Abstract
A variety of combination products composed of biomaterials and biologics have been developed for tissue regeneration or vaccine delivery. The host immune response to the immunogenic biological components in such products may be modulated by the biomaterial component. Distinct biomaterials have been shown to differentially affect the maturation of dendritic cells (DCs). DCs are professional antigen-presenting cells (APCs) that bridge innate and adaptive immunity and play a central role in inducing immunity or initiating immune tolerance. However, the biomaterials systems used to study DC response thus far have been insufficient to draw a clear conclusion as to which biomaterial properties are the key to controlling DC phenotype. In this study, we developed a 96-well filter plate-based high throughput (HTP) methodology to assess DC maturation upon biomaterial treatment. Equivalent biomaterial effects on DC phenotype were measured using the conventional flow cytometric and filter plate method, which validated the HTP methodology. This methodology will be used to screen a large number of biomaterials simultaneously and to draw correlations between material properties and DC phenotype, thereby providing biomaterial design criteria and immunomodulatory strategies for both tissue engineering and vaccine delivery applications.
Keywords: Dendritic cells, biomaterials, high throughput screening, tissue engineering, vaccine delivery
1. Introduction
Biomaterials are widely used as the carriers of biologics, such as cells, nucleic acids, and/or proteins, in combination products for tissue regeneration or vaccine delivery. These products may evoke both a non-specific inflammatory response against the biomaterial component and an adaptive immune response against the immunogenic biologics. Furthermore, the biomaterials also play a role in modulating the host responses due to their adjuvant or immunosuppressive effect. Obviously, the goal of tissue engineering is to minimize the host response to allow the proper functioning of the device and its integration to the host tissue. In contrast, vaccine delivery aims to enhance or maximize a protective immune response to the delivered antigen.
Dendritic cells (DCs) are the most potent antigen-presenting cells (APCs) that are specialized in the uptake, transport, processing, and presentation of antigens to T cells [1–3]. Among APCs, which also include macrophages and B cells, only DCs are believed to be capable of activating naïve T cells [4]. In their immature state, DCs act as sentinels in peripheral tissues, constantly sampling the environment for potentially dangerous pathogens and antigens [2, 5]. In fact, they detect the presence of pathogens by recognizing a limited number of conserved structures, called pathogen-associated molecular patterns (PAMPs), produced only by micro-organisms, viruses, and fungi [6]. In addition, DCs can be stimulated by “danger signals”, necrotic tissues and their byproducts such as tissue fragments and intracellular molecules [7]. Using pattern recognition receptors (PRRs) expressed on DCs, the ligation of pathogens or “danger signals” initiates signaling cascades (e.g., the activation of NF-κB, AP-1, MAPK) that lead to the maturation of DCs [7, 8]. Dendritic cell maturation results in the change in morphology, the up-regulation of co-stimulatory molecules (notably CD80 and CD86 in the B7 family) and major histocompatibility complex (MHC) molecules, and the release of pro-inflammatory cytokines [9, 10]. Activated DCs transiently enhance antigen uptake but down-regulate their endocytic capacity after several hours [11, 12], accompanied by a decreased expression of C-type lectin DC-SIGN (DC-specific intercellular adhesion molecule-grabbing nonintegrin), which is primarily expressed in certain subsets of DCs, including monocyte-derived DCs [13].
The primary mechanism by which adjuvants enhance an adaptive immune response is the maturation of DCs, which results in their efficient antigen presentation and T cell stimulation that generate an immune response to associated antigens. Enhanced immunogenicity is believed to occur when mineral salts (e.g., alum), liposomes, and biodegradable polymer microspheres cause a depot effect at the site of injection [14, 15]. Others, such as complete Freund’s adjuvant (CFA) and incomplete Freund’s adjuvant (IFA) act as immuno-stimulators but are not approved for human use [15–17]. Previous studies show that the polymer, poly(lactic-co-glycolic acid) (PLGA), acted as an adjuvant in the enhancement of humoral immunity against a co-delivered model antigen in vivo [18, 19]. In addition, depending on the biomaterial used in vitro to treat immature DCs (iDCs), differential levels of DC maturation were observed. For example, DC maturation was induced by treatment with PLGA or chitosan films, not induced by treatment with agarose or alginate films, and inhibited by treatment with hyaluronic acid films [20, 21]. These studies suggested the potential of biomaterials to modulate DC phenotype, thereby achieving distinct effects on immune responses. However, in order to translate a differential biomaterial effect on DC phenotype into design rules for biomaterials with distinct immunomodulatory effects, it is necessary to draw correlations between biomaterial physiochemical properties and effects on resultant DC phenotype. With the limited biomaterial systems that were used in the previous studies cited [20, 21], it was unclear which biomaterial properties caused such differential effects. If the effects of biomaterials on DCs are investigated using biomaterials with controlled graded variations in their properties in a combinatorial array, the correlations between DC phenotype and material properties can become more evident. Such correlations will serve as criteria for the biomaterial design of combination products to modulate the host responses.
