Abstract
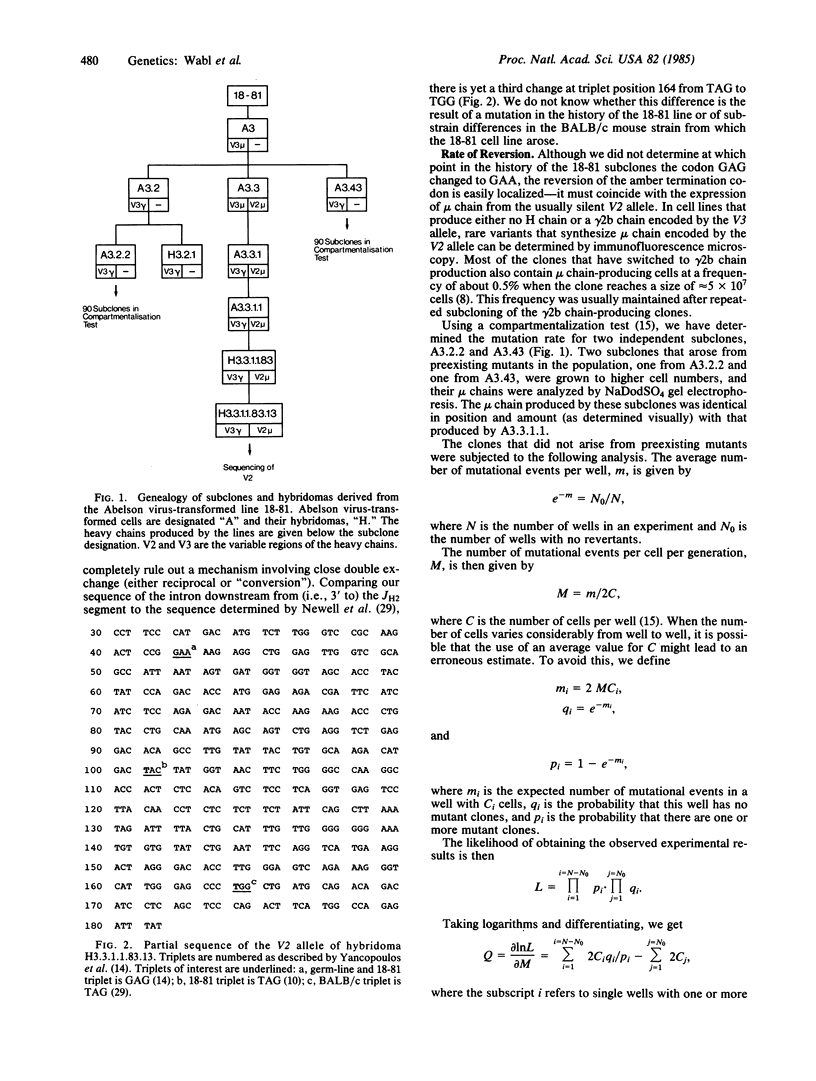
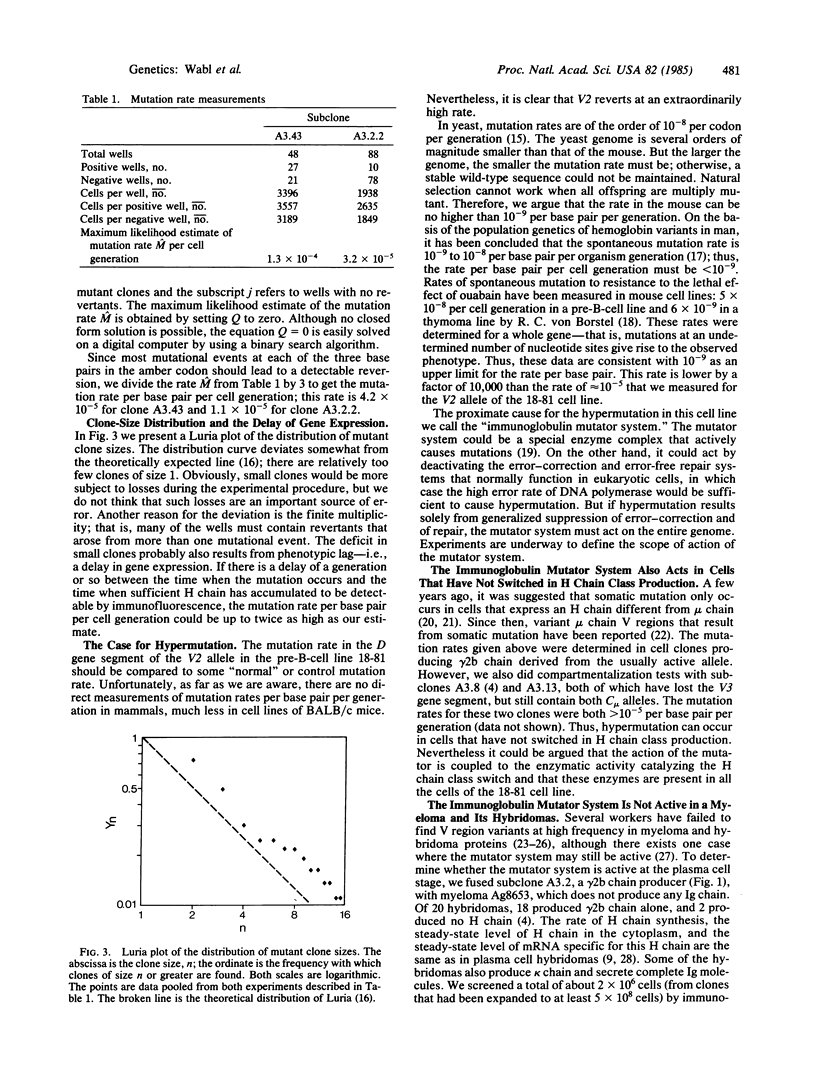
Most cells in the well-known pre-B-lymphocyte line 18-81 have correctly assembled genes for both alleles at the immunoglobulin heavy chain locus. Only one allele is "active"; the other, "silent" allele contains an amber termination codon. The rate of reversion of this amber codon was determined to be 0.3-1 X 10(-4) per cell generation. This high rate is termed hypermutation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Rosenberg N., Casanova R. J., Thomas E., Baltimore D. Immunoglobulin heavy-chain expression and class switching in a murine leukaemia cell line. Nature. 1982 Mar 25;296(5855):325–331. doi: 10.1038/296325a0. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Rosenberg N., Enea V., Siden E., Baltimore D. Multiple immunoglobulin heavy-chain gene transcripts in Abelson murine leukemia virus-transformed lymphoid cell lines. Mol Cell Biol. 1982 Apr;2(4):386–400. doi: 10.1128/mcb.2.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Brenner S., Milstein C. Origin of antibody variation. Nature. 1966 Jul 16;211(5046):242–243. doi: 10.1038/211242a0. [DOI] [PubMed] [Google Scholar]
- Burrows P. D., Beck-Engeser G. B., Wabl M. R. Immunoglobulin heavy-chain class switching in a pre-B cell line is accompanied by DNA rearrangement. Nature. 1983 Nov 17;306(5940):243–246. doi: 10.1038/306243a0. [DOI] [PubMed] [Google Scholar]
- Burrows P. D., Beck G. B., Wabl M. R. Expression of mu and gamma immunoglobulin heavy chains in different cells of a cloned mouse lymphoid line. Proc Natl Acad Sci U S A. 1981 Jan;78(1):564–568. doi: 10.1073/pnas.78.1.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D., Rudikoff S., Giusti A. M., Scharff M. D. Somatic mutation in a cultured mouse myeloma cell affects antigen binding. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1240–1244. doi: 10.1073/pnas.79.4.