Abstract
Schistosomiasis is an endemic parasite disease and praziquantel is the only drug currently in use to control this disease. Experimental and epidemiological evidence strongly suggests that Microtus fortis ( Mf ) is a naturally resistant vertebrate host of Schistosoma japonicum . In the present study, we found that Mf serum albumin ( Mf -albumin) and the conditioned medium of pcDNA3.1- Mf -albumin caused 46.2% and 38.7% schistosomula death rates in 96 h, respectively, which were significantly higher than that of the negative control (p < 0.05). We also found that mice injected with Mf -albumin had a 43.5% reduction in worm burden and a 48.1% reduction in liver eggs per gram (p < 0.05) in comparison to the control animals. To characterise the mechanisms involved in clearance, schistosomula were incubated with fluorescein isothiocyanate-labelled Mf -albumin and fluorescent enrichment effects were found in the gut lumen of schistosomula after 48 h of incubation. Next, digestive tract excretions from schistosomula were collected and the sensitivity of Mf -albumin to digestive tract excretions was evaluated. The results indicated that schistosomula digestive tract excretions showed indigestibility of Mf -albumin. The death of schistosomula could be partially attributed to the lack of digestion of Mf -albumin by digestive tract excretions during the development of the schistosomula stage. Therefore, these data indicate the potential of Mf -albumin as one of the major selective forces for schistosomiasis.
Keywords: Schistosoma japonicum, Microtus fortis, serum albumin
Schistosomiasis is a parasitic disease that has been reported to have affected more than 200 million individuals in 76 countries, while close to 800 million people are at risk ( Wu et al. 1962 ). It is a major public health problem worldwide. The disease can be controlled in areas where the financial resources exist to mount sustained national efforts, but eradication of the parasite has proved difficult. Schistosoma japonicum , one of the most common and most severely pathogenic parasites in China, could infect approximately 46 mammalian species including humans and excluding Microtus fortis ( He et al. 2001 ). During the 1950s, Chinese scientists anatomised thousands of M. fortis residing in the Dongting Lake region, the same location as a S. japonicum epidemic, but no eggs or adult worms were found in their bodies ( Wu et al. 1962 , Li et al. 1965 ). Further observations showed that M. fortis bred in laboratories or residing in the Ningxia region, an area where S. japonicum was not prevalent, had natural resistance to this disease as well. This finding indicated that resistance could be inherited and was not affected by the environment. It was more recently shown that M. fortis was the rodent mammal in China with the greater natural resistance to S. japonicum .
Various efforts have been undertaken to enhance the effectiveness of anti-schistosome treatment, usually involving the participation of an antibody or the expression of cytokine genes or hormone-like proteins. Recent experimental evidence has suggested that the hormonal microenvironment within the host can influence not only the course of parasitic infection by modulating the immune system, but it can also be exploited to favour the establishment, growth and reproduction of the parasites ( Escobedo et al. 2005 ). M. fortis has been shown to be a non-permissive host of S. japonicum in a complex host-parasite evolutionary relationship ( Li et al. 1965 ). However, the anti-schistosome mechanism of M. fortis remains under investigation. He et al. (1999) discovered in vitro a specific innate anti-schistosome antibody from M. fortis . Additionally, studies from other labs reported that mice that received M. fortis serum by passive transfer could achieve protection against S. japonicum ( Jiang et al. 2004 ). It was hypothesised that the anti-schistosome characteristics of M. fortis were due to an unknown, but heritable material. Previous studies from our lab found that the anti-schistosomula effects of M. fortis serum were stronger than those of the homogenate supernatants of the heart, liver, lung, spleen, kidney or muscle ( Shen et al. 2002 , Qin et al. 2004 , Gong et al. 2010 , Cheng et al. 2011 ). Thus, we focused on M. fortis serum, as it has been shown to contain a potential anti-schistosome substance. Additionally, to shed greater light on the anti-schistosome mechanism of M. fortis , we isolated and purified M. fortis serum proteins by Blue Sepharose chromatography to test their anti-schistosomula effects.
MATERIALS AND METHODS
Parasites and animals - Oncomelania hupensis hupensis snails infected with S. japonicum were purchased from the Jiangsu Institute of Parasitic Diseases, China. M. fortis in closed colonies, BALB/c mice weighing 18-20 g each and New Zealand white rabbits weighing 1-2 kg each were provided by the Lab Animal Centre of Central South University. The animal experiments were performed according to the protocols approved by the National Animal Care and Use Committee of China.
Serum preparation - Blood samples from M. fortis and mice were collected using an eye-detachment technique, while the New Zealand white rabbit blood samples were collected via heart puncture. The sera were separated by centrifugation and the sera of the rabbits and mice were then filtered (MILLEX ® GP Filter Unit, 0.22 μm, Millipore). All of the sera were stored at -70ºC until further use.
Schistosomula culture in vitro - Cercariae were collected from infected O. hupensis snails as described previously ( Salafsky et al. 1988 ). Cercariae were washed four times with Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, USA) containing 300 U/mL of penicillin G sodium and 300 μg/mL of streptomycin. After centrifugation at 322 g for 10 min, schistosomula were transformed from cercariae by culturing in DMEM containing 100 U/mL of penicillin G sodium and 100 μg/mL of streptomycin at 37ºC, 5% CO 2 with 25% New Zealand white rabbit sera for 24-48 h.
Schistosomula were next cultured in 48-well cell plates with 100 ± 20 schistosomula per well at 37ºC and 5% CO 2 . The viability of the incubated schistosomula was assessed directly with light microscopy (Olympus Co, Tokyo, Japan). Dead schistosomula showed a loss of motility, an increase in opacity and occasionally gross morphological changes in shape or obvious lesions on the tegument. Furthermore, there was no motion during a continuous 30 s observation. The viability assessment was ensured by methylene blue (MB) uptake testing after observation with the unstained schistosomula considered dead and the stained ones alive ( Gold & Flescher 2000 ).
