Abstract
Research on schizophrenia has provided evidence of both impaired attentional control and dysfunctional magnocellular sensory processing. The present study tested the hypothesis that these impairments may be related, such that people with schizophrenia would be differentially distracted by stimuli that strongly activate the magnocellular pathway. To accomplish this, we used a visual attention paradigm from the basic cognitive neuroscience literature designed to assess the capture of attention by salient but irrelevant stimuli. Participants searched for a target shape in an array of non-target shapes. On some trials, a salient distractor was presented that either selectively activated the parvocellular system (parvo-biased distractors) or activated both the magnocellular and parvocellular systems (magno+parvo distractors). For both manual reaction times and eye movement measures, the magno+parvo distractors captured attention more strongly than the parvo-biased distractors in people with schizophrenia, but the opposite pattern was observed in matched healthy control participants. These results indicate that attentional control deficits in schizophrenia may arise, at least in part, by means of an interaction with magnocellular sensory dysfunction.
Keywords: schizophrenia, visual attention, magnocellular, eye movements, visual search, attentional capture
1. Introduction
Schizophrenia is associated with significant deficits in everyday functioning. This is in large part the result of deficits in cognitive functioning (Green, Kern, & Heaton, 2004), which may partly reflect an impairment in selecting goal-relevant information from the many sources of salient information in the environment. Accordingly, attentional impairment has been a key concept in schizophrenia research since the earliest theories (Bleuler, 1911) as well as in more recent investigations (e.g., Braff, 1993; Nuechterlein & Dawson, 1984).
However, many laboratory tasks have shown surprisingly little impairment in selective visual attention in people with schizophrenia (PSZ) compared to matched healthy control subjects (HCS). The clearest evidence comes from variants of the Posner spatial cuing paradigm, in which the effectiveness of attentional selection can be quantified as the difference in performance for stimuli presented at cued versus uncued locations. Across a large number of studies, this cuing effect is typically just as large or even larger in PSZ than in HCS (Hahn, et al., 2011; Spencer, et al., 2011). In addition, Luck et al. (2006) found both behavioral and electrophysiological evidence that shifting attention to the location of a single, salient target in a visual search array is unimpaired in PSZ compared to HCS. Furthermore, both PSZ and HCS can efficiently encode task-relevant visual stimuli into working memory and suppress the encoding of equally salient distractors (Gold, et al., 2006). These results suggest that PSZ do not experience difficulty in implementing attentional selection if attention can be easily guided to the correct target. Instead, PSZ may be impaired in their ability to select task-relevant information in the presence of strong competition from highly salient distractors (Luck & Gold, 2008).
Consistent with this hypothesis, PSZ were worse than HCS at selectively encoding non-flickering, task-relevant objects into working memory in the presence of more salient flickering distractors (Hahn, et al., 2010). However, the failure of selective attention in this experiment may reflect the fact that flickering stimuli are particularly effective at stimulating the magnocellular pathway (Merigan & Maunsell, 1993). Because the magnocellular system appears to be dysregulated in PSZ (Butler & Javitt, 2005; Butler, et al., 2007; Martinez, et al., 2008), the finding of impaired filtering of flickering objects may reflect a specific interaction between attentional control and magnocellular processing rather than a general impairment in controlling attention in the face of salient distractors.
Given the many findings showing reduced sensitivity and neural activation for stimuli that activate the magnocellular pathway in PSZ (Butler, et al., 2007; Keri, Kelemen, Benedek, & Janka, 2004; Schechter, et al., 2005), one might expect PSZ to exhibit reduced rather than increased distraction by stimuli that activate the magnocellular pathway (although see Skottun & Skoyles, 2007 for a critique of the magnocellular hypothesis). However, increased distraction might be expected given previous research showing that PSZ show potentiated backward masking, an effect that arises when target discrimination is impaired by trailing distracting information (for a review see, Green, Lee, Wynn, & Mathis, 2011). One potential explanation is that magnocellular information from the mask catches up to and interferes with the detailed, sustained-channel processing of the target (Breitmeyer & Ganz, 1976). Indeed, dysregulated processing of magnocellular input has been hypothesized to be the cause of increased masking deficits in PSZ (Butler, et al., 2003; Cadenhead, Serper, & Braff, 1998; Green, Nuechterlein, & Mintz, 1994; Schechter, Butler, Silipo, Zemon, & Javitt, 2003; Slaghuis & Curran, 1999). However, given that dysregulated magnocellular processing appears to yield greater interference by magnocellular stimuli in tasks that involve masking, it is plausible that dysregulated magnocellular processing might also yield greater interference by magnocellular distractors during visual search paradigms in PSZ.
Here we sought to address this possibility by using a well-studied visual search task in which we have previously shown that healthy young adults show largely equivalent capture independent of whether or not the stimuli activate the magnocellular pathway (Leonard & Luck, 2011). This general paradigm is frequently used in the basic cognitive neuroscience literature to assess interference from a salient yet irrelevant distractor (e.g., Bacon & Egeth, 1994; Theeuwes, 1994; Yantis & Jonides, 1990). Typically, the target is an object that is unique in the shape dimension (i.e., a single circle among multiple diamonds or a single diamond among multiple circles) and the irrelevant salient distractor is a color singleton (see Figure 1). Under conditions in which the specific target shape is unknown (i.e., participants are instructed to look for the unique shape in the display but are not told whether it will be the circle or the diamond), the color singleton attracts attention and thus slows search times for the shape target (Bacon & Egeth, 1994; Theeuwes, 1991).
Figure 1.
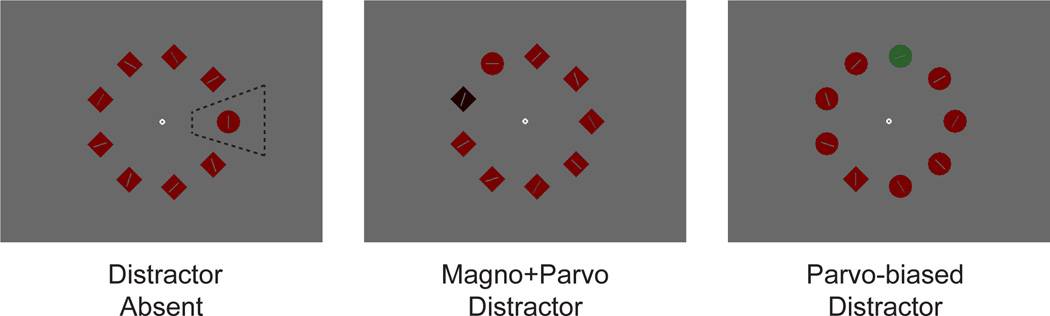
Example stimuli. The region indicated by the dotted line in the salient distractor absent panel shows an example interest area used for analysis, and is not part of the experimental display. Also note that printed colors do not accurately depict those used in the experiment.
