can result in differences in steady state total hemoglobin levels of as much as 1 gram (Galanello et al, 2011). Earlier γ-globin inducing therapeutics, such as 5-azacytidine, sodium phenylbutyrate, and arginine butyrate, demonstrated proof-of-concept of the benefits of increasing HbF in beta thalassemia, with subsequent rises in total hemoglobin, and even abolished transfusion requirements (Perrine, 2005; Steinberg et al, 2001; Schrier, 1997; Collins et al, 1995; Perrine et al, 1993; Cappellini et al, 2000; Reich et al, 2000; Hajjar et al, 1994; Singer et al, 2005; Fucharoen et al, 1996; Ley et al, 1982; Dunbar et al, 1989; Lowrey et al, 1993; Olivieri et al, 2011; Bourantas et al, 1997; Nisli et al, 1996; Rachmilewitz et al, 1998). Yet, this treatment approach has not become widely adopted due to requirements for parenteral administration or large drug doses, which are not easily tolerated for chronic use. While hydroxyurea has shown some benefit in select populations (Singer et al, 2005; Fucharoen et al, 1996), cytotoxic agents are less effective in beta thalassemia than in sickle cell disease. No HbF-inducing therapeutics are currently approved by regulatory authorities for treatment of β-thalassemias. An oral, non-cytotoxic agent that induces HbF expression could benefit many patients worldwide.
Sodium 2,2 dimethylbutyrate (SDMB, HQK-1001) is a promoter-targeted, short-chain fatty acid derivative which is orally bio-available, noncytotoxic, benign in toxicology testing, and has a t1/2 of 11 hours in normal human volunteers and sickle cell patients, favorable features for long-term use (Pace et al, 2002; Castaneda et al, 2005; Perrine et al, 2009; Perrine et al, 2011). We report here a dose-ranging trial of this oral therapeutic in β-thalassemia intermedia. An active dose was identified in patients from two geographic regions which are globally representative of molecular mutations common in Mediterranean, Middle Eastern, and Asian beta thalassemia patients. Pharmacokinetic (PK) profiles were favorable for a long-term therapeutic, and no safety concerns were found.
METHODS
This was a randomized, blinded, sequential dose-escalation trial (NCT 00790127, www.clinicaltrials.gov). The primary objective of this study was to assess the safety and tolerability of HQK-1001 when administered daily for 8 weeks in subjects with β-thalassemia intermedia (BTI). Secondary objectives were: 1) to evaluate pharmacokinetic (PK) profiles of HQK-1001, and 2) to assess potential effects of HQK-1001 on fetal globin expression and hemoglobin in subjects with BTI within a brief dosing period. The study was approved by the Institutional Review Boards of Sirijaj Hospital in Bangkok, Thailand, and the Rafik Hariri University Hospital and the Chronic Care Center in Beirut, Lebanon, and subjects were recruited over a two month period at each site. Eligibility criteria required a diagnosis of β-thalassemia intermedia with total hemoglobin levels between 5 and 9 gm/dl, ages 18–50, inclusive, and a maximal spleen dimension 2 cm below the left costal margin for non-splenectomized subjects, based on lower responses in extreme splenomegaly noted in prior trials of other HbF inducers (Perrine, 2005). Exclusion criteria included participation in regular red blood cell transfusions or other experimental medications. Female patients were required to have negative pregnancy tests before dosing and every 4 weeks. After written informed consent was obtained, subjects were randomized to receive active treatment or placebo according to a schedule prepared by independent biostatisticians at a contract research organization Quintiles, Inc., and employing a table of random numbers. Randomization was made to each of 4 dose levels in two subject cohorts, with a wash-out period between each dose level. Subjects were randomized to receive active study medication or placebo for 8 weeks (56 days) with a 4-week follow-up period and a wash-out period of at least 8 weeks. Randomization to HQK-1001 or placebo was made at a ratio of 7:2 at dose level 1, (10 mg/kg/dose), and a ratio of 6:1 at dose levels 2, 3, or 4. All subjects were re-randomized between HQK-1001 or placebo for the second study cycle. Cohort 1 received active study medication at 10 mg/kg/dose first, and then 30 mg/kg/dose, following the wash-out period. Cohort 2 received active medication at 20 mg/kg/dose and then 40 mg/kg/dose. Participants, caregivers, investigators and laboratory personnel were blinded to interventions. An Independent Safety Monitoring Committee reviewed data in 4 subjects treated for at least 28 days at each dose level before dosing was begun at a higher level. If no safety concerns were observed, randomization to the next dose level was allowed. Subjects who had ferritin levels <1000 ng/ml received supplementation once/daily with standard doses of ferrous fumarate, and all subjects received folic acid once daily to support erythropoiesis during the treatment and follow-up periods.
Safety assessments and HbF assays
The predetermined primary outcomes were safety assessments and PK profiles, and secondary outcomes were changes in HbF from baseline, within the context of a brief dose-ranging trial. Safety assessments were performed at screening and every 2 weeks during the study with physical examinations, complete blood counts, complete chemistry and coagulation panels, urinalyses, and electrocardiograms (ECG). All patients who received at least one dose were included in the safety population. Adverse event severity was graded by the blinded investigators using the NCI CTCAE v 3.0, with grades of 1 (mild), 2 (moderate), 3 (severe), 4 (life-threatening), and 5 (fatal). Two sets of HbF levels were obtained over a two-month period and averaged with the Day 0 levels for baseline values. HbF was assayed by HPLC, F-reticulocytes, and F-cells by flow cytometry as previously described (Garner et al, 1998). A serum pregnancy test was performed on women of child bearing potential (WCBP) prior to dosing and every 4 weeks. All subjects underwent blood sampling (at 2, 4, 6, 8, 12, and 24 hours following the dose) for PK analysis on day 13 and day 55, the last day of dosing, and a plasma level was obtained 1 week after the last dose of study drug. During the 56-day period of drug administration at each level, subjects were contacted between visits by study personnel to monitor safety and compliance with study drug. Pre-specified and exploratory analyses were performed using Wilcoxon sign rank tests to evaluate change from baseline values within each treatment group by the independent biostatisticians. Exploratory analyses were performed to evaluate mean and median differences between dose levels and not as formal hypothesis testing.