Screening of anti-schistosomula-associated protein - To obtain an effective schistosomula protein from M. fortis serum, molecular sieve chromatography was performed with a Sephadex G50 column. Chromatographic separations were performed by high-performance liquid chromatograph (HPLC) (Waters Company, USA). To confirm the anti-schistosome activity of HPLC separations further, eluted peaks were pooled, desalted and lyophilised after the separations were gradient-eluted with 50-80% acetonitrile containing 0.1% trifluoroacetic acid. Then, the anti-schistosomula protein was sequenced.
Affinity chromatography - Pre-swollen Blue Sepharose FF was mixed thoroughly and was poured into a chromatography column (3 cm i.d. 10 cm), which was then equilibrated thoroughly with solution A (0.05 M KH 2 PO 4 , pH 7.0). M. fortis serum was then diluted 1:1 (V/V) with solution A and was dialysed overnight against phosphate buffered saline (PBS) using Dalton membranes (8,000-14,000). After centrifugation at 8,050 g for 10 min, the supernatant was applied to the Blue Sepharose FF affinity chromatography column (4.8 cm × 40 cm) (Pharmacia) at a flow rate of 2 mL/min. The column was eluted with solution A, followed by solution B (0.05 M KH 2 PO 4 , 1.5 M KCl, pH 7.0). The elution peak was pooled and dialysed overnight at 4ºC against PBS using Dalton membranes (8,000-14,000). The solution was then lyophilised, desalted and re-lyophilised as above. Finally, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis was conducted to ensure the purity of the M. fortis- albumin ( Mf -albumin).
Anti-schistosomula experiment of Mf-albumin in vitro - The inhibitory effects of Mf- albumin on schistosomula were detected in vitro. After the live schistosomula were counted, samples corresponding to 1.25 M of Mf- albumin were added and then incubated at 37ºC. M. fortis serum (40%, final concentration) was used as the positive control and mice serum albumin was used as the negative control. Each experiment was duplicated in three wells. Schistosomula were cultivated for up to 96 h and the residual live schistosomula were counted and the mortality rate of schistosomula was calculated. The death rate of schistosomula was evaluated by the following formula: death rate % = (total number of schistosomula - live number of schistosomula)/total number of schistosomula × 100.
Preparation of the conditioned medium - Full-length Mf -albumin genes (GenBank accession AY885264) were amplified from the total RNA of M. fortis livers by reverse transcription polymerase chain reaction (PCR) using the following PCR primers: 5’-TATTGGATCCACCATGAGGGGCTTGTTTCGCCGAGA-3’ and 5’-TATTCTCGAGAGCAACAAGTTTTGGACCCTCTAG-3’ for Mf -albumin and 5’-TATTGGATCCACCATGAGGGGTGTGTTTCGCCGAG-3’ and 5’-TATTCTCGAGAGCAACAAGTTTTGGACCCTCTAG-3’ for mouse serum albumin ( Ms -albumin). The generated DNA fragment was cloned into plasmid pcDNA3.1 (+) and was transferred into the Escherichia coli strain DH5a. The recombinant plasmid (pcDNA3.1-albumin) was sequenced and transiently transfected into 293T cells using Lipofectamine TM 2000 (Invitrogen, USA). Twelve hours after DNA transfection, the cells were cultured with 2 mL of serum-free DMEM. Supernatant (conditioned medium) from each pool was collected 36 h later and was tested at a concentration of 50% for its anti-schistosomula potential.
Protective experiment in mice with Mf-albumin - Thirty-six-week-old male BALB/c mice were infected with 40 S. japonicum cercariae percutaneously and were randomly distributed into three groups containing 10 mice each: one blank PBS group, one Ms -albumin control group and one Mf -albumin test group. On the first day, the blank group was administered 0.3 mL of PBS, the control group was administered 0.3 mL of Ms -albumin and the test group with 0.3 mL Mf -albumin, all via intravenous injection. On the third and sixth days after the first injection, two other injections were administered using the same doses as above. All of the mice were sacrificed on day 42 by cervical dislocation. S. japonicum adult worms in the hepatic and portomesenteric veins were recovered and counted. The number of eggs in the liver was estimated after the tissue was digested with 4% KOH. The worm burdens and liver eggs per gram (LEPG) were calculated. The percentage changes in worm and egg burden were evaluated by the following formula: % change = (mean number in infected controls - mean number in infected, treated mice)/mean number in infected control × 100.
Isolation of digestive tract excretions from schistosomula and adult worms - After schistosomula were harvested and washed three times with distilled water, they were placed in distilled water at room temperature for 20 min until the water became turbid with dark granular material from the parasites’ digestive tracts. These excretions were collected, adjusted to a pH of 4.9 with 0.1 M citrate and then centrifuged at 8,050 g for 10 min, after which the supernatant was collected, lyophilised, sealed and kept at -70ºC until further use ( Chappell & Dresden 1986 ). Digestive tract excretions of adult worms were collected using the same method. Immediately before use, the powder was dissolved with PBS and the protein content was measured using bovine serum albumin as a standard ( Lowry et al. 1951 ).
On-site detection of Mf-albumin in schistosomula - Labelling of protein was performed with fluorescein isothiocyanate (FITC) according to a conventional protocol. FITC- Mf- albumin was added to the schistosomula cultures and was incubated for 24 h and 48 h in vitro. FITC-labelled Ms -albumin, fluorescein (30 μg/mL) and schistosomula without treatment were used as controls. To ascertain the site of fluorescence, schistosomula were collected after 24 h and 48 h of incubation, washed with 0.01 M PBS (pH 7.0) three times and then were observed under an Olympus fluorescence microscope.
Albumin digestion with digestive tract excretions in vitro - Samples of 5 μg/μL Mf- albumin and digestive tract excretions were incubated for 0 h, 1 h, 2 h, 4 h, 8 h, 16 h and 24 h, respectively, at 37ºC in citrate saline buffer solution, pH 4.0, in the presence of 20 mM cysteine. After digestion, the reaction was terminated by 100 μL of 0.5 M Tris buffer (pH 6.8, 10% SDS). Twenty microlitres of digestion products and 10 μL of loading buffer were run on 10% SDS-PAGE.