The assessment of DC maturation in response to biomaterials typically involves the treatment of immature DCs (iDCs) with biomaterials pre-placed in wells of a 6-well plate to allow for a sufficient number of cells for the assessment of DC phenotype using immunological assays such as flow cytometry for the expression of DC-specific or maturation surface markers or allostimulatory ability in a mixed lymphocyte reaction. Using extensive immunological assessment assays, the effect of different biomaterials on various aspects of DC phenotype and function have been assessed [21, 22]. However, our conventional method would be time-consuming and require large quantities of reagents for the assessment of DC responses to large libraries of polymers. Hence, the goal of this research was to develop and validate a high throughput (HTP) methodology to assess DC phenotype upon the contact with combinatorial libraries of biomaterials with graded material properties.
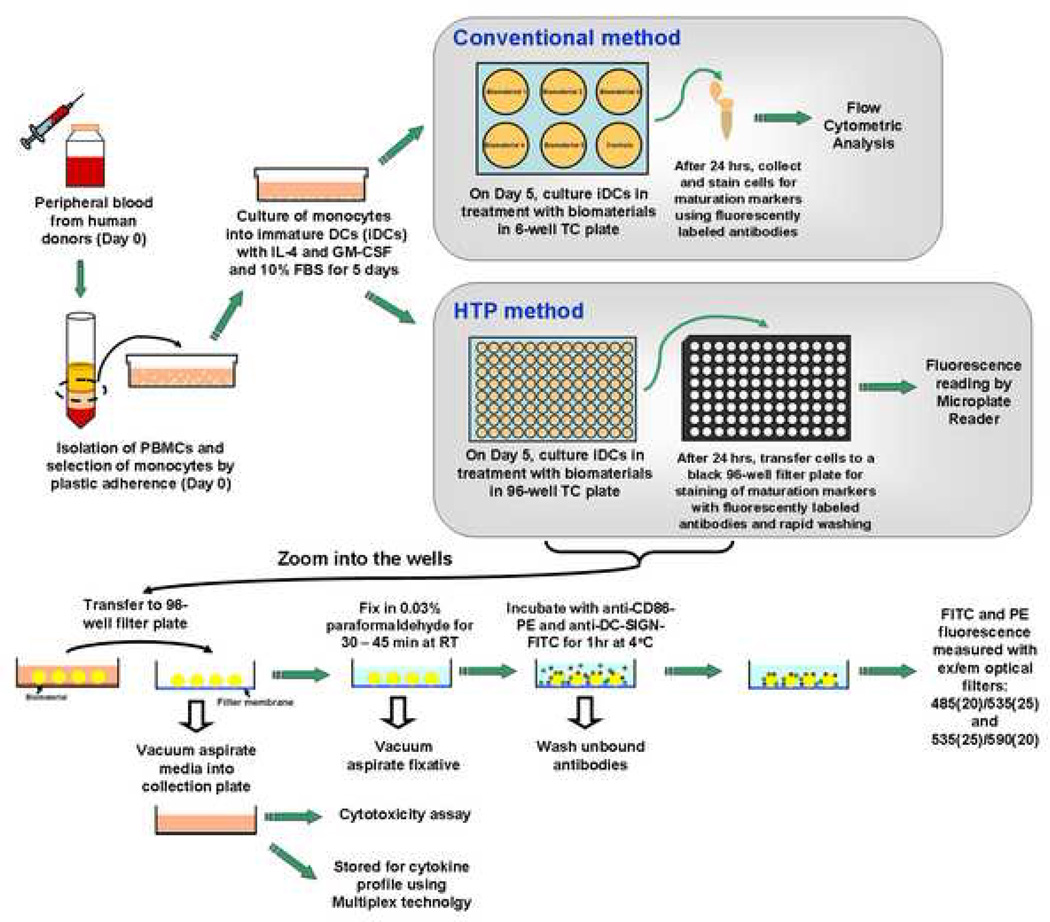
The culture characteristics of DCs presented a unique challenge in that the DCs are loosely-adherent or non-adherent in culture; hence, a traditional cell-based Enzyme-Linked ImmunoSorbent Assays (ELISAs) could not be used due to expected cell loss during wash steps. To enhance the efficiency in sample processing and subsequent measurement, many cell-based assays have been processed in filter plates [23, 24]. Such plates are 96- or 384-well standard-sized plates with an individual filter membrane welded in each well. Because the fast and simple removal of supernatants is assisted by the application of a vacuum manifold, we expected that the filter plates would provide a suitable platform for the development of a HTP screening methodology for the simultaneous quantification of maturation markers of many DC samples. By far, black 96-well filter plates have offered the most promising means to rapidly wash the cell samples without any cell loss and offered fluorescence detection in situ. Therefore, here, we present the validation of a 96-well filter plate-based HTP screening methodology for DC phenotype upon biomaterial contact. Briefly, after treatment with biomaterials in a 96-well plate, the DCs are transferred to a black 96-well filter plate and stained with anti-CD86-PE and DC-specific anti-DC-SIGN-FITC monoclonal antibodies. The ratio of CD86-PE/DC-SIGN-FITC, or ’maturation factor‘, is a DC number-independent parameter to represent DC maturation. The supernatants can be easily collected into a 96-well plate using a centrifuge, assayed for cytotoxicity, and stored for cytokine profiling. Figure 1 illustrates the experimental scheme of the conventional assessment of DC maturation as well as the filter plate-based HTP screening methodology.
