Abstract
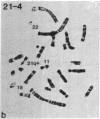
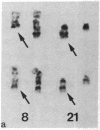
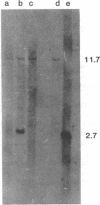
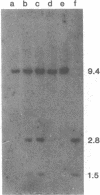
Acute myelogenous leukemia (AML), subgroup M2, is associated with a nonrandom chromosomal translocation, t(8;21)(q22,q22). The oncogene c-mos also has been localized to the q22 band on chromosome 8. There is also evidence that genes on chromosome 21 may be important in the development of leukemia. To determine whether the c-mos oncogene has been translocated in AML-M2 with this translocation and to isolate DNA sequences and genes from these two chromosomes that may be important in malignancy, we constructed somatic cell hybrids between a Chinese hamster ovary cell (CHO) mutant defective in glycine metabolism and myeloblasts with an 8;21 translocation from a patient with AML. We isolated the 21q+ chromosome of this translocation in a somatic cell hybrid and showed that the c-mos oncogene had not been translocated to chromosome 21, ruling out the possibility that translocation of c-mos to chromosome 21 is necessary for development of AML-M2. In addition, there was no detectable rearrangement of the c-mos locus within a 12.4-kilobase region surrounding the gene, indicating that rearrangement of the coding region of the gene itself or alteration of proximal 5' or 3' flanking sequences is not involved. We used this hybrid to determine whether specific DNA sequences and biochemical markers from chromosomes 8 and 21 had been translocated in this case.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhadeff B., Velivasakis M., Siniscalco M. Simultaneous identification of chromatid replication and of human chromosomes in metaphases of man-mouse somatic cell hybrids. (With 1 color plate). Cytogenet Cell Genet. 1977;19(4):236–239. doi: 10.1159/000130814. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Thierfelder W., Erikson J., Nishikura K., Finan J., Lenoir G. M., Nowell P. C. Transcriptional activation of an unrearranged and untranslocated c-myc oncogene by translocation of a C lambda locus in Burkitt. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6922–6926. doi: 10.1073/pnas.80.22.6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Bregni M., Erikson J., Patterson D., Gallo R. C., Croce C. M. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M., Malcolm S., Rabbitts T. H. Chromosome translocation can occur on either side of the c-myc oncogene in Burkitt lymphoma cells. Nature. 1984 Mar 15;308(5956):286–288. doi: 10.1038/308286a0. [DOI] [PubMed] [Google Scholar]
- Erikson J., Nishikura K., ar-Rushdi A., Finan J., Emanuel B., Lenoir G., Nowell P. C., Croce C. M. Translocation of an immunoglobulin kappa locus to a region 3' of an unrearranged c-myc oncogene enhances c-myc transcription. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7581–7585. doi: 10.1073/pnas.80.24.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. I., Steward J. K. Down's syndrome and leukaemia. Lancet. 1972 Dec 16;2(7790):1322–1322. doi: 10.1016/s0140-6736(72)92704-3. [DOI] [PubMed] [Google Scholar]
- Groffen J., Stephenson J. R., Heisterkamp N., de Klein A., Bartram C. R., Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984 Jan;36(1):93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- Gusella J., Varsanyi-Breiner A., Kao F. T., Jones C., Puck T. T., Keys C., Orkin S., Housman D. Precise localization of human beta-globin gene complex on chromosome 11. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5239–5242. doi: 10.1073/pnas.76.10.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieter P. A., Hollis G. F., Korsmeyer S. J., Waldmann T. A., Leder P. Clustered arrangement of immunoglobulin lambda constant region genes in man. Nature. 1981 Dec 10;294(5841):536–540. doi: 10.1038/294536a0. [DOI] [PubMed] [Google Scholar]
- Hollis G. F., Mitchell K. F., Battey J., Potter H., Taub R., Lenoir G. M., Leder P. A variant translocation places the lambda immunoglobulin genes 3' to the c-myc oncogene in Burkitt's lymphoma. Nature. 1984 Feb 23;307(5953):752–755. doi: 10.1038/307752a0. [DOI] [PubMed] [Google Scholar]
- Jones C., Patterson D., Kao F. T. Assignment of the gene coding for phosphoribosylglycineamide formyltransferase to human chromosome 14. Somatic Cell Genet. 1981 Jul;7(4):399–409. doi: 10.1007/BF01542985. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Induction and isolation of auxotrophic mutants in mammalian cells. Methods Cell Biol. 1974;8(0):23–39. doi: 10.1016/s0091-679x(08)60441-0. [DOI] [PubMed] [Google Scholar]