Statistical analysis - The differences between groups are presented as means ± standard deviation by one-way ANOVA, using SPSS statistical software version 13.0. A p-value < 0.05 was considered a significant difference.
RESULTS
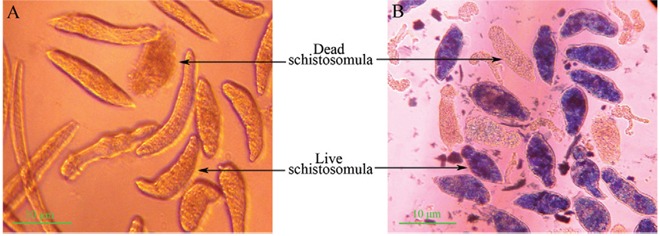
Schistosomula culture in vitro - The tails of cercariae were detached in DMEM medium containing 25% fresh New Zealand white rabbit serum for 24-48 h and the transformation rate remained greater than 90% with a less than 10% blank mortality rate. Under light microscopy, the live schistosomula were very vivacious with semitransparent bodies, crawling unhindered on the bottom of culture plate, whereas the dead schistosomula showed ankylosis and immobility, increased opacity and obvious lesions on the tegument and they did not display MB staining ( Fig. 1 ).
Fig. 1. : micrographic images of schistosomula: dead schistosomula showed an increased opacity and obvious lesions in the tegumental outer membrane. The live schistosomula took up the dye and stained into blue-black with orbicular tegumental outer membrane, whereas the dead ones have not taken up the dye and unstained. A: schistosomula cultured in Dulbecco’s modified Eagle’s medium (DMEM) without methylene blue; B: live (stained) and dead (unstained) schistosomula after introduction of methylene blue into the DMEM.
Identification of the protein associated with the inhibitory effects on schistosomula - The protein components of M. fortis serum were isolated by means of molecular sieve chromatography using a Sephadex G50 column and HPLC and the protein that induced resistance to schistosomula was acquired. The amino-terminal end was identified as DAHKSEIAHR by protein sequencing and BLAST showed that it had 85% homology with mouse albumin and was named Mf -albumin. At a protein concentration of 1 mg/mL, it induced a schistosomula mortality rate of 36.9% (χ 2 = 3.8594, p < 0.05) in vitro, significantly greater than that of the negative control.
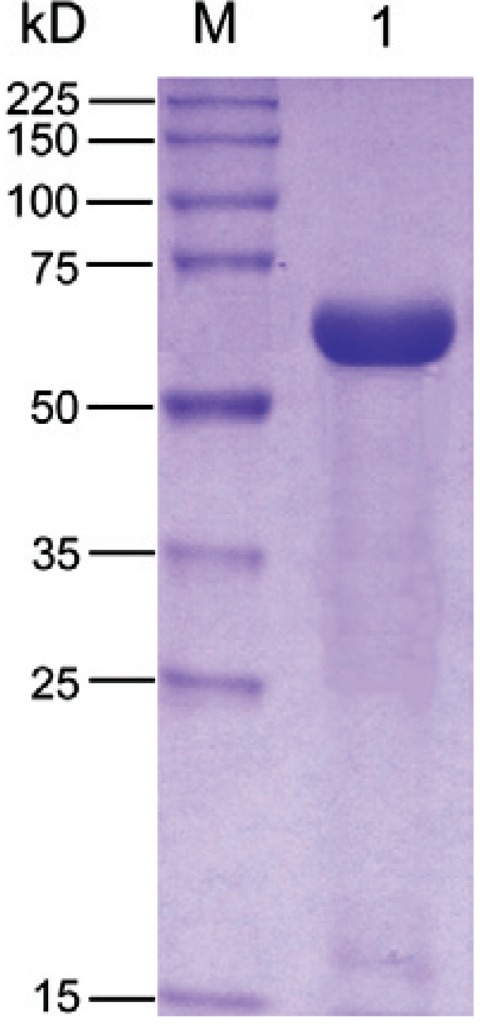
Albumin purification - Blue Sepharose FF was used to purify M. fortis and Ms -albumin. There were two main peaks in the Blue Sepharose FF affinity chromatography (data not shown). The albumin only existed at the second peak, theoretically. Thus, it was accumulated and dialysed thoroughly with distilled water and then was placed in a -80ºC ultra-low temperature freezer for at least 4 h. The purity of the albumin was detected by SDS-PAGE and the result showed a single monomeric protein migration at approximately 66 kDa ( Fig. 2 ). The purified Mf serum albumin was subjected to N-terminal amino acid sequencing for further verification.
Fig. 2. : sodium dodecyl sulfate polyacrylamide gel electrophoresis of Microtus fortis -albumin purified with Blue Sepharose FF affinity chromatography. Lane M: broad range protein molecular weight marker; 1: M. fortis serum albumin purified.
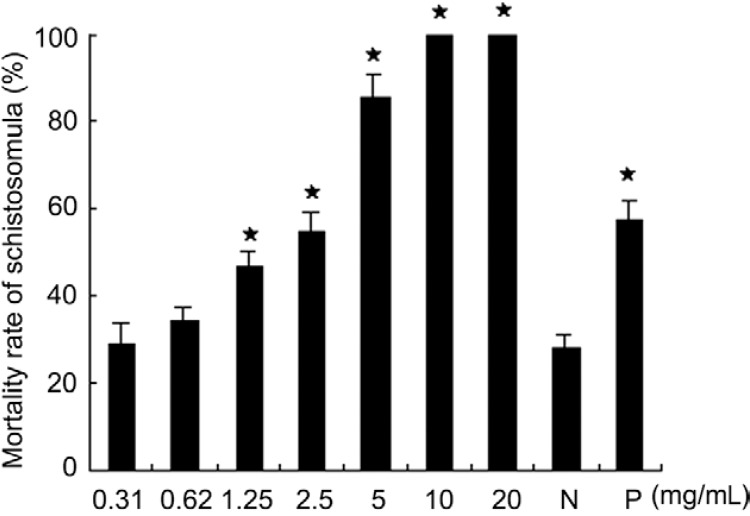
Inhibitory effects of Mf-albumin on schistosomula in vitro - To confirm the anti-schistosomula effects of Mf -albumin, Mf -albumin was added to cultured schistosomula at final concentrations of 0.31, 0.62, 1.25, 2.5, 5.0, 10.0 and 20.0 mg/mL, with Ms-albumin as a negative control and M. fortis serum as a positive control. We observed and calculated the death rate of schistosomula after 96 h of incubation. In addition to the morphological changes of the dead schistosomula previously described, we also observed that there were one or two large blebs on the bodies of schistosomula during the period of Mf -albumin treatment. The same change was observed in the positive control group, but no change was seen in the negative control group. Most of the schistosomula in the negative control group were vivacious with semitransparent bodies and we observed them to be crawling on the bottom of the cell plate ( Fig. 3 ). The results of the morphological observations and schistosomula death rate data showed that Mf -albumin had obvious inhibitory activity on schistosomula in vitro. Our results showed that when the concentration of Mf -albumin was 1.25 mg/mL, the death rate of schistosomula was 46.2% (χ 2 = 8.4703, p < 0.05), significantly greater than that of the negative control. The histography of the mortality rate for each group is presented in Fig. 4 .