Figure 1.
A schematic of the conventional method and the HTP method for analyzing DC response to biomaterials. For both of the analysis methods, DCs were derived from human peripheral blood mononuclear cells (PBMCs) using the same procedures until day 5. On day 5, for the conventional method, DCs were treated with biomaterials in a 6-well plate for 24 hours. The cells after treatment are then collected and stained, and flow cytometry is performed to analyze the cell surface marker expression. In contrast, for the HTP method, DCs are treated with biomaterials in a 96-well plate for 24 hours. On day 6, DCs are transferred to a 96-well filtration plate, fixed and then stained with anti-CD86-PE and anti-DC-SIGN-FITC antibodies for 1 hour and washed. The relative fluorescence intensity is subsequently measured by a Tecan Infinite F500 microplate reader. Simultaneously, the cell culture supernatants from each well can be aspirated into a collection plate and tested for cytotoxicity and stored for cytokine profiling using Multiplex technology.
2. Materials and methods
2.1. Derivation of immature DCs (iDCs)
Human blood was collected from donors with informed consent and heparinized (333 U/ml blood) (Abraxis Pharmaceutical Products, Schaumburg, IL) at the Student Health Center Phlebotomy Laboratory, in accordance with the protocol (No. H05012) of the Institutional Review Board of Georgia Institute of Technology. Dendritic cells were derived from human peripheral blood mononuclear cells (PBMCs) using a previously described method [25] with some modifications. Briefly, the collected blood was diluted with a 1:1 ratio in Mg2+- and Ca2+-free phosphate buffer saline (D-PBS; Invitrogen, Carlsbad, CA), and peripheral blood mononuclear cells (PBMCs) were isolated by differential centrifugation using lymphocyte separation medium (Cellgro MediaTech, Herndon, VA). After the lysis of erythrocytes with RBC lysing buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) and washing steps, PBMCs were resuspended at a concentration of 5 × 106 cells/ml in DC media, which was prepared by filter-sterilizing RPMI-1640 containing 25 mM HEPES and L-glutamine (Invitrogen), supplemented with a final concentration of 10% heat inactivated fetal bovine serum (h.i. FBS; Cellgro MediaTech, Herndon, VA) and 100 U/ml of Penicillin/Streptomycin (Cellgro MediaTech). The cells were plated in a volume of 10 ml/plate in a Primaria 100 × 20 mm2 tissue-culture dish (Becton Dickinson, Franklin Lakes, NJ) and incubated for 2 hours at 95% relative humidity and 5% CO2 at 37°C to select for adherent monocytes. After this incubation, the dishes were washed three times with warm DC media to remove non-adherent cells. The remaining adherent cells were supplied with 10 ml/plate new warm DC media, supplemented with 1000 U/ml GM-CSF and 800 U/ml IL-4 (PeproTech, Rocky Hill, NJ), and incubated for 5 days without changing the media to induce the differentiation of monocytes into iDCs. Immature DCs were treated with biomaterial films in the wells of 6-well or 96-well plates (the HTP methodology) with assessment of resultant DC phenotype using flow cytometry or fluorescent plate reader, as described in sections 2.3 or 2.4, respectively. As a part of culture characterization, DCs were purified using magnetic sorting based on manufacturer’s protocol (Miltenyi Biotec, Auburn, CA). Briefly, the cell population harvested on day 5 of DC culture first underwent CD19+ B cell depletion (negative selection), followed by CD1c+ DC isolation (positive selection). The purity of DC population was approximately 95% or above following this purification.
2.2. Preparation of PLGA and agarose film
Poly(DL-lactic-co-glycolic acid) (PLGA, molar ratio: 75:25, inherent viscosity: 0.70 dL/g in trichloromethane, MW = 100,000 Da; Birmingham Polymers, Birmingham, AL) films were prepared by solvent casting without a porogen as previously reported [20]. Briefly, PLGA was dissolved 20% w/v in dichloromethane (DCM) overnight at room temperature and poured into a 50-mm Teflon dish (Cole-Parmer, Vernon Hills, IL) in a chemical fume hood. After evaporation of the solvent and drying to form films (48–72 hours), the PLGA films were punched to fit into the wells of a 6-well plate or a 96-well plate, followed by three washing steps using endotoxin-free water (Cambrex, East Rutherford, NJ) and UV sterilization for 30 min on each side in the tissue culture hood before iDCs were plated on them. Agarose (type V, high gelling, gel strength of ≥800g/cm2 at 1.0%, MW unknown; Sigma, St. Louis, MO) was prepared to form 3% w/v aqueous solution by boiling of agarose in ddH2O in a microwave until the agarose was completely dissolved. The films were prepared by dispensing 1 ml of agarose solution into a well of a 6-well tissue culture plate (Corning, Corning, NY), or 50 µl in a well of a 96-well tissue culture plate (Corning). The films were allowed to solidify at 4°C for at least 30 min and brought back to room temperature for another 30 min in a tissue culture hood without any further sterilization step prior to culturing iDCs on them. The endotoxin content of PLGA and agarose films was measured using a chromogenic substrate (QCL-1000 LAL assay; Cambrex) and determined less than 0.l EU/mL, which is well below the FDA limit of 0.5 EU/ml [20, 21]. Previous study showed that a minimum Escherichia coli endotoxin concentration of 100 EU/ml or 10 ng/ml was required for DC maturation [26].