In the current experiment, we used two types of irrelevant distractors, one designed to activate both the magnocellular and parvocellular pathways (magno+parvo distractors), and the other designed to selectively activate the parvocellular pathway (parvo-biased distractors).1 The magnocellular system is largely blind to differences in hue between stimuli that are equal in luminance, but both the magnocellular and parvocellular systems can easily discriminate large luminance differences (Kaplan & Shapley, 1982; Merigan & Maunsell, 1993). Our magno+parvo distractor differed greatly in luminance from the other objects and the background and therefore activated both processing streams. In contrast, our parvo-biased distractor differed in hue from the other objects and background but was equal in luminance, therefore minimizing activation of the magnocellular system. It is impossible to be certain that the parvo-biased distractor was completely indistinguishable from the other stimuli by the magnocellular system, but it should have been much more salient to the parvo-cellular system than to the magnocellular system.
This design makes it possible to distinguish among three specific types of impairment that might plausibly be present in PSZ. First, PSZ might show more capture than HCS for both parvo-biased and magno+parvo distractors, which would indicate a general failure in using top-down control mechanisms to avoid distraction. Second, PSZ might show less capture than HCS for the magno+parvo distractor but not the parvo-biased distractors. This would indicate that reduced sensitivity to magnocellular stimulation in PSZ leads to reduced magnocellular-based salience. Third, PSZ might show exaggerated capture relative to HCS for the magno+parvo distractors but not the parvo-based distractors. This would reflect a more complex dysregulation of the magnocellular pathway, and it would be analogous to the increased magno-based masking observed in PSZ. To preview the results, we find evidence from multiple measures that supports the third alternative in which there is dysregulation of magnocellular input in schizophrenia, similar to that found in backward masking.
2. Methods
2.1 Participants
In this experiment, 26 HCS and 33 PSZ were tested. Two PSZ were excluded due to the reaction time criteria described below, and the subsequent demographics and analyses are from the remaining sample of 26 HCS and 31 PSZ. All participants passed Ishihara’s Test for Color Deficiency (2001, Kanehara Trading Inc., Tokyo, Japan).
There were no significant differences between HCS and PSZ in age, race, gender, or parental education (see Table 1 for statistics). As is typically found, PSZ completed significantly fewer years of education than HCS, likely due to interference in education attainment owing to disease onset in early adulthood.
Table 1.
Demographic information for sample. Means are shown with SEM in parentheses.
| HCS N = 26 |
PSZ N = 31 |
Stats | |
|---|---|---|---|
| Age (yrs) | 42.7 (1.90) | 41.0 (1.91) | t(55) = 0.59, p = 0.55 |
| Education (yrs) | 14.7 (0.41) | 12.3 (0.41) | t(55) = 4.26 p < 0.01 |
| Parental Education (yrs)1 | 12.98 (0.56) | 12.96 (0.48) | t(55) = 0.03, p = 0.98 |
| Male/Female (M:F) | 18:8 | 23:8 | χ2(1) = 0.17, p = 0.68 |
| Race (AA:W:O) | 9:17:0 | 9:21:1 | χ2(2) = 0.99, p = 0.61 |
Parental education is the average years of mother and father when both are available. Two participants in the HCS group and two participants in the PSZ group were only able to report education information about a single parent. One participant in the PSZ group could report no parental education information and was excluded from this analysis.
Material from past medical records, collateral informants (when available), and the results of the Structured Clinical Interview for DSM-IV-TR Axis 1 Disorders (First, Spitzer, Miriam, & Williams, 2002) were combined to make a diagnosis based on the standard operational criteria in the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV). Final diagnoses were reached at a consensus conference chaired by co-author J.M.G. All PSZ were clinically stable outpatients who had been receiving the same medications, at the same dose, for at least 4 weeks prior to study participation. Three were receiving typical antipsychotic medication, 27 atypical antipsychotic medication, and 1 both. Additionally, 18 of the PSZ were on an antidepressant, 4 were on a mood stabilizer, 11 were on an anxiolytic, 6 were on an antiparkinsonian, and 1 was on Modafinil for sleep apnea. The 31 PSZ were categorized into the following types: 14 paranoid, 10 undifferentiated, 2 bipolar, 2 residual, 1 catatonic, 1 disorganized, 1 depressed.
Control participants were recruited via random digit dialing, word of mouth, and limited use of online advertisements. They were screened using the complete Structured Clinical Interview for DSM-IV Axis 1 Disorders (SCID-I; (First, et al., 2002) and Axis II Personality Disorders (SCID-IV; (Pfhol, Blum, & Zimmerman, 1995). All had no current diagnosis of any Axis I disorder or any Axis II schizophrenia-spectrum disorder, and all denied a lifetime history of psychosis or any family history of psychotic disorders in first-degree relatives.
All participants (PSZ as well as HCS) were free of other medical or neurologic comorbidity that could reasonably influence test performance, including substance abuse or dependence within the last 12 months. This protocol was approved by the Institutional Review Board at the University of Maryland School of Medicine, and all participants gave written informed consent before taking part in this study.
2.2 Task and design
During the task, an SR Research Eyelink 1000 desk-mounted system recorded eye position from the right eye at 2000 Hz. At the beginning of each trial, a fixation point appeared at the center of the display, and the task was initiated when fixation was maintained for 500 ms within 1° of this point. Participants were told that they could move their eyes freely once the visual search stimuli were presented.
Participants searched for the unique shape, either a single red circle among multiple diamonds or a single red diamond among multiple circles (see Figure 1). The task was to make a speeded response about the orientation of the line inside the target shape (horizontal or vertical) by pressing one of two buttons on a gamepad. A salient color singleton distractor was present on two-thirds of trials and was equally likely to occur at any of the nontarget locations; participants were informed that this distractor was irrelevant and could never be the target.
Each participant received 144 no-distractor trials, 144 parvo-biased distractor trials, and 144 magno+parvo distractor trials, randomly intermixed. The target was equally likely to be a circle among diamonds or a diamond among circles, and it occurred at each location with equal probability. The experiment was divided into 3 blocks of trials, separated by long breaks, with short breaks approximately every 50 trials within a block.
2.3 Stimuli
On each trial, the search display consisted of nine objects presented evenly spaced on an imaginary circle with a radius of 3.6°. The circles and diamonds were each 1.2° in diameter. A line segment (0.7° in length) was presented inside of each object; the line within the target shape was either vertical or horizontal, and the line randomly tilted within the nontarget shapes. The search display was presented on a gray background (3.9 cd/m2, CIE X: 0.26, Y: 0.24) and all of the objects, with the exception of the singleton distractors, were a nearly isoluminant red (3.7 cd/m2, CIE X: 0.64, Y: 0.31).