Pharmacokinetic studies and analyses
The pharmacokinetic (PK) population for the study consisted of a total of 21 unique subjects across the two Cohorts with 8 subjects in Cohort 1, 10 mg/kg doses, 9 subjects in Cohort 2, 20 mg/kg dosing, 6 subjects in Cohort 1, 30 mg/kg doses, and 9 subjects in Cohort 2, 40 mg/kg doses. For each dose level, once daily oral dosing was conducted for 8 weeks. Pharmacokinetic (PK) profiles were obtained on Day 13 for all dose levels (10, 20, 30, and 40 mg/kg). Subjects who received placebo or had no or insufficient plasma PK samples taken on Day 13 were excluded from the PK population analysis for that specific cycle. Plasma HQP-1001 concentrations were summarized using descriptive statistics (including N, mean, standard deviation (SD), coefficient of variation (CV%), median, minimum, and maximum) for each dose level. Derived plasma PK descriptive statistics were tabulated by dosing group and summary statistics presented for PK parameters (CMAX, TMAX, CMIN, AUCτ, t½, λz, Cavg, and CLss_F) are the arithmetic and geometric mean, CV%, SD of the arithmetic mean, median, minimum, maximum, and N. Subjects who received HQP-1001 and had complete concentration-time profiles through at least Day 13 of one test cycle were included in the PK population for non-compartmental PK analysis.
RESULTS
Demographics and Safety Assessments
Twenty-four subjects were screened and twenty-one unique subjects enrolled on the trial; 14 patients with HbE/β0 thalassemia enrolled in Thailand; 7 subjects with βo/β+ thalassemia mutations were studied in Lebanon. There were 17 females and 4 males, ages from 19– 49 years. Patient demographics, baseline hematologic characteristics, and molecular mutations are shown in Table I (A and B). All the Lebanese BTI patients were splenectomized; 9 of 14 Thai subjects were splenectomized. The study enrolled over 3 months and was conducted during 2009–2010; data collection and analysis were completed in 2011 by Quintiles, Inc., and Premier Research Group.
Table I.
| A. Demographic and Baseline Characteristics: Safety Population | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (N = 8) |
HQK-1001 10 mg/kg (N = 8) |
HQK-1001 20 mg/kg (N = 9) |
HQK-1001 30 mg/kg (N = 6) |
HQK-1001 40 mg/kg (N = 9) |
||||||
| Characteristic | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) |
| Gender [n (%)] | ||||||||||
| Male | 2 | (25.0%) | 1 | (12.5%) | 2 | (22.2%) | 2 | (33.3%) | 2 | (22.2%) |
| Female | 6 | (75.0%) | 7 | (87.5%) | 7 | (77.8%) | 4 | (66.7%) | 7 | (77.8%) |
| Race [n (%)] | ||||||||||
| Caucasian | 2 | (25.0%) | 0 | (0%) | 6 | (66.7%) | 0 | (0%) | 6 | (66.7%) |
| Asian (Thai) | 6 | (75.0%) | 8 | (100%) | 3 | (33.3%) | 6 | (100%) | 3 | (33.3%) |
| Age (Years) | ||||||||||
| Mean (SD) | 32.0 (12.1) | 33.9 (8.2) | 26.8 (5.7) | 28.0 (6.3) | 31.8 (9.0) | |||||
| Median | 30.5 | 33.5 | 24.0 | 29.5 | 32.0 | |||||
| Min, Max | (17, 49) | (20, 45) | (20, 35) | (17, 35) | (21, 49) | |||||
| Weight (kg) | ||||||||||
| Mean (SD) | 51.6 (8.6) | 47.9 (8.6) | 52.7 (5.9) | 54.2 (8.9) | 52.5 (7.9) | |||||
| Median | 51.95 | 45.95 | 51.00 | 58.05 | 51.00 | |||||