2.3 Treatment of DCs with biomaterials in 6-well plates with assessment of DC phenotype using Flow Cytometry
After 5 days of cell culture, the PLGA films were placed into the wells of a 6-well plate with sterilized gaskets (Cole-Parmer, Vernon Hills, IL) to secure the films. Agarose films were prepared as described in Section 2.2 directly in the wells. Non-adherent and loosely-adherent cells were collected, resuspended in new pre-warmed DC media at a concentration of 5 × 105 cells/ml, and plated at the volume of 3 ml (1.5 × 106 cells/well) in each well. The cells were then supplemented with cytokines (1000 U/ml GM-CSF, 800 U/ml IL-4). Dendritic cells were treated for 24 hours with biomaterials with known effects on DCs (i.e., PLGA or agarose), treated with 1 µg/ml of lipopolysaccharide (LPS) (E. coli 055:B5; Sigma) to become mature DCs (mDCs; positive control), or left untreated to remain iDCs (negative control). The levels of surface marker expression were monitored after 24 hours of biomaterial treatment by flow cytometry per the methods described previously [22] and compared to the controls. The cells were collected by centrifugation at 1100 rpm for 10 min, resuspended in 0.1 % BSA and 2 mM EDTA in PBS, pH = 7.2 (cell-staining buffer), and stained with fluorescently-labeled antibodies CD40 (clone B-B20; mouse IgG1κ), CD86 (clone BU63; mouse IgG1κ) (Ancell Corporation, Bayport, MN), CD83 (clone HB15a; mouse IgG2aκ) (Immunotech, Marseille, France), CD80 (clone BB1; IgMκ), HLA-DQ (clone TU169; mouse IgG2aκ), HLA-DR (clone TU36; mouse IgG2aκ) (BD Biosciences), CD1c (clone AD5-8E7; mouse IgG2a) (Miltenyi Biotec, Auburn, CA), or DC-SIGN (clone 120507; mouse IgG2b) (R&D Systems). The cells were stained for 1 hour at 4°C, and analyzed using a BDLSR flow cytometer (Beckton Dickinson, San Jose, CA). Data analysis was performed using FlowJo (Tree Star, Ashland, OR) based on the differential shift of histograms compared to the controls unless otherwise indicated. The ‘maturation factor’ values were determined by dividing the gMFIs of CD86-PE by that of DC-SIGN-FITC. The antibody binding capacity of CD86 expressed on DCs or B cells were determined by staining the cells with anti-CD86-PE, gating the distinct DC and B cell populations on the scatter plot, measuring the gMFIs of the populations, and comparing the gMFIs to a standard curve created by beads with known number of CD86 antibody binding sites (Quantum Simply Cellular® kit; Bangs Laboratory, Fishers, IN). To quantify the percentage of DCs and B cells or B cells and T cells in the culture system, the cells were double-stained with anti-CD19-APC (clone HIB19; mouse IgG1κ) (BD Biosciences) and anti-DC-SIGN-FITC or anti-CD19-APC and anti-CD3-PE (clone UCHT1; BD Biosciences). The percentage of B cells (CD19+), T cells (CD3+) and DCs (DC-SIGN+) were analyzed using FlowJo based on the differential shift of cell populations in the dot plots.