The magno+parvo distractor was a very dark red (0.5 cd/m2, CIE X: 0.32, Y: 0.46) (magno+parvo). Thus, when this distractor was present, it was a very different luminance from the background (Michelson contrast = 0.77), but the target and other non-target shapes were nearly the same luminance as the background. A luminance decrement was chosen over a luminance increment such that increased capture by the magno+parvo distractor would not be due to a higher intensity, but rather due to the fact that the magnocellular system would be sensitive to the contrast between this object and the rest of the display.
The parvo-biased distractor was green, such that it differed in color from the other objects in the display. However, the luminance of this distractor was adjusted for each participant to the point of subjective isoluminance to these red objects. This was done using heterochromatic flicker fusion that adjusted the depth of modulation of the green gun. The magnocellular system is relatively insensitive to isoluminant color differences (Kaplan & Shapley, 1982; Merigan & Maunsell, 1993), and therefore this type of singleton should selectively activate the parvocellular stream. The stimulus parameters were chosen on the basis of a previous study (Leonard & Luck, 2011) and additional pilot testing, with the goal that the parvo-biased and magno+parvo singletons would be approximately equally salient (i.e., would produce approximately equal amounts of capture in HCS).
It should be noted that a magno-biased condition was not included because of the difficulty in developing a stimulus that isolates the magnocellular system and can also be used in this type of search paradigm. Our stimuli also do not isolate contributions from the koniocellular pathway, which has more idiosyncratic tuning properties (Hendry & Reid, 2000). No evidence of specific koniocellular dysfunction has been reported in PSZ.
Note that the distance between the target and the salient distractor varied from trial to trial, and capture effects are typically largest when the distractor is near the target (Caputo & Guerra, 1998; Mounts, 2000). We found the same pattern in our data, but this effect did not interact with any of the other factors, so we have collapsed across target-distractor distance in the analyses presented here.
2.4 Data Analysis
For each participant, the median reaction time from correct trials for each trial type was the primary behavioral measure of interest. The mean and standard deviation of performance in the distractor-absent trials was calculated across all participants, and any participant whose individual performance fell outside of 3 standard deviations was excluded from all analyses. Two participants from the PSZ group with exceptionally slow manual response times (4100 ms and 5018 ms averaged across trial types) met this outlier criterion and were excluded from analysis (and the demographic information in Table 1).
Saccades and fixations were detected using the default Eyelink parser, with saccade onset detection settings of minimum eye velocity threshold at 30°/s and a minimum eye acceleration threshold at 9500°/s/s. A wedge shaped interest area was created for each object that subtended 1.8° both inward and outward from the center of the object, such that each of the 9 interest areas encompassed 40° of the circumference of the circle on which the stimuli were distributed (see Figure 1, left panel, for an example). Fixations whose mean position fell within this interest area were categorized as a visit to that object. Fixation latencies were measured as the time that elapsed between stimulus onset and the beginning of the first fixation in which the eyes were within one of the 9 interest areas.
3. Results
3.1 Accuracy
Target discrimination accuracy was near ceiling across all trial types for both HCS and PSZ, with errors on fewer than 3% of trials (see Table 2). An ANOVA with factors of condition (no distractor, magno+parvo, parvo-biased) and group (HCS, PSZ) showed no main effect of condition (F < 1), no main effect of group, F(1, 55) = 2.82, p = 0.10, and no significant interaction (F < 1). Only correct trials were used in further analyses.
Table 2.
Accuracy (% correct) of manual response for task (standard error in parentheses).
| No Distractor | Magno+Parvo | Parvo-biased | |
|---|---|---|---|
| HCS | 98.5 (0.33) | 98.4 (0.25) | 98.2 (0.24) |
| PSZ | 97.6 (0.47) | 97.3 (0.51) | 97.6 (0.48) |
3.2 Manual reaction time and latency to target fixation
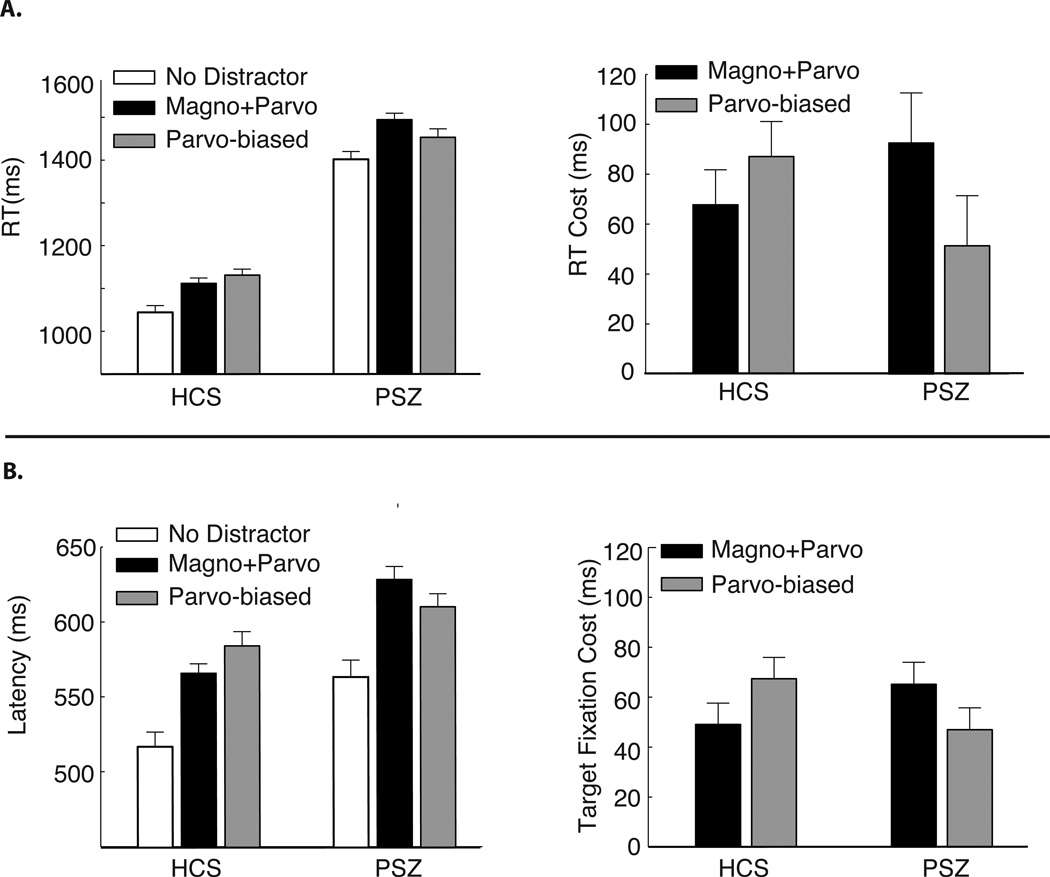
Figure 2a shows the manual reaction times (RT). Overall, RTs were elevated in PSZ compared to HCS, as is typical. In addition, both groups exhibited slower RTs when an irrelevant singleton distractor was present in the display compared to no-distractor trials, indicating that the irrelevant singleton effectively attracted attention away from the target. To focus on distractor interference, the reaction time (RT) measure from the no-distractor trials were subtracted from the RT measures in the magno+parvo and parvo-biased distractor trials for each participant, creating RT Cost for each distractor type (shown at right of Figure 2a).