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dildrop R., Brüggemann M., Radbruch A., Rajewsky K., Beyreuther K. Immunoglobulin V region variants in hybridoma cells. II. Recombination between V genes. EMBO J. 1982;1(5):635–640. doi: 10.1002/j.1460-2075.1982.tb01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart P. J., Johnson N. D., Douglas R., Hood L. IgG antibodies to phosphorylcholine exhibit more diversity than their IgM counterparts. Nature. 1981 May 7;291(5810):29–34. doi: 10.1038/291029a0. [DOI] [PubMed] [Google Scholar]
- Haas I. G., Wabl M. R. Immunoglobulin heavy chain toxicity in plasma cells is neutralized by fusion to pre-B cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7185–7188. doi: 10.1073/pnas.81.22.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner H., Meo T., Müller E. Assignment of genes for immunoglobulin kappa and heavy chains to chromosomes 6 and 12 in mouse. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4494–4498. doi: 10.1073/pnas.75.9.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIA S. E. The frequency distribution of spontaneous bacteriophage mutants as evidence for the exponential rate of phage reproduction. Cold Spring Harb Symp Quant Biol. 1951;16:463–470. doi: 10.1101/sqb.1951.016.01.033. [DOI] [PubMed] [Google Scholar]
- Meo T., Johnson J., Beechey C. V., Andrews S. J., Peters J., Searle A. G. Linkage analyses of murine immunoglobulin heavy chain and serum prealbumin genes establish their location on chromosome 12 proximal to the T (5;12) 31H breakpoint in band 12F1. Proc Natl Acad Sci U S A. 1980 Jan;77(1):550–553. doi: 10.1073/pnas.77.1.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Milstein C., Adetugbo K., Cowan N. J., Köhler G., Secher D. S., Wilde C. D. Somatic cell genetics of antibody-secreting cells: studies of clonal diversification and analysis by cell fusion. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):793–803. doi: 10.1101/sqb.1977.041.01.090. [DOI] [PubMed] [Google Scholar]