2.4 Treatment of DCs with biomaterials in the 6 or 96-well plate HTP format with assessment of DC phenotype using Fluorescent Microplate Reader
On day 5 of DC culture, the PLGA or agarose films were prepared as described in Section 2.2, and iDCs (3 ml, 5 × 105 cells/ml) were plated onto each well in the 6-well plate. In the 96-well format, the PLGA films were slightly wetted on one side with endotoxin-free water and adhered to the wells of the 96-well tissue-culture plate in triplicate, while agarose films were formed by dispensing 50 µl of agarose solution directly into the wells and solidified. One hundred microliters of iDCs (5 × 105 cells/ml) was plated onto each well in the 96-well plate, and secure adherence of the PLGA film was checked by visual inspection. The wells for the negative control of iDCs remained untreated and those for the positive control of mDCs were treated with lipopolysaccharide (LPS; E. coli 055:B5; Sigma). The DCs cultured in a 96-well plate were pre-incubated at room temperature for 30 min to reduce the edge effect by minimizing thermal gradients in the edge wells [27]. Subsequently, the DCs were cultured in an incubator at 95% relative humidity and 5% CO2 at 37°C for 24 hours. On day 6, the DCs treated in the 6-well plate were harvested, and 100 µl of the cell suspension was transferred to wells of a 96-well black filter plate, while the DCs treated in the 96-well plate were transferred directly to other wells in the same filter plate. The supernatants were then removed by a vacuum manifold (Millipore, Bedford, MA) with vacuum pressure pre-adjusted to 2–4 inHg. To each well, 100 µl of cold working fixation solution (0.03 % paraformaldehyde) was added, and the plate was incubated for at least 30 min at room temperature on a microplate shaker at 600 rpm (VWR, West Chester, PA), followed by the removal of the fixative by the vacuum manifold. Subsequently, 100 µl of staining solution containing 1.5 µg/ml anti-CD86-PE and 1.5 µg/ml anti-DC-SIGN-FITC (monoclonal antibodies as used for flow cytometry) in cell staining buffer was added into each well containing sample to be stained. IgG1-PE (clone MOPC31C) (Ancell) and IgG2B-FITC (clone 133303) (R&D Systems) isotype-stained DCs were used for background fluorescence subtraction in separate treatment or control wells. The plates were washed three times with 200, 250, and 300 µl/well of cell staining buffer. Again, the vacuum manifold was used for each supernatant removal. The relative fluorescence units (RFUs) were measured with a Tecan Infinite F500 microplate reader (Tecan US, Durham, NC) using excitation filters of 535/25 and 485/20, and emission filters of 590/20 and 535/25, for PE and FITC, respectively. Because no difference in the RFUs from the isotype controls among iDCs, mDCs, PLGA-treated, and agarose-treated DCs was observed, only the isotype control of iDCs was used for the subtraction from the raw data to eliminate the background fluorescence. The ratio of background-subtracted CD86-PE to background-subtracted DC-SIGN-FITC from each well was determined, and the average ratio (‘maturation factor’) was calculated from the triplicate.
2.5. Assessment of Biomaterial-induced Cytotoxicity
Cytotoxicity associated with biomaterial treatment was assessed by measuring the release of cytosolic enzyme glucose-6-phosphate dehydrogenase (G6PD) into the media from cells cultured in the presence or absence of biomaterials. G6PD is released from damaged or dead cells, and its presence was measured using the Vybrant Cytotoxicity Assay (Molecular Probes, Eugene, OR). The supernatants were easily collected from cells cultured with or without biomaterials of PLGA or agarose films or from controls from a 96-well plate filter plate into a 96-well collection plate by stacking the filter plate on top of the collection plate and centrifuging at 250 × g for 2 min. Fifty microliters of the supernatants were assayed immediately according to the manufacturer’s protocol, because freeze-thawing the supernatants decreased the enzymatic activity substantially. The medium from cells lysed with 0.5% Triton X served as a positive control. The fluorescence readings were taken after 30 min incubation at 37°C with excitation and emission filters 535/25 and 590/20, respectively. This experiment was repeated 3 times.
2.6. Statistical analysis
Two-sided pairwise student t-test was used to compare the sample group to the appropriate control group. To observe any significant differences between all sample groups in pairs, pairwise general linear model of the two-way ANOVA with a mixed model and repeated measure followed by Tukey post test was used. For all statistical methods, the Minitab software (Version 14, State College, PA) was used. If otherwise indicated, the p-value equal to or less than 0.05 was considered significant.
3. Results
3.1. DC-SIGN was a suitable marker for the definition of ’maturation factor‘, which represents the degree of DC maturation
Biomaterials could not be cast onto the filter plates because the filter membranes would be clogged. For this reason, the cell samples cultured on biomaterials in a regular 96-well tissue-culture plate were required to be transferred to a 96-well black filter plate for staining and analysis. Hence, the cell numbers in the wells in the filter plate may vary significantly and a cell number normalization method was required to account for the variations in cell number. Cell number normalization by total DNA or a DC-specific surface marker was investigated. CD86, a costimulatory molecule, was used as the maturation marker because of its high expression level and its large fold change upon DC maturation, including upon biomaterial treatment [21, 22].