Figure 2.
A) Manual reaction times. RT across the no distractor, magno+parvo, and parvo-biased trial types are shown on the left, and the RT Cost relative to the no distractor trials is shown at the right. B) Target fixation latencies. Time at which the target interest area was first entered across the no distractor, magno+parvo, and parvo-biased trial types are shown on the left, and the Target Fixation Cost relative to the no distractor trials is shown on the right. Error bars here represent the within-subjects standard error of the mean, calculated separately for each group (Cousineau, 2005; Morey, 2008).
After the target object is fixated, the participant must still identify the line segment, select the appropriate response, and execute the button-press. To provide a more refined measure of the initial allocation of attention that excludes these later processes, we also analyzed the latency of the first fixation that landed within the target interest area on each trial. These target fixation latencies were much faster than the manual RTs, but they showed the same pattern as the manual response times (Figure 2b). As with manual RT, we subtracted target fixation latency for the no-distractor trials from target fixation latency for the distractor trials to create a Target Fixation Cost measure.
To examine the nature of these interference effects, the RT Cost and Target Fixation Cost measures were entered into separate ANOVAs with factors of distractor type (magno+parvo, parvo-biased) and group (HCS, PSZ). The main effect of group was not significant for either measure (Fs < 1), indicating that the overall costs were no greater in PSZ than in HCS. In addition, the main effect of distractor type was not significant for either measure (Fs < 1), indicating that the overall amount of capture was approximately equivalent for the two distractor types. However, PSZ exhibited greater capture for magno+parvo distractors whereas HCS exhibited greater capture for parvo-biased distractors, which led to significant interactions between distractor type and group for manual RT (F(1,55) = 5.88, p = 0.02, η2p = 0.10) and for target fixation latency (F(1,55) = 8.99, p < 0.01, η2p = 0.14).
Follow-up within-group comparisons were used to isolate the sources of these interactions. Target Fixation Cost was significantly greater for magno+parvo distractors than for parvo-biased distractors in PSZ (t(30) = 2.11, p = 0.04, d = 0.38), whereas it was significantly greater for parvo-biased distractors than for magno+parvo distractors in HCS (t(25) = 2.18, p = 0.04, d = 0.43). RT Cost showed the same numerical pattern, although the differences did not reach significance for both groups (HCS: t(25) = 1.41, p = 0.17, d = 0.28; PSZ: t(30) = 2.09, p = 0.05, d = 0.38). The fact that the same pattern of means was observed for both the eye movement and manual response measures, but did not reach significance for the manual response measure, likely reflects the fact that additional sources of variance contribute to the manual response measure. That is, the time required to localize the target contributes to both measures, but the manual response also requires discrimination of the target orientation and the selection of an appropriate choice response, which adds variance that may overshadow the effect of interest (S. J. Luck, et al., 2009).
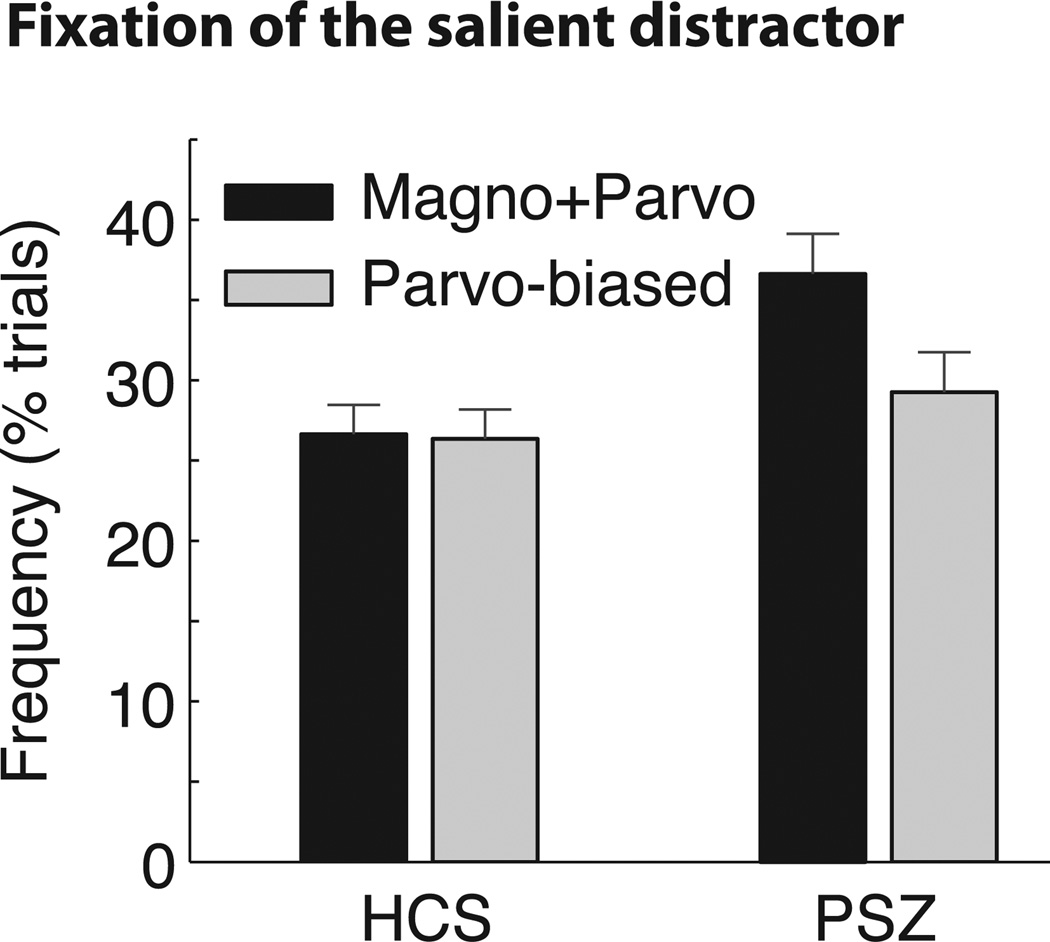
3.3 Fixations of the salient distractor
To provide a more direct measure of attention capture, we examined the percentage of trials on which a given distractor type was fixated. PSZ fixated the magno+parvo distractor more often than they did the parvo-biased distractor, whereas there was little or no difference between distractor types for HCS (Figure 3). An ANOVA of this measure showed a significant main effect of distractor type, F(1,55) = 5.94, p = 0.02, η2p = 0.10, and a significant interaction of distractor type and group, F(1,55) = 5.05, p = 0.03, η2p = 0.08. The main effect of group was not significant, F(1,55) = 1.73, p = 0.19.