Fig. 3. : micrographic images of schistosomula cultured in 96 h: dead schistosomula showed an increased opacity and obvious lesions in the tegumental outer membrane. Sometimes the blebs appeared in their tegumental outer membrane (arrowed). A: schistosomula treated with 5 mg/mL Microtus fortis ( Mf )-albumin; B: schistosomula treated with 10 mg/mL Mf -albumin; C: schistosomula treated with represented 40% M. fortis serum; D: schistosomula treated with 10 mg/mL mouse serum albumin.
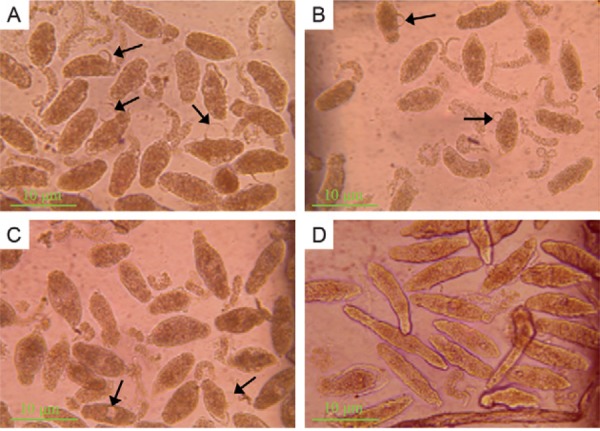
Fig. 4. : inhibitory effect of Microtus fortis -albumin on schistosomula in vitro. This diagram showed that when the target protein concentration was equal or greater than 1.25 mg/mL, significant difference of schistosomula mortality rate was observed when compared with negative control and the dead rate of schistosomula raised markedly along with the rise of albumin concentration (asterisk means p < 0.05, compare with negative control). Results represented mean ± standard deviation for triplicates. N: negative control; P: positive control.
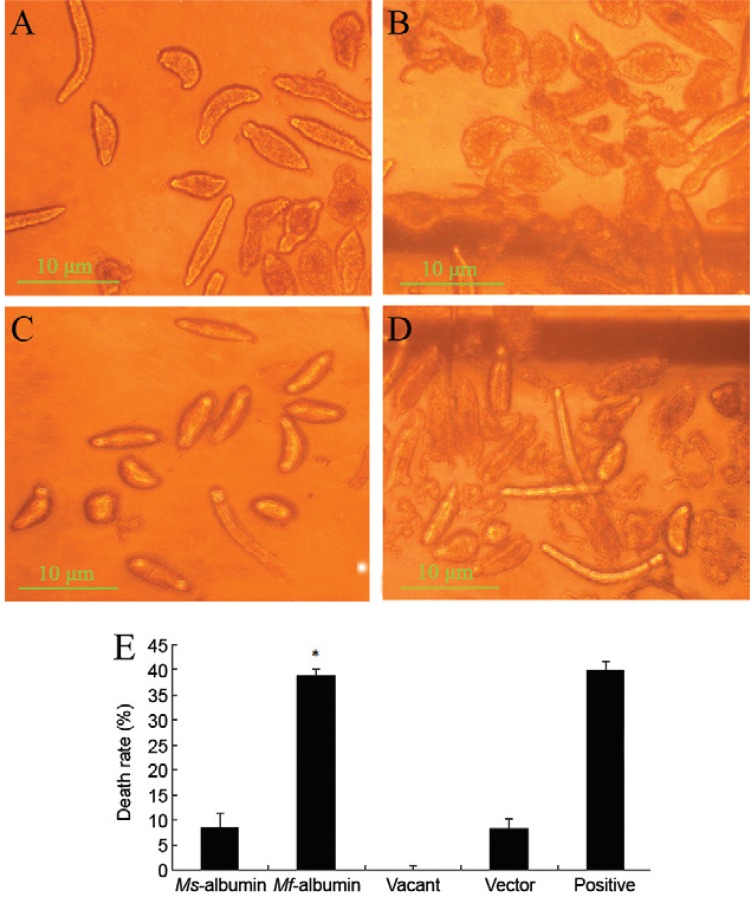
Effects of conditioned media on schistosomula - To verify further the anti-schistosome effects of Mf -albumin, conditioned medium containing Mf -albumin or Ms -albumin was added to schistosomula culture at a concentration of 50%. We observed and calculated the death rate of schistosomula at 96 h. Our results showed the average schistosomula-killing rate of pcDNA3.1/ Mf -albumin conditioned medium was 38.7%, which was significantly higher than that of the negative control ( Fig. 5 ).