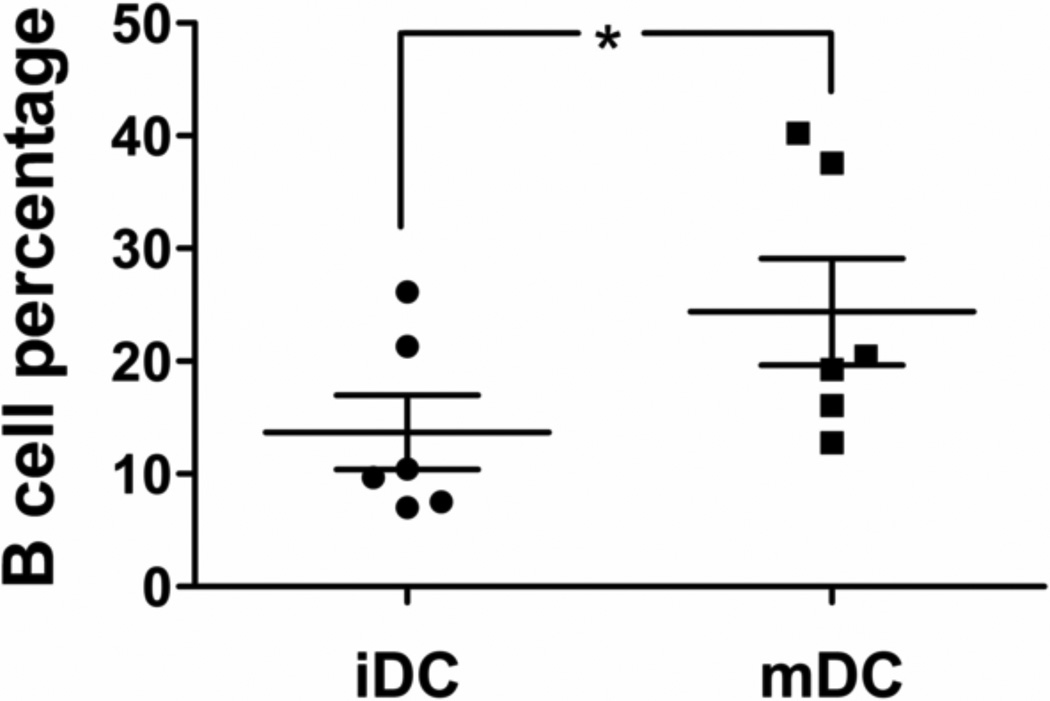
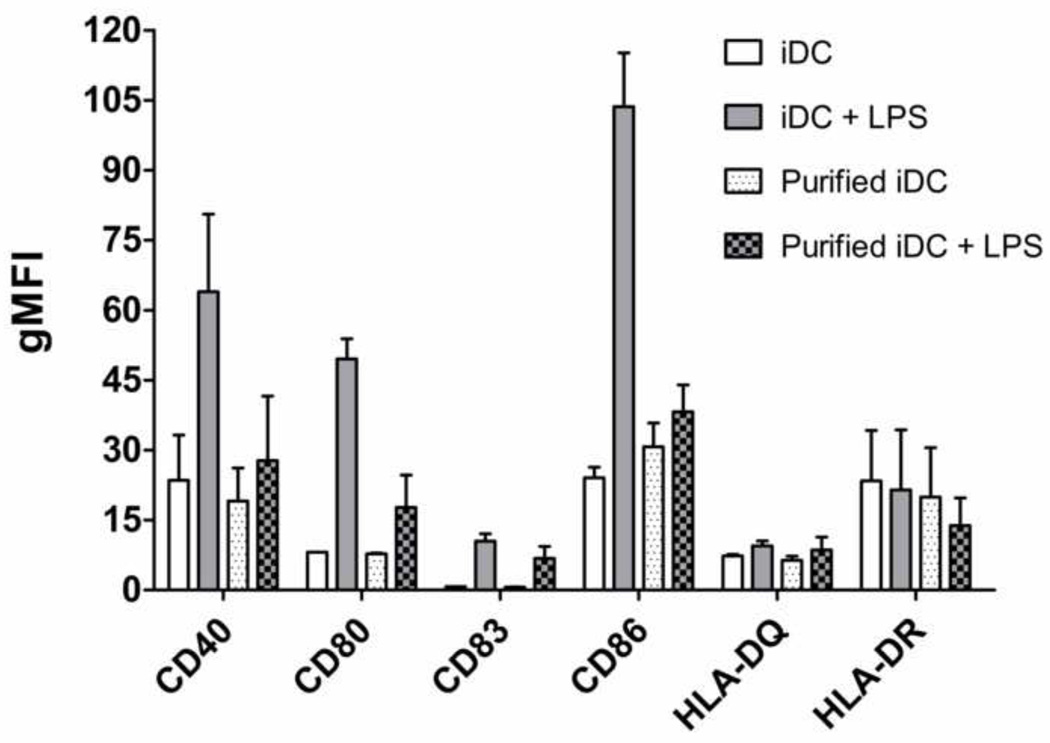
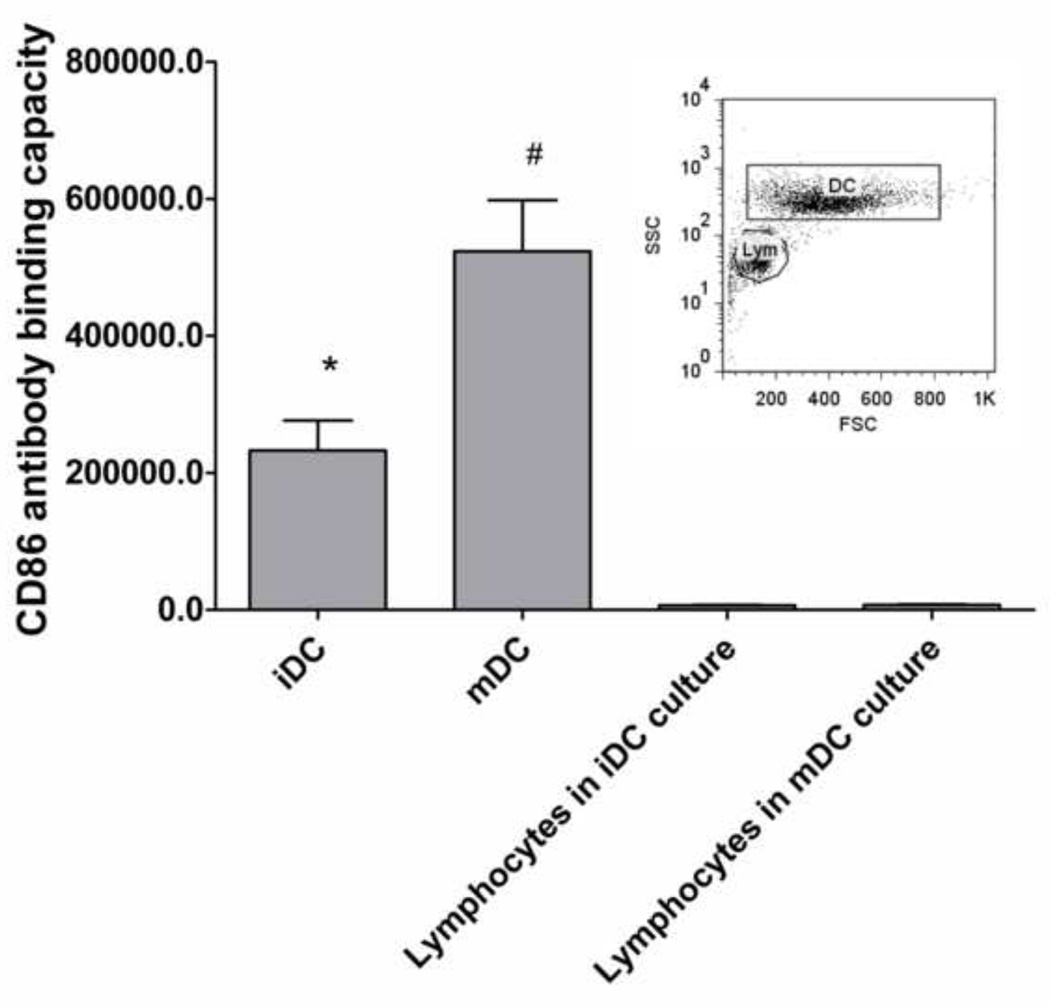
The conventional method was used to derive iDCs and mDCs, and the DC culture was previously determined to primarily consist of DCs and lymphocytes by day 5 and day 6 [28], and ≥ 90% of the lymphocytes were found to be CD19+ B cells based on dual antibody staining for CD3+ T cells and CD19+ B cells. The same culture system was used in this study. It was determined that the DC:B-cell ratios and B cell percentages changed significantly between iDCs and mDCs, and the B cell content varied significantly among different donors (Figure 2). Such variations could introduce substantial noise in the analysis of the DC maturation results. To eliminate the variability in the cell population ratios (and remove B cells) in the culture system, DCs were purified by removing CD19+ B cells (negative selection) and then positively selecting CD1c+ using magnetic beads. However, the purified DCs were significantly less responsive to the LPS treatment than the unpurified population (Figure 3); hence purified DCs could not be used for treatment with biomaterials. B cells were necessary in the culture system to achieve full responsiveness of DCs, possibly due to the profile of cytokines and natural antibodies produced by B cells even in the absence of support from T cells in the culture system [29–31]. A potential issue with B cell presence in the culture system is that they can also express the maturation marker, CD86 [32]. However, it was determined that the B cells in this culture system expressed very low CD86 compared to the DCs. Although approximately 9.4% and 7.3% of B cells were CD86+ in the iDC culture and mDC culture, respectively, the contribution of CD86 from B cells was less than 5% of the CD86 that is expressed on iDCs and less than 2% on mDCs (Figure 4). Furthermore, no DNA stain was found to be compatible with the filter plate assay, primarily due to their broad excitation and emission spectra and the strong background fluorescence generated by the possible binding of the stain to the filter membrane. Consequently, the strategy of data normalization by total DNA was not further pursued.
Figure 2.
B-cell percentage in the iDC and mDC cultures by flow cytometric analysis. The B cell percentages in the DC culture are shown with mean ± SEM, n=6 different donors. *: p<0.05 and represents statistical difference between iDCs and mDCs.
Figure 3.