| Min – Max | (40, 62) | (38, 62) | (46, 65) | (40, 62) | (40, 65) | |||||
| BMI (kg/m2) | ||||||||||
| N | 7 | 8 | 8 | 6 | 7 | |||||
| Mean (SD) | 19.0 (1.3) | 19.6 (2.1) | 19.6 (2.3) | 20.8 (1.3) | 19.4 (2.5) | |||||
| Median | 19.06 | 19.36 | 19.50 | 20.45 | 19.43 | |||||
| Min – Max | (17, 21) | (16, 23) | (17, 24) | (20, 23) | (17, 24) | |||||
| B. Baseline characteristics of individual subjects is shown. | ||||||
|---|---|---|---|---|---|---|
| ID | Gender | Age (yrs) |
Spleen Status |
Thalassemia Genotype |
Total Hgb (g/dL) |
% HbF |
| 01002 | Female | 32 | S | β17(A->T) / βE | 6.6 | 35 |
| 01003 | Female | 44 | S | β17(A->T) / βE | 6.8 | 9 |
| 01004 | Female | 35 | NS | β105bp deletion / βE | 9.8 | 22 |
| 01005 | Female | 36 | NS | β105bp deletion / βE | 8.9 | 54 |
| 01007 | Female | 30 | NS | β110(T->C) / βE | 8.5 | 50 |
| 01008 | Male | 25 | S | β17(A->T) / βE | 6.0 | 30 |
| 01011 | Male | 21 | S | β41/42(-nCT) / βE | 6.2 | 20 |
| 01012 | Female | 30 | NS | βIVSI-1 (G->T) / βE | 9.3 | 41 |
| 01013 | Female | 45 | S | β17(A->T) / βE | 6.3 | 28 |
| 01014 | Female | 17 | S | β41/42(-nCT) / βE | 9.2 | 40 |
| 01015 | Female | 40 | S | β41/42(-TTCT) / βE | 5.3 | 15 |
| 01016 | Female | 21 | S | β17 (+A) / βE | 7.2 | 37 |
| 01017 | Male | 32 | S | β17(A->T) / βE | 5.8 | 24 |
| 01018 | Female | 32 | NS | β41/42(-nCT) / βE | 6.1 | 30 |
| 02001 | Female | 21 | S | βIVS I-110/-87 | 7.9 | 58 |
| 02002 | Female | 23 | S | βIVSII-1/CD29 | 7.7 | 62 |
| 02003 | Female | 29 | S | βIVSI-6/IVSI-6 | 6.0 | 9.5 |
| 02004 | Male | 22 | S | βIVSI-6/IVSI-6 | 9.3 | 20 |
| 02005 | Female | 32 | S | βCD29/CD29 | 5.9 | 21 |
| 02006 | Female | 35 | S | βCD30/CD30 | 7.5 | 91 |
| 02007 | Female | 39 | S | βCD29/CD29 | 5.9 | 29 |
S= splenectomized; NS= non-splenectomized
Thai HbE/β0 thalassemia patients only were enrolled in Cohort 1 at dose levels 10 mg/kg and 30 mg/kg, because the study opened first at the Thailand site. In Cohort 2, 6 Lebanese subjects and 3 Thai subjects were randomized to receive active study drug and 2 were randomized to receive the placebo. Eighteen patients completed 2 dose levels on the study; 3 subjects discontinued early due to events which were considered not drug-related by the blinded Investigators. All subjects were included in the subgroup analyses. A tolerable safety profile was observed at all dose levels; no dose-related patterns for incidence and severity of adverse events (AEs) by dose of HQK-1001 or differences from placebo treatment were observed. The most frequent adverse events noted were headache, upper respiratory infection, fatigue, nausea, and dizziness, as shown in Table 2. Upper respiratory tract infection and headache were more common in the 10 mg/kg group. Nausea, vomiting, and abdominal pain were reported in the 20 and 40 mg/kg groups. Fatigue was reported more frequently in the 40 mg/kg group. Five subjects experienced 5 severe adverse events (SAEs); (3 HQK-1001 subjects had 3 SAEs, and 2 placebo subjects had 2 SAEs). Severe adverse events (SAEs) included upper abdominal pain, gastritis, suprapubic pain, and back pain in one HQK-1001 treated subject each; headache and gastroenteritis occurred in one placebo subject each. All