Fig. 5. : inhibitory effect of pcDNA3.1/ Microtus fortis conditioned medium on schistosomula after 96 h of incubation. A: schistosomula cultured with control pcDNA3.1/mouse serum albumin ( Ms -albumin) conditioned medium; B: schistosomula cultured with pcDNA3.1/ M. fortis -albumin ( Mf -albumin) conditioned medium; C: schistosomula cultured with pcDNA3.1/Amp conditioned medium (negative control); D: schistosomula cultured with 20% M. fortis sera (positive control) (more dead schistosomula were observed in Mf-albumin group than the negative control group in 96 h); E: histogram of schistosomula mortality rate after incubation with conditioned medium (p < 0.05 compared with negative control). Results represented mean ± standard deviation for triplicates.
Anti-schistosome effects of Mf-albumin in vivo - BALB/c mice were randomly divided into three groups and were infected with S. japonicum cercariae. Adult worms were quantified in these mice after heart perfusion 42 d after infection. The resulting worm burden and number of LEPG in BALB/c mice treated with Mf -albumin and Ms -albumin are presented in Table I. BALB/c mice administered Mf -albumin (15 mg/animal) showed a significant worm burden reduction of 43.5% (p < 0.05) compared with Ms -albumin administration. Moreover, we also observed a highly significant reduction in LEPG in the Mf -albumin treated group, which had a 48.1% (p < 0.05) egg reduction. Additionally, there were no differences in the numbers of egg granuloma observed between the controls (data not shown). All of these results showed that Mf -albumin possessed significant inhibitory activity on S. japonicum in vivo.
TABLE. The anti-schistosome effect of Microtus fortis (Mf )-albumin on Schistosoma japonicum cercariae infection in BALB/c mice.
| Groups | Worm burden (mean ± SE) | Worms reduction rate (%) | LEPG (mean ± SE) | Egg reduction rate (%) | p |
|---|---|---|---|---|---|
| PBS | 24.9 ± 4.8 | - | 59.543 ± 6.887 | - | > 0.05 |
| Ms -albumin | 24.6 ± 4.8 | - | 59.510 ± 6.256 | - | > 0.05 |
| Mf -albumin | 13.9 ± 2.6 | 43.5 | 30.900 ± 3.298 | 48.1 | < 0.05 |
worm burden rate and egg reduction rate were calculated relative to phosphate buffered saline (PBS) control. Worm burden and liver eggs per gram (LEPG) between PBS and mouse serum albumin ( Ms -albumin) groups were not statistically different (p > 0.05). Worm burden and LEPG of Mf -albumin group were statistically different from those of PBS and Ms -albumin groups (p < 0.05). SE: standard error.
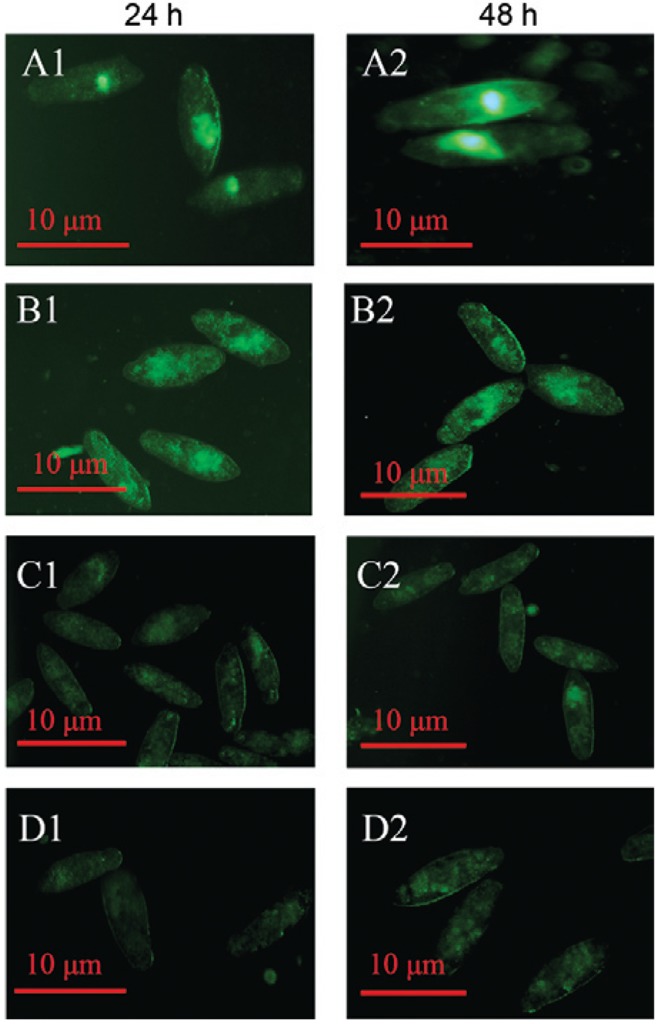
Detection of the deposit site of Mf-albumin in schistosomula - Schistosomula culture was incubated with FITC-labelled proteins or other control proteins. The results, provided in Fig. 6 , clearly showed that a fluorescent enrichment effect could be found in the gut lumen of schistosomula after incubation with FITC- Mf -albumin for 24 h. After 48 h of incubation, the fluorescent enrichment effect became even stronger in the gut of schistosomula by fluorescence microscopy. However, there was no distinct fluorescence enrichment in schistosomula that were treated with the FITC-labelled Ms -albumin, fluorescence and the blank control.
Fig. 6. : on-site of Microtus fortis -albumin ( Mf -albumin) in schistosomula (24 h and 48 h post-treated). A1: treated with fluorescein isothiocyanate (FITC)-labelled Mf -albumin after 24 h incubation; A2: treated with FITC-labelled Mf -albumin after 48 h incubation; B1: treated with FITC-labelled mouse serum albumin ( Ms -albumin) after 24 h incubation; B2: treated with FITC-labelled Ms -albumin after 48 h incubation; C1: fluorescein control after 24 h incubation; C2: fluorescein control after 48 h incubation; D1: blank control after 24 h incubation; D2: blank control after 48 h incubation.