Figure 3.
Distractor fixation frequency. Percent of trials the irrelevant distractor was fixated before the target for each group. Error bars here represent the within-subjects standard error of the mean, calculated separately for each group (Cousineau, 2005; Morey, 2008).
Within-subjects follow-up comparisons of the two distractor types showed that PSZ visited the magno+parvo distractor significantly more than the parvo-biased distractor, t(30) = 3.00, p < 0.01, d = 0.54, whereas HCS showed no significant difference between distractor types, t(25) = 0.17, p = 0.87. No significant difference between groups was found for the number of fixations of the parvo-biased distractor, t(55) = 0.61, p = 0.54. This provides converging evidence that PSZ have difficulty suppressing shifts of attention to stimuli that activate both magnocellular and parvocellular pathways but show normal capture of attention to stimuli that selectively activate the parvocellular pathway. However, the distractor fixation results did not perfectly mirror the RT results: whereas HCS exhibited more RT capture for the parvo-biased distractor than for the magno+parvo distractor, they did not show evidence for greater fixation of the parvo-biased distractor than the magno+parvo distractor. This may indicate that the parvo-biased distractor captured covert attention without capturing overt attention in HCS.
3.4 Medication Analyses
To address the possible role of medication in explaining the magnitude of attentional capture, we calculated chlorpromazine equivalents for each participant in the PSZ group (Andreasen, Pressler, Nopoulos, Miller, & Ho, 2010). We examined how this correlated with the RT Cost and Target Fixation Cost measures and with the frequency of fixating the singleton distractor, separately for parvo-biased and magno+parvo distractors. None of the correlations approached significance (see Table 3).
Table 3.
Correlations with chlorpromazine equivalent measure.
| Magno+Parvo | Parvo-biased | |
|---|---|---|
| RT Cost | r = −0.03, p = 0.88 | r = −0.05, p = 0.79 |
| Target Fixation Cost | r = 0.05, p = 0.77 | r = 0.01, p = 0.94 |
| Frequency of Salient Distractor Fixation | r = 0.19, p = 0.28 | r = 0.05, p = 0.81 |
Previous research suggests that anxiolytics may influence contrast sensitivity (e.g., Giersch, Speeg-Schatz, Tondre, & Gottenkiene, 2006; Harris & Phillipson, 1995). Because 11 of our 31 PSZ were taking anxiolytics, it is important to ensure that these medications were not somehow responsible for the finding of greater capture by magno+parvo singletons than by parvo-biased singletons. To assess the role of anxiolytics, we took our three main measures of capture (RT Cost, Target Fixation Cost, and Salient Distractor Fixation Probability) and conducted an ANOVA on the PSZ with factors of Anxiolytic use (present, absent) and distractor type (parvo-biased, magno+parvo). For all three measures, the difference in capture was similar for the two subgroups of PSZ, and none of the Anxiolytic × distractor type interactions approached significance (p’s > 0.3).
We also conducted additional analyses to determine whether our finding that PSZ exhibited greater capture for magno+parvo distractors than for parvo-biased distractors could be observed in the absence of anxiolytic use. Specifically, we performed t tests comparing the two distractor types in the subset of PSZ who were not using anxiolytics. As in the whole group of PSZ, this subgroup had a larger capture effect for magno+parvo distractors than for parvo-biased distractors. This effect was statistically significant for the Target Fixation Cost measure, t(19), 2.5, p = 0.02. It was only marginally significant for the RT Cost measure, t(19) = 1.9, p = 0.07, and for the probability of fixating the salient distractor, t(19) = 1.8, p = 0.08. The fact that two of these three measures were only marginally significant is unsurprising given the smaller N in these analyses than in the whole-group analyses. The overall pattern indicates that the use of anxiolytics among a subset of our sample of PSZ is not a likely explanation for the finding of greater capture by magno+parvo singletons.
4. Discussion
This study was designed to distinguish among three plausible hypotheses regarding the relationship between attentional control and magnocellular sensory processing in PSZ. Specifically, we sought to determine whether PSZ exhibit a general increase in capture of attention by salient distractors, a reduced capture of attention by salient distractors that activate the magnocellular pathway, or an increased capture of attention by salient distractors that activate the magnocellular pathway. We found several pieces of converging evidence for the last of these three possibilities. Specifically, the overall capture of attention by salient distractors was no different in PSZ and HCS, ruling out a general impairment in the suppression of salient but irrelevant information. However, PSZ showed greater capture for stimuli that activated the magnocellular pathway (plus the parvocellular pathway) than for stimuli that were designed to selectively activate the parvocellular pathway, whereas HCS did not show this effect (and in fact showed the opposite pattern). This pattern was observed for three different measures of capture: a) slowing of manual RT produced by the presence of a distractor; b) slowing of the arrival of the eyes to the target; and c) likelihood that the distractor was fixated prior to the target.
In healthy individuals, it is known that information from the magnocellular and parvocellular streams are both capable of capturing attention and triggering eye movements (Leonard & Luck, 2011; Sumner, Adamjee, & Mollon, 2002). In the present study, the parvo-biased stimuli produced somewhat greater capture of attention than did the magno+parvo stimuli for HCS, but this just means that the luminance contrast of the magno+parvo stimuli was not perfectly equated to the color contrast of the parvo-biased stimuli for these subjects (although it was equated for the two groups combined). This enhances the interpretability of the finding of greater capture by the magno+parvo distractors in PSZ, because it led to a crossover interaction for the RT Cost and Target Fixation Cost measures (see the bottom of Figure 2). In other words, PSZ did not merely show an exaggeration of the pattern shown by HCS, but instead showed an opposite-direction pattern. This would be extremely difficult to explain in terms of a generalized deficit.