the SAEs were considered unrelated to the study drug by the blinded Investigators, and no apparent dose-dependent pattern was observed. The most frequent AEs considered possibly drug-related by the Investigators included fatigue and nausea, with no clear dose dependent pattern observed. No significant adverse differences in laboratory studies for hematology, chemistry, coagulation, or urinalysis were observed between the treatment groups. Weight measurements showed a slight decrease from baseline at the end of the treatment period for the placebo, 10, 30, and 40 mg/kg HQK-1001 groups, while the 20 mg/kg treatment group showed a slight increase in weight. An independent expert cardiology review performed to evaluate ECGs determined there was no treatment effect for RR, PR, QRS, QT, or QTc interval values by dose or time. No clinically relevant findings were observed with other safety parameters of physical examinations or concomitant medications.
Table II.
A. Adverse events which occurred in >10% of subjects are shown by dose cohort in the top panel and serious adverse events are shown in the lower panel.
| PLACEBO | HQK-1001 | ||||
|---|---|---|---|---|---|
| EVENT | N (%) | 10 mg/kg N (%) |
20 mg/kg N (%) |
30 mg/kg N (%) |
40 mg/kg N (%) |
| Headache | 2 (25) | 5 (63) | 3 (33) | 2 (33) | 1 (11) |
| Upper Respiratory Infection | 3 (38) | 5 (63) | 2 (22) | 1 (17) | 1 (11) |
| Pyrexia | 2 (25) | 3 (38) | 0 | 2 (33) | 1 (11) |
| Fatigue | 1 (13) | 1 (13) | 0 | 0 | 4 (44) |
| Nausea | 0 | 0 | 2 (22) | 0 | 1 (11) |
| Dizziness | 0 | 2 (25) | 2 (22) | 0 | 1 (11) |
|
Serious Adverse Event |
Placebo N, (%) |
HQK-1001 Dose N (%), dose |
Treatment Relatedness |
||
| Palpitations | 1 (13), 10 mg/kg | Unrelated | |||
| Suprapubic Pain | 1 (13), 20 mg/kg | Unrelated | |||
| Gastritis | 1 (13), 30 mg/kg | Unrelated | |||
| Gastroenteritis | 1 (13) | Unrelated | |||
| Upper Respiratory Tract Infection | 1 (13) | Unrelated | |||
Pharmacokinetic profiles
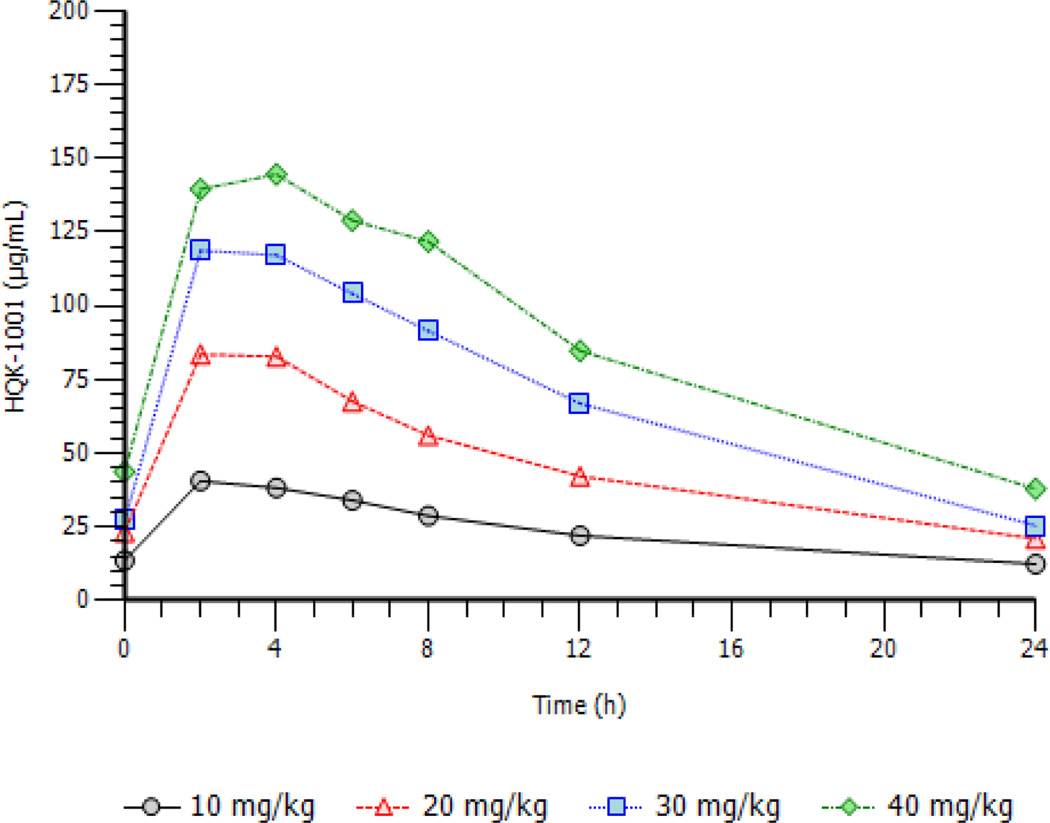
Anemia ranges from moderate to severe in BTI patients and plasma volumes vary accordingly. To evaluate potential metabolic differences in this diverse patient population, PK profiles were studied over multiple time points. Drug concentrations which induce fetal globin expression in preclinical studies in vitro were readily maintained at 10–20 mg/kg doses and were highly exceeded at 30–40 mg/kg doses. HQK-1001 has a low clearance and a relatively long half-life; the plasma concentrations are quite high, with steady-state concentrations averaging 24.0 to 88.1 µg/mL over the 10 to 40 mg/kg dose range. Both half-life and CLss/F were constant over the dose range tested, indicating the PK profiles likely will be applicable to others. Steady state PK profiles per dose cohort obtained on day 13 are illustrated in Figure 1. Dose proportional increases in overall exposure as measured by AUC, ranged from 579 to 2110 h* µg/mL over the dose ranges studied. Minimum and maximum plasma concentrations increased with dose levels; Cmax means ranged from 41 to 154 µg/mL over the 10–40 mg/kg dose group. Median TMAX occurred at 2 to 4 hours across the four dose levels. Terminal half-life ranged from 12 hours at the 10 mg/kg dose to 10 hours at the 40 mg/kg dose. Concentrations associated with optimal HbF induction in vitro were observed at the 20 mg/kg dose level (Boosalis et al, 2011).