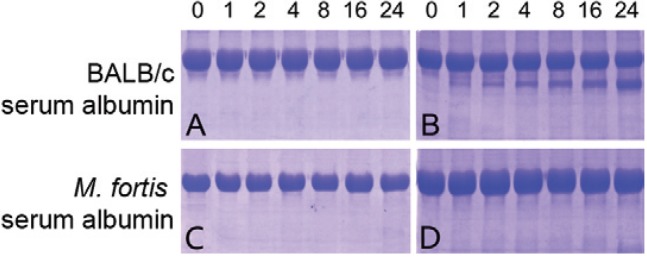
Digestion of Mf-albumin by digestive tract excretions from schistosomula - Digestive tract excretions obtained from schistosomula were used to evaluate the ability of the digestion processes of M. fortis and mice albumin. The digestion of the potentially digestible fraction could be detected by SDS-PAGE. The results showed that evident digestion could be observed when Ms -albumin was incubated with digestive tract excretions of schistosomula for only 2 h, but no evident digestion of Mf -albumin was detected until co-incubation for 24 h ( Fig. 7 ).
Fig. 7. : digestion of albumins by digestive tract excretions from schistosomula and adult worms. Albumins Microtus fortis and mice were incubated with digestive tract excretions from schistosomula and adult worms at 37ºC for 0 h, 1 h, 2 h, 4 h, 8 h, 16 h and 24 h, respectively. Then the products were collected and tested by sodium dodecyl sulfate polyacrylamide gel electrophoresis. A: mouse serum albumin ( Ms -albumin) treated with distilled water; B: Ms -albumin treated with digestive tract excretions of schistosomula; C: M. fortis -albumin ( Mf -albumin) treated with distilled water; D: Mf -albumin treated with digestive tract excretions of schistosomula.
DISCUSSION
Schistosomiasis is a fairly prevalent communicable disease in the tropics and subtropics. It is well known that M. fortis possesses natural resistance to S. japonicum and this characteristic could be steadily inherited. Host resistance to parasites can be behavioural, physiological, mechanical or immunological. In addition, there can be variations in host resistance and parasite infectivity within a species or across different species ( Schwarzenbach et al. 2005 ). This resistance can be based on the genetic makeup of the animals. However, the degree of resistance, which can be a plastic response to environmental variation in the natural population, is not well understood ( Nagel et al. 2010 ). Some studies have shown that environmental variation might increase ischaemia-modified albumin concentrations, which are a form of human serum albumin (HSA) ( Bar-Or et al. 2001 ). In addition, there has also been evidence of possible genetic variants of HSA, particularly structural changes at the N-terminus, which abolish transition metal binding ( Sheat et al. 1991 , Bhagavan et al. 2003 , Lippi et al. 2005 ). Therefore, we hypothesised that a genetic difference in albumin between M. fortis and mice would result in functional disparity between them, giving Mf -albumin stronger anti-schistosome potential.
Laboratory studies have shown that M. fortis sera possessed anti-schistosome activity in vitro and in vivo. In the present investigation, we showed that extract of albumin from M. forti s sera exhibited anti-schistosomal activity both in vitro and in vivo. It is well known that serum albumin is the most abundant protein in the circulatory system and it possesses many important physiological functions that contribute to colloid osmotic blood pressure and aid in the transport, distribution and metabolism of many endogenous and exogenous substances. In addition, it was reported that albumin might play an important role in innate immunity ( Fassi et al. 1993 , Brummer et al. 1999 , Ahluwalia et al. 2001 ). Giles and Czuprynski (2003) showed that the albumin from several mammalian species had obvious inhibitory activity against Blastomyces dermatitidis , while analbuminaemic rat serum failed to inhibit B. dermatitidis growth in vivo. Takami et al. (1992) indicated that maleylated HSA inhibited human immunodeficiency virus-1 infection and syncytia formation via binding to target cells with high affinity in vitro. Milojevic et al. (2009) found that HSA could inhibit Aβ fibrillisation through a “monomer-competitor” mechanism by nuclear magnetic resonance and intrinsic albumin fluorescence experiments. Recently, a series of studies suggested that fluid resuscitation with albumin was associated with higher mortality in patients ( Schierhout & Roberts 1998 , Vincent et al. 2005 , Myburgh et al. 2007 , Wheeler et al. 2011 ). All of these data indicated that, apart from the basic functions, albumin possesses an immunological effect related to the innate immunity of living things.
M. fortis is a non-permissive host, the serum of which has been found to possess innate resistance to S. japonicum in vitro and in vivo. In this study, we isolated and purified M. fortis albumin. The inhibitory activity of the albumin to schistosomula was examined in vitro and in vivo. Schistosomula culture treated with 1.25 mg/mL of Mf -albumin caused a significantly higher schistosomula death rate when compared to the negative controls. The mortality rate of schistosomula approached 100% when the Mf -albumin concentration was increased to 10 mg/mL. Mf -albumin was administered to mice infected with cercariae by intravenous injection through the tail vein. After perfusion of the mice 42 d after infection, we found that mice injected with Mf- albumin had 43.5% worm burden reduction and 48.1% LEPG reduction compared with the control group. All of the results showed that Mf -albumin possessed significant inhibitory activity against schistosomula.
Until now, the possible mechanism of Mf -albumin anti-schistosomula had been unclear. It is well known that the definitive hosts of S. japonicum are widespread, infecting more than 40 mammalian species. However, M. fortis was shown to be the only non-permissive host of schistosome among mammalians in China ( Li et al. 1965 ). When infected, the growth and development of S. japonicum are hindered and no adult worms and eggs could be found in both wild and laboratory-bred M. fortis ( He et al. 1995 ). In this study, FITC-labelled Mf -albumin accumulated in the gut lumen of schistosomula after incubation for 24 h and 48 h. Thus, Mf -albumin must be transported intact and be metabolised slowly by schistosomula. However, little is known about the mechanisms of protein transport in schistosomula. We speculated that the anti-schistosomal activity would be accompanied by a series of proteins involved in signal transduction and protein trafficking; these proteins might activate or strengthen the immune system and induce anti-schistosome effects during different stages of the schistosome life cycle in the vertebrate host. Recent experimental evidence has shown that hormonal-like proteins can regulate a variety of cellular and physiological functions of schistosome in establishment, growth and reproduction, such as adrenal steroid hormones and testosterone ( Barrabes et al. 1986 , Fallon et al. 1998 , Morales-Montor et al. 2001 , Remoué et al. 2002 ). In combination with the results of FITC-labelled Mf -albumin accumulated in the intestines of schistosomula, it is possible that Mf -albumin could influence the microenvironment or be indigested by the digestive tract excretions of schistosomula. Thus, we presumed Mf -albumin might play an important role in resistance to schistosome, by acting as a kind of a stressor.