- Krumlauf R., Jeanpierre M., Young B. D. Construction and characterization of genomic libraries from specific human chromosomes. Proc Natl Acad Sci U S A. 1982 May;79(9):2971–2975. doi: 10.1073/pnas.79.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumlauf R., Jeanpierre M., Young B. D. Construction and characterization of genomic libraries from specific human chromosomes. Proc Natl Acad Sci U S A. 1982 May;79(9):2971–2975. doi: 10.1073/pnas.79.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Beau M. M., Larson R. A., Bitter M. A., Vardiman J. W., Golomb H. M., Rowley J. D. Association of an inversion of chromosome 16 with abnormal marrow eosinophils in acute myelomonocytic leukemia. A unique cytogenetic-clinicopathological association. N Engl J Med. 1983 Sep 15;309(11):630–636. doi: 10.1056/NEJM198309153091103. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Harris L. J., Stanton L. W., Erikson J., Watt R., Croce C. M. Transcriptionally active c-myc oncogene is contained within NIARD, a DNA sequence associated with chromosome translocations in B-cell neoplasia. Proc Natl Acad Sci U S A. 1983 Jan;80(2):519–523. doi: 10.1073/pnas.80.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera Khan P. Enzyme electrophoresis on cellulose acetate gel: zymogram patterns in mgh-mouse and man--Chinese hamster somatic cell hybrids. Arch Biochem Biophys. 1971 Aug;145(2):470–483. doi: 10.1016/s0003-9861(71)80007-3. [DOI] [PubMed] [Google Scholar]
- Moore E. E., Jones C., Kao F. T., Oates D. C. Synteny between glycinamide ribonucleotide synthetase and superoxide dismutase (soluble). Am J Hum Genet. 1977 Jul;29(4):389–396. [PMC free article] [PubMed] [Google Scholar]
- Morse H. G., Hays T., Patterson D., Robinson A. Giemsa-11 technique. Applications in the chromosomal characterization of hematologic specimens. Hum Genet. 1982;61(2):141–144. doi: 10.1007/BF00274204. [DOI] [PubMed] [Google Scholar]
- Prakash K., McBride O. W., Swan D. C., Devare S. G., Tronick S. R., Aaronson S. A. Molecular cloning and chromosomal mapping of a human locus related to the transforming gene of Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5210–5214. doi: 10.1073/pnas.79.17.5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puck T. T., Kao F. T. Genetics of somatic mammalian cells. V. Treatment with 5-bromodeoxyuridine and visible light for isolation of nutritionally deficient mutants. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1227–1234. doi: 10.1073/pnas.58.3.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechavi G., Givol D., Canaani E. Activation of a cellular oncogene by DNA rearrangement: possible involvement of an IS-like element. Nature. 1982 Dec 16;300(5893):607–611. doi: 10.1038/300607a0. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Identification of the constant chromosome regions involved in human hematologic malignant disease. Science. 1982 May 14;216(4547):749–751. doi: 10.1126/science.7079737. [DOI] [PubMed] [Google Scholar]
- Sinet P. M., Couturier J., Dutrillaux B., Poissonnier M., Raoul O., Rethore M. O., Allard D., Lejeune J., Jerome H. Trisomie 21 et superoxyde dismutase-1 (IPO-A). Tentative de localisation sur la sous bande 21Q22.1. Exp Cell Res. 1976 Jan;97:47–55. doi: 10.1016/0014-4827(76)90653-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taub R., Kirsch I., Morton C., Lenoir G., Swan D., Tronick S., Aaronson S., Leder P. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7837–7841. doi: 10.1073/pnas.79.24.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. T., Hanna M. L. Folate-dependent enzymes in cultured Chinese hamster ovary cells: impaired mitochondrial serine hydroxymethyltransferase activity in two additional glycine--auxotroph complementation classes. Arch Biochem Biophys. 1982 Sep;217(2):609–623. doi: 10.1016/0003-9861(82)90543-4. [DOI] [PubMed] [Google Scholar]
- Watson R., Oskarsson M., Vande Woude G. F. Human DNA sequence homologous to the transforming gene (mos) of Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4078–4082. doi: 10.1073/pnas.79.13.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Klein A., van Kessel A. G., Grosveld G., Bartram C. R., Hagemeijer A., Bootsma D., Spurr N. K., Heisterkamp N., Groffen J., Stephenson J. R. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982 Dec 23;300(5894):765–767. doi: 10.1038/300765a0. [DOI] [PubMed] [Google Scholar]
- van Someren H., Beijersbergen van Henegouwen H., Los W., Wurzer-Figurelli E., Doppert B., Vervloet M., Meera Khan P. Enzyme electrophoresis on cellulose acetate gel. II. Zymogram patterns in man-Chinese hamster somatic cell hybrids. Humangenetik. 1974;25(3):189–201. doi: 10.1007/BF00281426. [DOI] [PubMed] [Google Scholar]