Figure 1.
Pharmacokinetic profiles of HQK-1001 shown by dose cohort.
Fetal globin assays
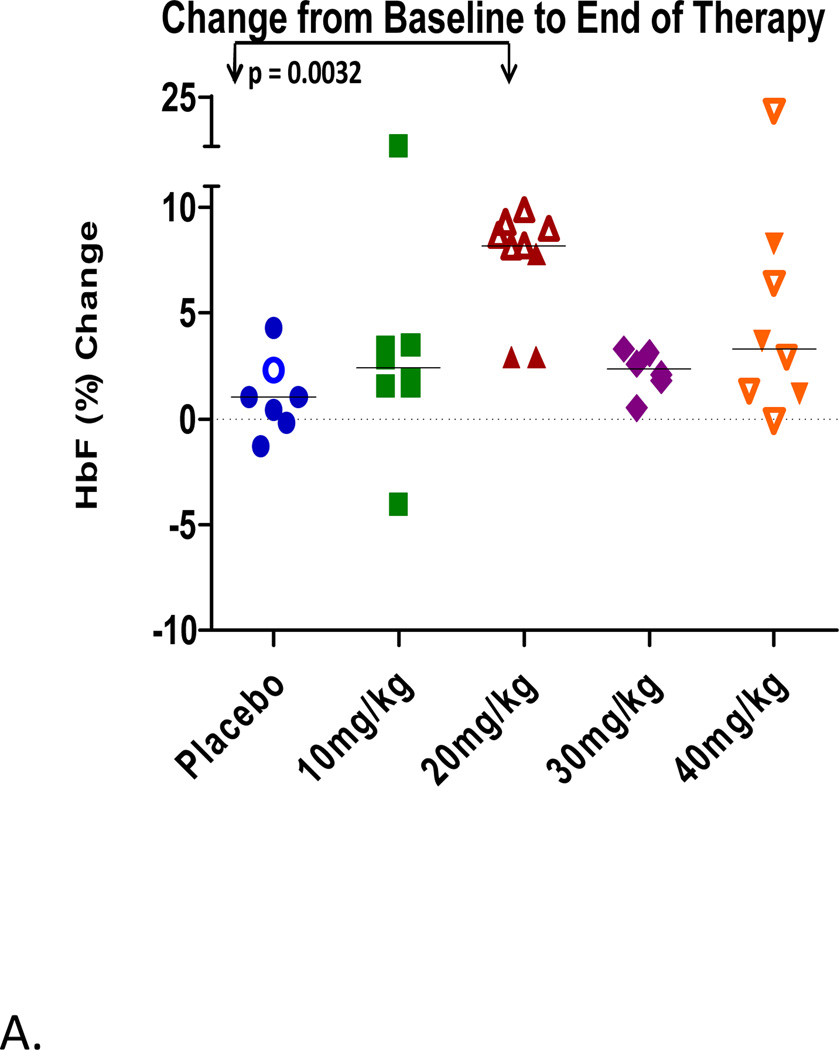
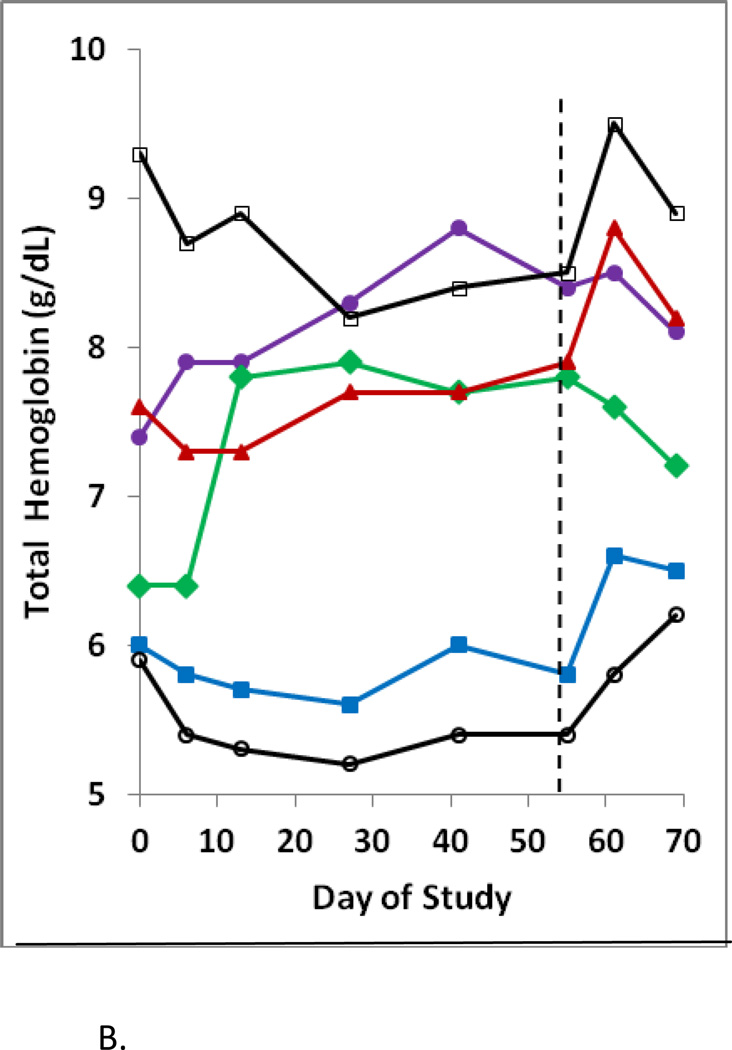
In this first clinical evaluation of HQK-1001 in beta thalassemia, increases in HbF above baseline were observed in some individuals in all study drug dose cohorts, ranging from 3% to 22% above individual subjects’ averaged baseline levels, while differences were not observed in the placebo-treated subjects, shown in Figure 2A. On Day 55, the last day of dosing, change from baseline in percent HbF in placebo, 10, 20, 30 and 40 mg/kg HQK-1001 dose groups was 0.7, 3.2, 6.6, 2.4, and 3.0%, respectively. The dose level with the largest median increase in HbF was the 20 mg/kg group. Median absolute HbF at Day 55 decreased from baseline by a median of −0.4 g/L in the placebo group and increased above baseline by 2.0, 4.4, 0.7, and 1.0 g/L in the 10, 20, 30, and 40 mg/kg HQK-1001 dose groups, respectively. Changes in HbF above baseline were evident in 4/8 subjects treated at 10 mg/kg and in 8/9 subjects treated with 20 mg/kg doses of SDMB (2/3 subjects in Thailand and 6/6 subjects in Lebanon). Lesser changes were observed at 30 mg/kg doses, administered only in Thai patients). The highest single HbF response, 21.8% increase above the subject’s baseline, was observed at 40 mg/kg/dose in a Lebanese BTI patient. Rises in total Hgb have not generally occurred in β-thalassemia patients within an 8-week time-frame with other therapeutics; longer treatment courses of 3–6 months were required for butyrate, phenylbutyrate, hydroxyurea, or decitabine. Median changes in total hemoglobin (Hgb) of most dose groups in this study overall were not positive. However, increases in total Hgb were observed in 4/6 Lebanese BTI subjects or (4/9 total subjects) treated at 20 mg/kg doses, the same dose which produced the highest HbF responses (Figure 2B). Changes in F-cells were detected in both subject groups. Per cent F-reticulocytes increased by a median of 6.6% in the 30 mg/kg dose in HbE/β thalassemia subjects and were not significantly changed from baseline (−0.34) in the placebo group. Per cent F-cells increased maximally above baseline by a median of 7.4% with 10 mg/kg doses in HbE/β thalassemia subjects and by a mean of 14.6% in the 7 Lebanese BTI β0/β+ thalassaemia subjects from a mean baseline of 66% to 81% with treatment, (p< 0.01, paired t-test), shown in Table III.
Figure 2.
A. Changes in HbF from baseline by dose cohort. Values in Lebanese subjects are shown by the open symbols; values in Thai subjects are shown by the closed symbols.
B. Total hemoglobin levels increased in 4 of 6 Lebanese beta thalassaemia intermedia subjects treated with HQK-1001 at 20 mg/kg/dose for 8 weeks. Hemoglobin levels are shown in the 6 subjects who received active study drug at 20 mg/kg/dose. The end of the dosing period is designated by the dotted line.
Table III.