This pattern also supports the contention that our parvo-biased and magno+parvo stimuli activated the parvocellular and magnocellular pathways to different degrees. That is, the present results cannot be explained by assuming that the two distractor types activated the same pools of neurons but to different degrees. In addition, a previous study using these same stimuli in college students found that the magno+parvo stimuli triggered faster saccades than the parvo-biased stimuli (Leonard & Luck, 2011), consistent with the faster transmission of information in the magnocellular system than in the parvocellular system (Nowak, Munk, Girard, & Bullier, 1995; Schmolesky, et al., 1998; White & Munoz, 2011). Thus, not only were the stimuli designed to differentially activate the two pathways, the pattern of results was consistent with differential activation. However, it should be noted that the effect sizes of the interaction between stimulus type and group across the different measures were relatively small in this study (η2p ranged from 0.08 to 0.14).
Our finding that attention is more likely to be drawn to distractors that activate the magnocellular pathway in PSZ is consistent with previous results showing that a stimulus that preferentially activates the magnocellular pathway (and not one that preferentially activates the parvocellular pathway) is a more effective backward mask in PSZ compared to HCS (Schechter, et al., 2003). Other studies have shown complimentary evidence that PSZ are biased toward processing low-spatial frequency information during visual categorization tasks (Laprevote, Oliva, Delerue, Thomas, & Boucart, 2010; Laprevote, et al., 2013). Furthermore, Ducato, et al. (2008) found that PSZ could not inhibit interference from motion distractors, which likely activate the magnocellular pathway, even when occurrence of the distracting signal was predictable. These findings contrast with several studies in which PSZ exhibited reduced sensory responses to stimuli that were designed to selectively activate the magnocellular pathway (Butler, et al., 2007; Schechter, et al., 2005). It also contrasts with the finding of impaired performance in perceptual closure tasks that rely on the magnocellular pathway (Doniger, Foxe, Murray, Higgins, & Javitt, 2002; Sehatpour, et al., 2010).
The finding that stimuli that activate the magnocellular pathway produce greater interference but smaller sensory responses and poorer perceptual closure seems contradictory. One possible explanation for this apparent discrepancy is that a chronic weakness of signals from the magnocellular pathway leads to an increased weighting of information from this pathway when it is integrated with other information at higher levels of the visual system. That is, the magnocellular information is weak and of poor quality, producing small electrophysiological responses and poor contrast sensitivity, but as a compensatory mechanism it might be given greater weight in higher-level processes, leading to exaggerated masking and attentional capture. Why, then, doesn’t this higher weight lead to improved perceptual closure? The answer may be that perceptual closure requires high-fidelity information, whereas high-fidelity information is not needed to produce masking or capture attention. The combination of poorer quality and higher weight for magnocellular information in PSZ could therefore lead to poorer perceptual closure but greater masking and increased capture.
The general idea of dysregulated top-down weighting in schizophrenia has been discussed previously (Herzog, Roinishvili, Chkonia, & Brand, 2013; Laycock, Crewther, & Crewther, 2007). Furthermore, top-down dysregulation is supported by Harvey, et al. (2011), who showed that PSZ and HCS had a similar degree of connectivity between frontal regions and the lateral occipital area, in which magnocellular and parvocellular information about objects combine. However, the strength of this connectivity did not significantly vary as a function of stimulus visibility in PSZ as it did in HCS, suggesting some type of dysregulated attentional weighting. Additional evidence for increased attention to magnocellular information comes from Coleman, et al. (2009), who showed that PSZ are impaired at shifting from global processing (which is more related to low-spatial frequency and the magnocellular pathway) to local processing (which is related to high-spatial frequency processing and the parvocellular pathway).
In contrast to the current results, Leonard, et al. (in press) found that the antisaccade deficit in PSZ was equivalent for stimuli designed to preferentially activate the magnocellular, parvocellular, or both pathways. Unlike the antisaccade task, in which only a single object is presented on the screen, the current task involves simultaneous competition between the target and multiple distractors. Larger effects of top-down attention occur under conditions of simultaneous competition (Luck, Chelazzi, Hillyard, & Desimone, 1997; Moran & Desimone, 1985; Treue & Maunsell, 1996; Zhang & Luck, 2008). When the target is presented in isolation in the antisaccade task, it captures attention so strongly that the weighting of the magnocellular and parvocellular signals will have little or no effect on the initial capture. The exaggerated antisaccade effect in PSZ appears to reflect impairments in higher-level executive processes rather than dysregulation of the magnocellular pathway (Leonard, et al., in press).
On the other hand, Martinez et al. (2012) found electrophysiological evidence of both weaker sensory responses and impaired attentional selection for magnocellular stimuli. Specifically, participants were asked to attend either to high-frequency gratings (parvocellular stimuli) or low-frequency gratings (magnocellular stimuli) and look for subtle changes in spatial frequency within the attended gratings. Unlike in the antisaccade task, a detailed discrimination was required and, likely due to poor quality sensory information, PSZ exhibited increased difficulty compared to HCS in performing this task with low-frequency gratings. Moreover, the first 300 ms of the event-related potential showed reduced amplitude attention effects for low-spatial frequency stimuli in the PSZ compared to the HCS. This suggests that any increased weighting of the magnocellular signals does not amplify this early sensory response.
5. Conclusions
Overall, these results suggest that PSZ do not have a global impairment in top-down control mechanisms that increases distraction under all circumstances. Instead, there may be a specific increase in the amount of attention directed to irrelevant stimuli that activate the magnocellular system. This is consistent with previous results that suggest a specific impairment in filtering flickering distractors (Hahn, et al., 2010), but not other types of irrelevant stimuli (Gold, et al., 2006). Moreover, our findings clearly relate to previous evidence showing a specific impairment in the processing of visual inputs by the magnocellular system in schizophrenia (Butler & Javitt, 2005; Butler, et al., 2007; Martinez, et al., 2008), and suggest a disruption in the interface between attentional prioritization and specific types of sensory information in schizophrenia.
What effects might this have in the everyday lives of PSZ? Recurrent models suggest that information processed in the magnocellular stream provides the visual system with a rapid yet coarse estimate of identity information, creating a framework that shapes the slower parvocellular-based processing in the ventral stream (Bar, et al., 2006; Kveraga, Boshyan, & Bar, 2007). Increased weighting of such signals in crowded environments may be particularly damaging, in that details may be overpowered by coarse, and often inaccurate, visual information. This may lead to increased involuntary capture of attention and masking by magnocellular signals, accompanied by impairments in perception when low-fidelity magnocellular signals overpower the more precise parvocellular signals.
Acknowledgements
We also thank Leeka Hubzin, Sharon August, Bradley Gray, and Alexander Harvey for assistance in the conduct of this study and all of our volunteers who participated. Address correspondence to Carly J. Leonard, Center for Mind and Brain, 267 Cousteau Place, Davis, CA 95618, USA. cjleonard@ucdavis.edu. This work was supported by grant R01MH065034 from the National Institute of Mental Health to JMG and SJL
Footnotes
Magno+parvo distractors were used instead of magno-specific distractors because it is difficult to create a small but potent visual search object that selectively activates the magnocellular pathway.