- Newell N., Richards J. E., Tucker P. W., Blattner F. R. J genes for heavy chain immunoglobulins of mouse. Science. 1980 Sep 5;209(4461):1128–1132. doi: 10.1126/science.6250219. [DOI] [PubMed] [Google Scholar]
- Rudikoff S., Pawlita M., Pumphrey J., Mushinski E., Potter M. Galactan-binding antibodies. Diversity and structure of idiotypes. J Exp Med. 1983 Nov 1;158(5):1385–1400. doi: 10.1084/jem.158.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secher D. S., Milstein C., Adetugbo K. Somatic mutants and antibody diversity. Immunol Rev. 1977;36:51–72. doi: 10.1111/j.1600-065x.1977.tb00382.x. [DOI] [PubMed] [Google Scholar]
- Siden E. J., Baltimore D., Clark D., Rosenberg N. E. Immunoglobulin synthesis by lymphoid cells transformed in vitro by Abelson murine leukemia virus. Cell. 1979 Feb;16(2):389–396. doi: 10.1016/0092-8674(79)90014-x. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Von Borstel R. C., Cain K. T., Steinberg C. M. Inheritance of spontaneous mutability in yeast. Genetics. 1971 Sep;69(1):17–27. doi: 10.1093/genetics/69.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabl M. R., Beck-Engeser G. B., Burrows P. D. Allelic inclusion in the pre-B-cell line 18-81. Proc Natl Acad Sci U S A. 1984 Feb;81(3):867–870. doi: 10.1073/pnas.81.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabl M. R., Burrows P. D. Expression of immunoglobulin heavy chain at a high level in the absence of a proposed immunoglobulin enhancer element in cis. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2452–2455. doi: 10.1073/pnas.81.8.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Desiderio S. V., Paskind M., Kearney J. F., Baltimore D., Alt F. W. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984 Oct 25;311(5988):727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]