% F-cells in Lebanese β-thalassemia intermedia subjects at baseline and with HQK-1001 treatment at 20 mg/kg doses; subject 2007 received only 40 mg/kg/doses. Mean F-cells increased from 66% at baseline to 81% with treatment, (p<0.01, paired t-test).
| Subject | Baseline F-cells |
Treatment F-cells |
|---|---|---|
| 2001 | 84 | 89 |
| 2002 | 82 | 94 |
| 2003 | 32 | 58 |
| 2004 | 48 | 76 |
| 2005 | 55 | 70 |
| 2006 | 89 | 96 |
| 2007 | 75 | 85 |
DISCUSSION
BTI patients comprise 30–50% of beta thalassemia populations in some regions (Cappellini et al, 2004; Taher et al 2010; Gallo et al, 1979). Serious complications are increasingly recognized in BTI, including cardiac failure, osteoporosis, cord compression, pulmonary hypertension, iron overload, thromboses, refractory leg ulcers, and ischemic brain lesions (Cappellini et al, 2004; Taher et al 2010). Intermittent or regular transfusions often become indicated (Cappellini et al, 2004; Taher et al 2010).
A non-cytotoxic oral therapeutic which increases fetal globin expression, reduces globin chain imbalance, and is tolerable for long–term patient use should be beneficial in reducing anemia in affected individuals. Preclinical studies with HQK-1001 in thalassemic erythroid progenitors and anemic baboons indicated two potential therapeutic effects: fetal globin induction and enhanced erythroid survival through the pro-survival Bcl-family proteins (Bcl-xL) (Perrine, 2005, 2009; Pace et al, 2002; Castaneda et al, 2005). The latter effect is associated with improved hematologic responses with rhu-Erythropoietin in β+ thalassemia subjects with relatively low levels of erythropoietin (Perrine, 2005). Thus, this SCFAD offers a dual rationale for evaluation in BTI. HQK-1001 has a wide safety margin in formal toxicology studies, is non-mutagenic and non-cytotoxic (Perrine et al, 2011). Variable metabolism is a primary factor which limits the efficacy of many drugs (Wilkinson, 2005); however, the PK profiles of HQK-1001 in β-thalassemia intermedia patients treated here were consistent within and between subjects, and drug half-lives and clearance were constant over the dose range of 10–40 mg/kg. These patterns should reduce variation in responses related to drug metabolism. The 20 mg/kg doses may have been associated with higher responses because this dose produced plasma concentrations in the range (20–800 µM) that is most effective in vitro, whereas peak plasma concentrations achieved at 30–40 mg/kg doses (> 1 mM, 117 µg/ml), may suppress erythroid proliferation in vitro (Boosalis, 2011).
β-thalassemia intermedia subjects have variably accelerated rates of erythroid cell apoptosis, and translation of fetal globin induction into increases in total hemoglobin has required dosing periods of at least 3–6 months with parenteral drugs 5-azacytidine, decitabine, or butyrate to achieve significance, perhaps allowing time for higher-F cells to accumulate and selectively survive (Collins et al, 1995; Perrine et al, 1993; Cappellini et al, 2000; Reich et al, 2000; Hajjar et al, 1994; Singer et al, 2005; Fucharoen et al, 1996; Ley et al, 1982; Dunbar et al, 1989; Lowrey et al, 1993; Olivieri et al, 2011). While median changes in total hemoglobin were not significant in this brief trial, it is encouraging that increases in total hemoglobin in 4/9 subjects at the 20 mg/kg dose level occurred within 8-weeks. Why some subjects had more rapid responses is not clear in this small group; however, the rapid responders had higher basal levels of HbF, >58%, compared to other subjects. What level of hematologic response is required for definitive benefit and amelioration of clinical disease in BTI will depend in part on the magnitude of baseline anemia in any individual subject. A wide spectrum of clinical severity occurs in the β-thalassemias, related to the >200 globin molecular mutations and secondary genetic modifiers (Thein et al, 2009; Nuinoon et al, 2010; Jiang et al, 2006; Garner et al, 1998).28–36 Splenomegaly, EPO levels, and age affect baseline severity and influence responses to other HbF-inducing therapeutics in BTI patients (Perrine, 2005); larger and longer trials are required to evaluate their influence on hematologic parameters such as hemoglobin levels. In summary, this study identifies a new oral therapeutic that can be easily administered long-term, has favorable PK profiles with little variability between subjects, and which induced HbF in 8/9 treated BTI subjects at the optimal dose identified. Longer studies of HQK-1001 in diverse thalassemia populations should now be performed to determine which genetic subtypes or patient characteristics may respond most favorably with more extended use.
Acknowledgments
Supported by HemaQuest Pharmaceuticals
Footnotes
Authors’ contribution
RB and SP participated in study design and were involved in all aspects of the trial conduct. SP, SF, AI, and SLT participated in the writing and review of the manuscript. WW analyzed data. SLT and MB performed laboratory studies. SF, AI, NS, SK, AT, NC were significant clinical contributors to the trial.