References
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon WF, Egeth HE. Overriding stimulus-driven attentional capture. Perception & Psychophysics. 1994;55:485–496. doi: 10.3758/bf03205306. [DOI] [PubMed] [Google Scholar]
- Bar M, Kassam KS, Ghuman AS, Boshyan J, Schmid AM, Dale AM, Hamalainen MS, Marinkovic K, Schacter DL, Rosen BR, Halgren E. Top-down facilitation of visual recognition. Proc Natl Acad Sci U S A. 2006;103:449–454. doi: 10.1073/pnas.0507062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuler E. Dementia Praecox or the Group of Schizophrenias. New York: International Universities Press; 1911. [Google Scholar]
- Braff DL. Information processing and attention dysfunctions in schizophrenia. Schizophrenia Bulletin. 1993;19:233–259. doi: 10.1093/schbul/19.2.233. [DOI] [PubMed] [Google Scholar]
- Breitmeyer BG, Ganz L. Implications of sustained and transient channels for theories of visual pattern masking, saccadic suppression, and information processing. Psychological Review. 1976;83:1–36. [PubMed] [Google Scholar]
- Butler PD, DeSanti LA, Maddox J, Harkavy-Friedman JM, Amador XF, Goetz RR, Javitt DC, Gorman JM. Visual backward-masking deficits in schizophrenia: relationship to visual pathway function and symptomatology. Schizophr Res. 2003;59:199–209. doi: 10.1016/s0920-9964(01)00341-3. [DOI] [PubMed] [Google Scholar]
- Butler PD, Javitt DC. Early-stage visual processing deficits in schizophrenia. Curr Opin Psychiatry. 2005;18:151–157. doi: 10.1097/00001504-200503000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Martinez A, Foxe JJ, Kim D, Zemon V, Silipo G, Mahoney J, Shpaner M, Jalbrzikowski M, Javitt DC. Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain. 2007:417–430. doi: 10.1093/brain/awl233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenhead KS, Serper Y, Braff DL. Transient versus sustained visual channels in the visual backward masking deficits of schizophrenia patients. Biol Psychiatry. 1998;43:132–138. doi: 10.1016/S0006-3223(97)00316-8. [DOI] [PubMed] [Google Scholar]
- Caputo G, Guerra S. Attentional selection by distractor suppression. Vision Res. 1998;38:669–689. doi: 10.1016/s0042-6989(97)00189-2. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Cestnick L, Krastoshevsky O, Krause V, Huang Z, Mendell NR, Levy DL. Schizophrenia patients show deficits in shifts of attention to different levels of global-local stimuli: evidence for magnocellular dysfunction. Schizophr Bull. 2009;35:1108–1116. doi: 10.1093/schbul/sbp090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousineau D. Confidence intervals in within-subject designs: A simple alternative to Loftus and Masson's method. Tutorials in Quantitative Psychology. 2005;1:42–45. [Google Scholar]
- Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002;59:1011–1020. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- Ducato MG, Michael GA, Thomas P, Despretz P, Monestes JL, Loas G, Boucart M. Attentional capture in schizophrenia: failure to resist interference from motion signals. Cogn Neuropsychiatry. 2008;13:185–209. doi: 10.1080/13546800701706530. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition with Psychotic Screen. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Giersch A, Speeg-Schatz C, Tondre M, Gottenkiene S. Impairment of contrast sensitivity in long-term lorazepam users. Psychopharmacology (Berl) 2006;186:594–600. doi: 10.1007/s00213-006-0378-3. [DOI] [PubMed] [Google Scholar]
- Gold JM, Fuller RL, Robinson B, McMahon RP, Braun EL, Luck SJ. Intact attentional control of working memory encoding in schizophrenia. Journal of Abnormal Psychology. 2006;115:658–673. doi: 10.1037/0021-843X.115.4.658. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern MS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophrenia Research. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Green MF, Lee J, Wynn JK, Mathis KI. Visual masking in schizophrenia: overview and theoretical implications. Schizophr Bull. 2011;37:700–708. doi: 10.1093/schbul/sbr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. II. Specifying the visual channels. Arch Gen Psychiatry. 1994;51:945–951. doi: 10.1001/archpsyc.1994.03950120017004. [DOI] [PubMed] [Google Scholar]
- Hahn B, Robinson B, Harvey AN, Kaiser ST, Leonard CJ, Luck SJ, Gold JM. Visuospatial attention in schizophrenia: Deficits in broad monitoring. Journal of Abnormal Psychology. 2011 doi: 10.1037/a0023938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Robinson BM, Kaiser ST, Harvey AN, Becky VM, Leonard CJ, Kappenman ES, Luck SJ, Gold JM. Failure of schizophrenia patients to overcome salient distractors during working memory encoding. Biological Psychiatry. 2010;68:603–609. doi: 10.1016/j.biopsych.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JP, Phillipson OT. Effects of lorazepam on human contrast sensitivity. Psychopharmacology. 1995;117:379–384. doi: 10.1007/BF02246113. [DOI] [PubMed] [Google Scholar]
- Harvey PO, Lee J, Cohen MS, Engel SA, Glahn DC, Nuechterlein KH, Wynn JK, Green MF. Altered dynamic coupling of lateral occipital complex during visual perception in schizophrenia. Neuroimage. 2011;55:1219–1226. doi: 10.1016/j.neuroimage.2010.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SH, Reid RC. The koniocellular pathway in primate vision. Annual Review of Neuroscience. 2000;23:127–153. doi: 10.1146/annurev.neuro.23.1.127. [DOI] [PubMed] [Google Scholar]
- Herzog MH, Roinishvili M, Chkonia E, Brand A. Schizophrenia and visual backward masking: a general deficit of target enhancement. Front Psychol. 2013;4:254. doi: 10.3389/fpsyg.2013.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Shapley RM. X and Y cells in the lateral geniculate nucleus of macaque monkeys. J Physiol. 1982;330:125–143. doi: 10.1113/jphysiol.1982.sp014333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri S, Kelemen O, Benedek G, Janka Z. Vernier threshold in patients with schizophrenia and in their unaffected siblings. Neuropsychology. 2004;18:537–542. doi: 10.1037/0894-4105.18.3.537. [DOI] [PubMed] [Google Scholar]