To explore further possible mechanisms of Mf -albumin against S. japonicum , we collected digestive tract excretions of schistosomula to test their digestion differences from M. fortis and mice serum albumin in vitro. The results showed that Mf -albumin was more difficult to be digested by the digestive tract excretions of schistosomula, compared with Ms -albumin. When infected, the growth and development of S. japonicum were hindered and no adult worms or eggs could be found in either wild or laboratory-bred M. fortis ( He et al. 1995 ). Therefore, we presumed that Mf -albumin might be an important factor of the anti-schistosomula effects of M. fortis during S. japonicum development. In combination with the results of FITC-labelled Mf -albumin accumulated in the intestines of S. japonicum , it also provided insight that digestive tract excretions could be used for rapid immunodiagnosis, even during early stages of infection.
It is worthwhile to point out that when we studied the inhibitory effects of Mf -albumin on schistosomula in vitro, we observed some large blebs appearing in the tegumental outer membrane. The appearance of these blebs was reported and was suggested to be the result of the action of complement ( McLaren et al. 1978 ). However, in our in vitro experimental system, there was no complement or the complement was inactivated. Thus, we suggest that the presence of Mf -albumin created an environment that was not suitable for the survival of schistosomula because of the obvious morphological changes to the tegument of the schistosomula.
In summary, we screened proteins of M. fortis serum by molecular sieve chromatography, HPLC and Blue Sepharose FF and identified Mf -albumin as having anti-schistosomula effects, both in vitro and in vivo. We preliminarily explored the possible mechanism of Mf -albumin anti-schistosomula and concluded that Mf -albumin was an effective protein against schistosome.
ACKNOWLEDGEMENTS
To Saiqun Luo, Qingren Zeng, Shunke Zhang, Jingjing Chu and Gang Cheng, for providing technical assistance and suggestions.
Funding Statement
Financial support: NSFC (30070403, 30400256, 31100887)
Footnotes
Financial support: NSFC (30070403, 30400256, 31100887)
REFERENCES
- Ahluwalia M, Brummer E, Sridhar S, Singh R, Stevens DA. Isolation and characterization of an anticryptococcal protein in human cerebrospinal fluid. J Med Microbiol. 2001;50:83–89. doi: 10.1099/0022-1317-50-1-83. [DOI] [PubMed] [Google Scholar]
- Bar-Or D, Rael LT, Lau EP, Rao NK, Thomas GW, Winkler JV, Yukl RL, Kingston RG, Curtis CG. An analog of the human albumin N-terminus (Asp-Ala-His-Lys) prevents formation of copper-induced reactive oxygen species. Biochem Biophys Res Commun. 2001;284:856–862. doi: 10.1006/bbrc.2001.5042. [DOI] [PubMed] [Google Scholar]
- Barrabes A, Goma-Mouanda J, Reynouard F, Combescot C. 17-b estradiol receptors in Schistosoma mansoni . Contribution to the explanation of the protective power of this hormone in Schistosoma mansoni bilharziasis in the mouse. Preliminary study. Ann Parasitol Hum Comp. 1986;61:637–641. doi: 10.1051/parasite/1986616637. [DOI] [PubMed] [Google Scholar]
- Bhagavan NV, Lai EM, Rios PA. Evaluation of human serum albumin cobalt binding assay for the assessment of myocardial ischemia and myocardial infarction. Clin Chem. 2003;49:581–585. doi: 10.1373/49.4.581. [DOI] [PubMed] [Google Scholar]
- Brummer E, Drasin T, Adler JD. Antifungal activity of cerebrospinal fluid against Cryptococcus neoformans and Candida species. Med Mycol. 1999;37:339–344. doi: 10.1046/j.1365-280x.1999.00240.x. [DOI] [PubMed] [Google Scholar]
- Chappell CL, Dresden MH. Schistosoma mansoni : proteinase activity of “hemoglobinase” from the digestive tract of adult worms. Exp Parasitol. 1986;61:160–167. doi: 10.1016/0014-4894(86)90148-7. [DOI] [PubMed] [Google Scholar]
- Cheng G, Gong Q, Gai N, Xiong DH, Yu YJ, Zeng QR, Hu WX. Karyopherin alpha 2 (KPNA2) is associated with the natural resistance to Schistosoma japonicum infection in Microtus fortis. Biomed Pharmacother. 2011;65:230–237. doi: 10.1016/j.biopha.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Escobedo G, Roberts CW, Carrero JC, Morales-Montor J. Parasite regulation by host hormones: an old mechanism of host exploitation? Trends Parasitol. 2005;21:588–593. doi: 10.1016/j.pt.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Richardson EJ, Jones FM, Dunne DW. Dehydroepiandrosterone sulfate treatment of mice modulates infection with Schistosoma mansoni. Clin Diagn Lab Immunol. 1998;5:251–253. doi: 10.1128/cdli.5.2.251-253.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassi FO, Mohanty J, Elazhary Y. Bovine serum albumin inhibits the adsorption of respiratory syncytial virus on MDBK cells. Vet Res. 1993;24:488–493. [PubMed] [Google Scholar]
- Giles S, Czuprynski C. Novel role for albumin in innate immunity: serum albumin inhibits the growth of Blastomyces dermatitidis yeast form in vitro. Infect Immun. 2003;71:6648–6652. doi: 10.1128/IAI.71.11.6648-6652.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold D, Flescher E. Influence of mechanical tail-detachment techniques of schistosome cercariae on the production, viability and infectivity of resultant schistosomula: a comparative study. Parasitol Res. 2000;86:570–572. doi: 10.1007/s004360000199. [DOI] [PubMed] [Google Scholar]