References
- Boosalis MS, Castenada S, Bohacek R, Trudel M, Mabaera R, Emery D, Lowrey C, Shen L, Faller DV, Perrine SP. Novel therapeutic candidates, identified by molecular modeling, induce fetal globin gene expression in vivo. Blood Cells. Mol. Diseases. 2011;47:107–116. doi: 10.1016/j.bcmd.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourantas K, Economou G, Georgiou J. Administration of high doses of recombinant human erythropoietin to patients with beta- thalassemia intermedia: a preliminary trial. European Journal of Haematology. 1997;58:22–25. doi: 10.1111/j.1600-0609.1997.tb01405.x. [DOI] [PubMed] [Google Scholar]
- Cappellini D, Graziadei MG, Ciceri L, Comino A, Bianchi P, Porcella A, Fiorelli G. Oral isobutyramide therapy in patients with thalassemia intermedia: results of a phase II open study. Blood Cells, Molecules and Diseases. 2000;26:105–111. doi: 10.1006/bcmd.2000.0283. [DOI] [PubMed] [Google Scholar]
- Cappellini MD, Marelli S. Approaches to therapy of thalassemia intermedia. In: Maggio A, Hoffbrand AV, editors. Clinical aspects and therapy of hemoglobinopathies. Firenze: SEE Societa Editrice Europea di Nicodemo Maggiulli & C. Snc; 2004. pp. 203–213. [Google Scholar]
- Castaneda S, Boosalis MS, Emery D, Thies A, Faller DV, Perrine SP. Enhancement of growth and survival and alterations in Bcl-family proteins in beta-thalassemic erythroid progenitors by novel short-chain fatty acid derivatives. Blood Cells, Molecules and Diseases. 2005;35:217–226. doi: 10.1016/j.bcmd.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Luo HY, Steinberg MH, Chui DHK. BCL11A represses HBG transcription in K562 cells. Blood Cells, Molecules and Diseases. 2009;42:144–149. doi: 10.1016/j.bcmd.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Collins AF, Pearson HA, Giardina P, McDonagh KT, Brusilow SW, Dover GJ. Oral sodium phenylbutyrate therapy in homozygous beta thalassemia: a clinical trial. Blood. 1995;85:43–49. [PubMed] [Google Scholar]
- Dunbar C, Travis W, Kan YW, Nienhuis A. 5-Azacytidine treatment in a beta0-thalassaemic patient unable to be transfused due to multiple alloantibodies. British Journal of Haematology. 1989;72:467–468. doi: 10.1111/j.1365-2141.1989.tb07734.x. [DOI] [PubMed] [Google Scholar]
- Fucharoen S, Siritanaratkul N, Winichagoon P, Chowthaworn J, Siriboon W, Muangsup W, Chiacharoen S, Poolsup P, Piankijagum A, Schechter AN, Rodgers GP. Hydroxyurea increases hemoglobin F levels and improves the effectiveness of erythropoiesis in beta-thalassemia/hemoglobin E disease. Blood. 1996;87:887–892. [PubMed] [Google Scholar]
- Galanello R, Perseu L, Satta S, Demartis FR, Campus S. Phenotype-genotype correlation in beta thalassemia. In: Maggio A, editor. Thalassemia Reports, Proceedings of the 12th International Conference on Thalassemia and Hemoglobinopathies; 2011, May 11–14; Antalya, Turkey. Italy: PagePress; 2011. pp. e6pp. 16–20. [Google Scholar]
- Gallo E, Massaro P, Miniero R, David D, Tarella C. The importance of the genetic picture and globin synthesis in determining the clinical and haematological features of thalassaemia intermedia. British Journal of Haematology. 1979;41:211–221. doi: 10.1111/j.1365-2141.1979.tb05850.x. [DOI] [PubMed] [Google Scholar]
- Garner C, Mitchell J, Hatzis T, Reittie J, Farrall M, Thein SL. Haplotype mapping of a major quantitative-trait locus for fetal hemoglobin production, on chromosome 6q23. American Journal of Human Genetics. 1998;62:1468–1474. doi: 10.1086/301859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar FM, Pearson HA. Pharmacologic treatment of thalassemia intermedia with hydroxyurea. The Journal of Pediatrics. 1994;125:490–492. doi: 10.1016/s0022-3476(05)83304-9. [DOI] [PubMed] [Google Scholar]
- Jiang J, Best S, Menzel S, Silver N, Lai MI, Surdulescu GL, Spector TD, Thein SL. cMYB is involved in the regulation of fetal hemoglobin production in adults. Blood. 2006;108:1077–1083. doi: 10.1182/blood-2006-01-008912. [DOI] [PubMed] [Google Scholar]
- Labie D, Pagnier J, Lapoumeroulie C, Rouabhi F, Dunda-Belkhodja O, Chardin P, Beldjord C, Wajcmman H, Fabry ME, Nagel RL. Common haplotype dependency of high G gammaglobin gene expression and high Hb F levels in beta-thalassemia and sickle cell anemia patients. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:2111–2114. doi: 10.1073/pnas.82.7.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley TJ, DeSimone J, Anagnou NP, Keller GH, Humphries RK, Turner PH, Young NS, Keller P, Nienhuis AW. 5-azacytidine selectively increases gamma-globin synthesis in a patient with beta+ Thalassemia. The New England Journal of Medicine. 1982;307:1469–1475. doi: 10.1056/NEJM198212093072401. [DOI] [PubMed] [Google Scholar]
- Lowrey CH, Nienhuis AW. Brief report: treatment with azacitidine of patients with end-stage beta-thalassemia. The New England Journal of Medicine. 1993;329:845–848. doi: 10.1056/NEJM199309163291205. [DOI] [PubMed] [Google Scholar]
- Nisli G, Kavakli K, Vergin C, Ōztop S, Çetingül N. Recombinant human erythropoietin trial in thalassemia intermedia. Journal of Tropical Pediatrics. 1996;42:330–334. doi: 10.1093/tropej/42.6.330. [DOI] [PubMed] [Google Scholar]
- Nuinoon M, Makarasara W, Mushiroda T, Setianingsih I, Wahidiyat P, Sripichai O, Kumasaka N, Takahashi A, Svasti S, Munkonqdee T, Mahasirimonqkol S, Peerapittayamonqkol C, Viprakasit V, Kamatani N, Winichaqoon P, Kubo M, Nakamura Y, Fucharoen S. A genome-wide association identified the common genetic variants influence disease severity in beta0-thalassemia/hemoglobin E. Human Genetics. 2010;127:303–314. doi: 10.1007/s00439-009-0770-2. [DOI] [PubMed] [Google Scholar]