- Kveraga K, Boshyan J, Bar M. Magnocellular projections as the trigger of top-down facilitation in recognition. J Neurosci. 2007;27:13232–13240. doi: 10.1523/JNEUROSCI.3481-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprevote V, Oliva A, Delerue C, Thomas P, Boucart M. Patients with schizophrenia are biased toward low spatial frequency to decode facial expression at a glance. Neuropsychologia. 2010;48:4164–4168. doi: 10.1016/j.neuropsychologia.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Laprevote V, Oliva A, Ternois AS, Schwan R, Thomas P, Boucart M. Low Spatial Frequency Bias in Schizophrenia is Not Face Specific: When the Integration of Coarse and Fine Information Fails. Front Psychol. 2013;4:248. doi: 10.3389/fpsyg.2013.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laycock R, Crewther SG, Crewther DP. A role for the 'magnocellular advantage' in visual impairments in neurodevelopmental and psychiatric disorders. Neurosci Biobehav Rev. 2007;31:363–376. doi: 10.1016/j.neubiorev.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Leonard CJ, Luck SJ. The role of magnocellular signals in oculomotor attentional capture. J Vis. 2011;11:1–12. doi: 10.1167/11.13.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CJ, Robinson BM, Kaiser ST, Hahn B, McClenon C, Harvey AN, Luck SJ, Gold JM. Testing sensory and cognitive explanations of the antisaccade deficit in schizophrenia. Journal of Abnormal Psychology. doi: 10.1037/a0034956. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. Journal of Neurophysiology. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Fuller RL, Braun EL, Robinson B, Summerfelt A, Gold JM. The speed of visual attention in schizophrenia: Electrophysiological and behavioral evidence. Schizophrenia Research. 2006;85:174–195. doi: 10.1016/j.schres.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Gold JM. The construct of attention in schizophrenia. Biological Psychiatry. 2008;64:34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Kappenman ES, Fuller RL, Robinson B, Summerfelt A, Gold JM. Impaired response selection in schizophrenia: evidence from the P3 wave and the lateralized readiness potential. Psychophysiology. 2009;46:776–786. doi: 10.1111/j.1469-8986.2009.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Hillyard SA, Bickel S, Dias EC, Butler PD, Javitt DC. Consequences of magnocellular dysfunction on processing attended information in schizophrenia. Cereb Cortex. 2012;22:1282–1293. doi: 10.1093/cercor/bhr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Hillyard SA, Dias EC, Hagler DJ, Jr, Butler PD, Guilfoyle DN, Jalbrzikowski M, Silipo G, Javitt DC. Magnocellular pathway impairment in schizophrenia: evidence from functional magnetic resonance imaging. J Neurosci. 2008;28:7492–7500. doi: 10.1523/JNEUROSCI.1852-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JH. How parallel are the primate visual pathways? Annu Rev Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Morey RD. Confidence intervals from normalized data: A correction to Cousineau (2005) Tutorial in Quantitative Methods for Psychology. 2008;4:61–64. [Google Scholar]
- Mounts JR. Evidence for suppressive mechanisms in attentional selection: feature singletons produce inhibitory surrounds. Percept Psychophys. 2000;62:969–983. doi: 10.3758/bf03212082. [DOI] [PubMed] [Google Scholar]
- Nowak LG, Munk MH, Girard P, Bullier J. Visual latencies in areas V1 and V2 of the macaque monkey. Visual Neuroscience. 1995;12:371–384. doi: 10.1017/s095252380000804x. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophrenia Bulletin. 1984;10:160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- Pfhol B, Blum M, Zimmerman M. Structured Interview for DSM-IV Personality Disorders (SIDP-IV) Iowa City: University of Iowa, Dept. of Psychiatry; 1995. [Google Scholar]
- Schechter I, Butler PD, Silipo G, Zemon V, Javitt DC. Magnocellular and parvocellular contributions to backward masking dysfunction in schizophrenia. Schizophr Res. 2003;64:91–101. doi: 10.1016/s0920-9964(03)00008-2. [DOI] [PubMed] [Google Scholar]
- Schechter I, Butler PD, Zemon VM, Revheim N, Saperstein AM, Jalbrzikowski M, Pasternak R, Silipo G, Javitt DC. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Cliniical Neurophysiology. 2005;116:2204–2215. doi: 10.1016/j.clinph.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Hanes DP, Thompson KG, Leutgeb S, Schall JD, Leventhal AG. Signal timing across the macaque visual system. Journal of Neurophysiology. 1998;79:3272–3278. doi: 10.1152/jn.1998.79.6.3272. [DOI] [PubMed] [Google Scholar]
- Sehatpour P, Dias EC, Butler PD, Revheim N, Guilfoyle DN, Foxe JJ, Javitt DC. Impaired visual object processing across an occipital-frontal-hippocampal brain network in schizophrenia: an integrated neuroimaging study. Arch Gen Psychiatry. 2010;67:772–782. doi: 10.1001/archgenpsychiatry.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skottun BC, Skoyles JR. Contrast sensitivity and magnocellular functioning in schizophrenia. Vision Res. 2007;47:2923–2933. doi: 10.1016/j.visres.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Slaghuis WL, Curran CE. Spatial frequency masking in positive- and negative-symptom schizophrenia. J Abnorm Psychol. 1999;108:42–50. doi: 10.1037//0021-843x.108.1.42. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Valdman O, Niznikiewicz MA, Shenton ME, McCarley RW. Enhanced facilitation of spatial attention in schizophrenia. Neuropsychology. 2011;25:76–85. doi: 10.1037/a0020779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner P, Adamjee T, Mollon JD. Signals invisible to the collicular and magnocellular pathways can capture visual attention. Curr Biol. 2002;12:1312–1316. doi: 10.1016/s0960-9822(02)01020-5. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Cross-dimensional perceptual selectivity. Percept Psychophys. 1991;50:184–193. doi: 10.3758/bf03212219. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Stimulus-driven capture and attentional set: selective search for color and visual abrupt onsets. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:799–806. doi: 10.1037//0096-1523.20.4.799. [DOI] [PubMed] [Google Scholar]
- Treue S, Maunsell JHR. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- White BJ, Munoz DP. Separate Visual Signals for Saccade Initiation during Target Selection in the Primate Superior Colliculus. Journal of Neuroscience. 2011;31:1570–1578. doi: 10.1523/JNEUROSCI.5349-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S, Jonides J. Abrupt visual onsets and selective attention: voluntary versus automatic allocation. J Exp Psychol Hum Percept Perform. 1990;16:121–134. doi: 10.1037//0096-1523.16.1.121. [DOI] [PubMed] [Google Scholar]
- Zhang W, Luck SJ. Feature-based attention modulates feedforward visual processing. Nat Neurosci. 2008;12:24–25. doi: 10.1038/nn.2223. [DOI] [PubMed] [Google Scholar]