- Gong Q, Cheng G, Qin ZQ, Xiong DH, Yu YJ, Zeng QR. Identification of the resistance of a novel molecule heat shock protein 90a (HSP90a) in Microtus fortis to Schistosoma japonicum infection. Acta Trop. 2010;115:220–226. doi: 10.1016/j.actatropica.2010.03.007. [DOI] [PubMed] [Google Scholar]
- He HB, Zuo JZ, Liu BS. Comparison of infection with Schistosoma japonicum between wild and laboratory bred Microtus fortis. J Pract Parasitic Dis. 1995;3:72–74. [Google Scholar]
- He Y, Luo X, Zhang X, Yu X, Lin J, Li Y, Li Y, Liu S. Chin Med J. Vol. 112. Engl: 1999. Immunological characteristics of natural resistance in Microtus fortis to infection with Schistosoma japonicum; pp. 649–654. [PubMed] [Google Scholar]
- He YX, Salafsky B, Ramaswancy K. Host-parasite relationship of Schistosoma japonicum in mammalian hosts. Trends Parasitol. 2001;17:320–324. doi: 10.1016/s1471-4922(01)01904-3. [DOI] [PubMed] [Google Scholar]
- Jiang SF, Wei MX, Lin JJ, Pan CE, Li H, Cao L, He YY, Fu ZQ. Study on protection against Schistosoma japonicum induced by passively transferred sera from Microtus fortis in the mouse. Chin J Parasit Dis Con. 2004;17:298–300. [Google Scholar]
- Li SK, Zhu ZL, Jin BR. Uninfectibility to Schistosoma japonicum of Microtus fortis. Acta Parasitol Sinica. 1965;2:103. [Google Scholar]
- Lippi G, Brocco G, Salvagno GL, Montagnana M, Dima F, Guidi GC. High-workload, endurance training may increase serum ischemia-modified albumin concentrations. Clin Chem Lab Med. 2005;43:741–744. doi: 10.1515/CCLM.2005.126. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- McLaren DJ, Ramalho-Pinto FJ, Smithers SR. Ultrastructural evidence for complement and antibody-dependent damage to schistosomula of Schistosoma mansoni by rat eosinophils in vitro. Parasitology. 1978;77:313–324. doi: 10.1017/s0031182000050277. [DOI] [PubMed] [Google Scholar]
- Milojevic J, Radiatsis A, Melacini G. Human serum albumin inhibits Aβ fibrillization through a “monomer-competitor” mechanism. Biophys J. 2009;97:2585–2594. doi: 10.1016/j.bpj.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Montor J, Mohamed F, Ghaleb AM, Baiq S, Hallal-Calleros C, Damian RT. In vitro effects of hypothalamic-pituitary-adrenal axis (HPA) hormones on Schistosoma mansoni. J Parasitol. 2001;87:1132–1139. doi: 10.1645/0022-3395(2001)087[1132:IVEOHP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Myburgh J, Cooper DJ, Finfer S. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. New Engl J Med. 2007;357:874–884. doi: 10.1056/NEJMoa067514. [DOI] [PubMed] [Google Scholar]
- Nagel L, Robb T, Forbes MR. Inter-annual variation in prevalence and intensity of mite parasitism relates to appearance and expression of damselfly resistance. BMC Ecol. 2010;10:5. doi: 10.1186/1472-6785-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin ZQ, Hu WX, Wu GJ, Xu B, Shen QX, Gong Q, Wu C. Construction of gene pool of Microtus fortis and screening resistance-associated genes to infection of Schistosoma japonicum. Life Sci Space Res. 2004;8:333–338. [Google Scholar]
- Remoué F, Mani JC, Pugnière M, Schacht AM, Capron A, Riveau G. Functional specific binding of testosterone to Schistosoma haematobium 28-kilodalton glutathione S-transferase. Infect Immun. 2002;70:601–605. doi: 10.1128/IAI.70.2.601-605.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salafsky B, Fuscom AC, Whitley K, Nowicki D, Ellenberger B. Schistosoma mansoni: analysis of cercarial transformation methods. Exp Parasitol. 1988;67:116–127. doi: 10.1016/0014-4894(88)90014-8. [DOI] [PubMed] [Google Scholar]
- Schierhout G, Roberts I. Fluid resuscitation with colloid or crystalloid solutions in critically ill patients: a systematic review of randomised trials. BMJ. 1998;316:961. doi: 10.1136/bmj.316.7136.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzenbach GA, Hosken DJ, Ward PI. Sex and immunity in the yellow dung fly Scathophaga stercoraria. J Evol Biol. 2005;18:455–463. doi: 10.1111/j.1420-9101.2004.00820.x. [DOI] [PubMed] [Google Scholar]
- Sheat JM, Peach RJ, George PM. Rapid detection and initial characterization of genetic variants of human serum albumin. Clin Chem. 1991;37:1221–1224. [PubMed] [Google Scholar]
- Shen QX, Hu WX, Xu B. Killing effect of schistosomula of Schistosoma japonicum by tissues/organs of Microtus fortis in vitro. Bull Hunan Med Univ. 2002;27:198–200. [PubMed] [Google Scholar]
- Takami M, Sone T, Mizumoto K, Kino K, Tsunoo H. Maleylated human serum albumin inhibits HIV-1 infection in vitro. Biochim Biophys Acta. 1992;1180:180–186. doi: 10.1016/0925-4439(92)90066-v. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Sakr YK, Reinhart CL, Sprung HG, Ranieri VM. Is albumin administration in the acutely ill associated with increased mortality? Results of the SOAP study. Crit Care. 2005;9:745–754. doi: 10.1186/cc3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DS, Giuliano Jr JS, Lahni PM, Denenberg A, Wong HR, Zingarelli The immunomodulatory effects of albumin in vitro and in vivo. Adv Pharmacol Sci. 20112011: doi: 10.1155/2011/691928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Zhang R, Yang YQ. Laboratory infects Microtus fortis with Schistosoma japonicum. Acta Parasitol. 1962;1:189–190. [Google Scholar]