- Olivieri NF, Saunthararajah Y, Thayalasuthan V, Kwiatowski J, Ware RE, Kuypers FA, Kim H, Trachtenberg FL, Vichinsky EP for the Thalassemia Clinical Network. A pilot study of subcutaneous decitabine in beta-thalassemia intermedia. Blood. 2011;118:2708–2711. doi: 10.1182/blood-2011-03-341909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace BS, White GL, Dover GJ, Boosalis MS, Faller DV, Perrine SP. Short-chain fatty acid derivatives induce fetal globin expression and erythropoiesis in vivo. Blood. 2002;100:4640–4648. doi: 10.1182/blood-2002-02-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine SP, Ginder GD, Faller DV, Dover GH, Ikuta T, Witkowska HE, Cai T, Vichinsky EP, Olivieri NF. A short- term trial of butyrate to stimulate fetal-globin-gene expression in the beta-globin disorders. The New England Journal of Medicine. 1993;328:81–86. doi: 10.1056/NEJM199301143280202. [DOI] [PubMed] [Google Scholar]
- Perrine SP. Fetal globin induction--can it cure beta thalassemia? Hematology/the Education Program of the American Society of Hematology. 2005:38–44. doi: 10.1182/asheducation-2005.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine SP, Mankidy R, Boosalis MS, Bieker JJ, Faller DV. Erythroid Krüppel-like factor (EKLF) is recruited to the gamma-globin gene promoter as a co-activator and is required for gamma-globin gene induction by short-chain fatty acid derivatives. European Journal of Haematology. 2009;82:466–476. doi: 10.1111/j.1600-0609.2009.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine SP, Wargin WA, Boosalis MS, Wallis W, Case S, Keefer JR, Faller DV, Welch WC, Berenson RJ. Evaluation of safety and pharmacokinetics of sodium 2, 2 dimethylbutyrate, a novel short chain fatty acid derivative, in a phase 1, double-blind, placebo-controlled, single- and repeat- dose studies in healthy volunteers. The Journal of Clinical Pharmacology. 2011;51:1186–1194. doi: 10.1177/0091270010379810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmilewitz EA, Aker M. The role of recombinant human erythropoietin in the treatment of thalassemia. Annals of the New York Academy of Sciences. 1998;850:129–138. doi: 10.1111/j.1749-6632.1998.tb10470.x. [DOI] [PubMed] [Google Scholar]
- Reich S, Buhrer C, Henze G, Ohlendorf P, Mesche M, Sinha P, Kage A, Müller C, Vetter B, Kulozik AE. Oral isobutyramide reduces transfusion requirements in some patients with homozygous beta-thalassemia. Blood. 2000;96:3357–3363. [PubMed] [Google Scholar]
- Sankaran VG, Xu J, Ragoczy T, Ippolito GC, Walkley CR, Maika SD, Fujiwara Y, Ito M, Groudine M, Bender MA, Tucker PW, Orkin SH. Developmental and species-divergent globin switching are driven by BCL11A. Nature. 2009;460:1093–1097. doi: 10.1038/nature08243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier SL. Pathobiology of thalassemic erythrocytes. Current Opinion in Hematology. 1997;4(2):75–78. doi: 10.1097/00062752-199704020-00001. [DOI] [PubMed] [Google Scholar]
- Singer ST, Kuypers FA, Olivieri NF, Weatherall DJ, Miqnacca R, Coates TD, Davies S, Sweeters N, Vichinsky EP. Fetal haemoglobin augmentation in E/beta0 thalassaemia: clinical and haematological outcome. British Journal of Haematology. 2005;131:378–388. doi: 10.1111/j.1365-2141.2005.05768.x. [DOI] [PubMed] [Google Scholar]
- Steinberg MH, Rodgers GP. Pharmacologic modulation of fetal hemoglobin. Medicine. 2001;80:328–344. doi: 10.1097/00005792-200109000-00007. [DOI] [PubMed] [Google Scholar]
- Taher A, Mussallem KM, Karimi M, El-Beshlaway A, Cappellini M. Overview on practices in thalassemia management aiming for lowering complication rates across a region of endemicity: the OPTIMAL CARE study. Blood. 2010;115:1886–1892. doi: 10.1182/blood-2009-09-243154. [DOI] [PubMed] [Google Scholar]
- Thein SL, Menzel S. Discovering the genetics underlying foetal haemoglobin production in adults. British Journal of Haematology. 2009;145:455–467. doi: 10.1111/j.1365-2141.2009.07650.x. [DOI] [PubMed] [Google Scholar]
- Thein SL, Menzel S, Lathrop M, Garner C. Control of fetal hemoglobin: new insights emerging from genomics and clinical implications. Human Molecular Genetics. 2009;18:R216–R223. doi: 10.1093/hmg/ddp401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uda M, Galanello R, Sanna S, Lettre G, Sankaran VG, Chen W, Usala G, Busonero F, Maschio A, Albai G, Piras MG, Sestu N, Lai S, Dei M, Mulas A, Crisponi L, Naitza S, Asunis I, Deiana M, Naqaraja R, Perseu L, Satta S, Cipollina MD, Sollainoc C, Moi P, Hirshhom JN, Orkin SH, Abecasis GR, Schlessinger D, Cao A. Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta- thalassemia. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1620–1625. doi: 10.1073/pnas.0711566105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherall DJ. The inherited diseases of hemoglobin are an emerging global health burden. Blood. 2010;115:4331–4336. doi: 10.1182/blood-2010-01-251348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber A, Nienhuis AW, Persons DA. Transcriptional regulation of fetal to adult hemoglobin switching: new therapeutic opportunities. Blood. 2011;117:3945–3953. doi: 10.1182/blood-2010-11-316893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GR. Drug metabolism and variability among patients in drug response. The New England Journal of Medicine. 2005;352:2211–2221. doi: 10.1056/NEJMra032424. [DOI] [PubMed] [Google Scholar]