Dendritic cells purified by magnetic sorting were less responsive to LPS stimulation in comparison to unpurified counterparts. On day 5 of DC culture, DCs were magnetically isolated by removing CD19+ B cells and then positively selecting CD1c+ DCs, treated with LPS for mDCs or left untreated for iDCs. The geometric mean fluorescence intensity (gMFI) of these purified DCs was analyzed by flow cytometry for surface marker expression after 24 hrs and compared to the unpurified counterparts with mean ± range, n=2 donors.
Figure 4.
CD86 expression on DCs and lymphocytes in the culture system. The antibody binding capacity of CD86 on DCs and B cells was measured by comparing the gMFIs of CD86 expression to a standard curve created by beads that bound known numbers of antibodies using the BD FACSDiva software with mean ± SEM, n=6 donors. The inset shows the gating of DCs and lymphocytes (Lym) based on the forward (FSC) and side scatters (SSC) of the two distinct cell populations for which CD86 expression levels were determined. *: p<0.05, lower than mDCs and higher than lymphocytes; #: p<0.05, higher than iDCs and lymphocytes.
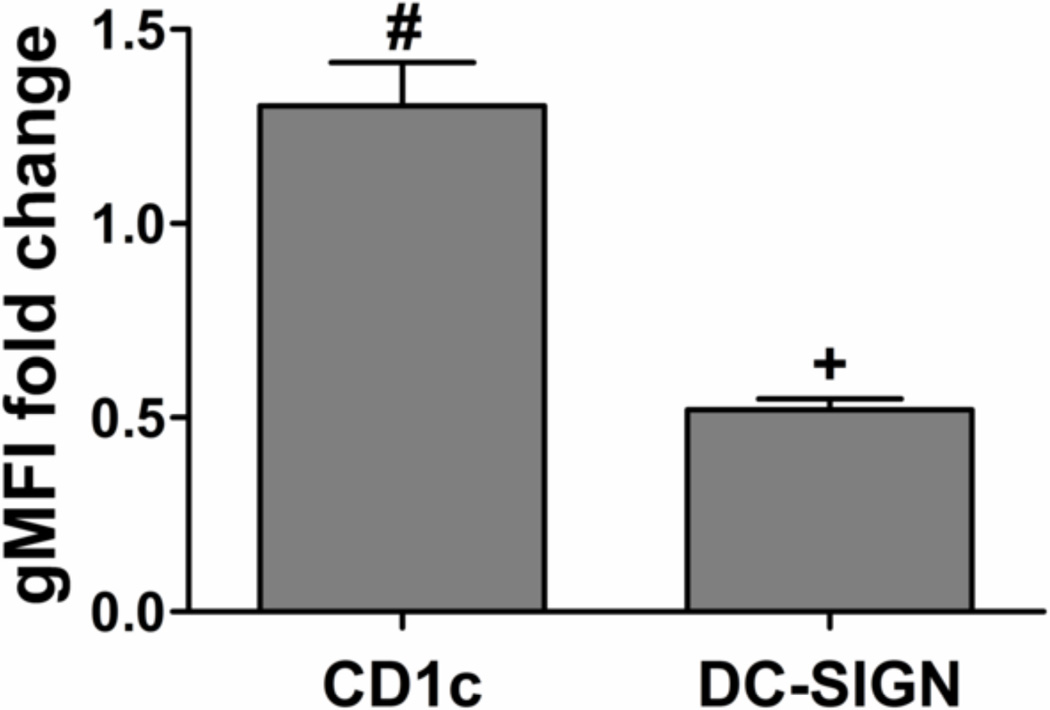
CD1c was initially considered as a normalization factor because some studies reported that CD1c expression only slightly increased during DC maturation [33] and because previously we observed negligible levels of CD1c expression on B cells in the culture system making it specific for DCs (data not shown). However, here CD1c expression increased significantly upon DC maturation (Figure 5). This may offset the increase in CD86 expression, if used as a normalization factor, which is undesirable. That is, when CD1c increases upon maturation along with CD86, the ratio of CD86/CD1c may show no difference compared to iDCs. As a result, CD1c is not suitable as a normalization factor.
Figure 5.
Dendritic cell expression of CD1c and DC-SIGN by flow cytometric analysis. The fold change of gMFI for mDCs was compared to that for iDCs among donors with mean ± SEM, n=6 donors. #: p<0.05, compared to iDCs and higher than iDCs; +: p<0.05, compared to iDCs and lower than iDCs.
As an alternative marker for DC phenotype, C-type lectin DC-SIGN was then examined for its applicability as a normalization factor in the HTP assay. Consistent with the literature [13], DC-SIGN expression level was lowered upon DC maturation (Figure 5). Furthermore, DC-SIGN is only expressed by DCs in the culture system, and the ratio of CD86/DC-SIGN was DC number-independent in the assay. Therefore, DC-SIGN was used in the definition of ‘maturation factor’, the ratio of CD86/DC-SIGN, to represent DC maturation.
3.2. Equivalent assessment of biomaterial effects on dendritic cells were observed validating the filter-plate method
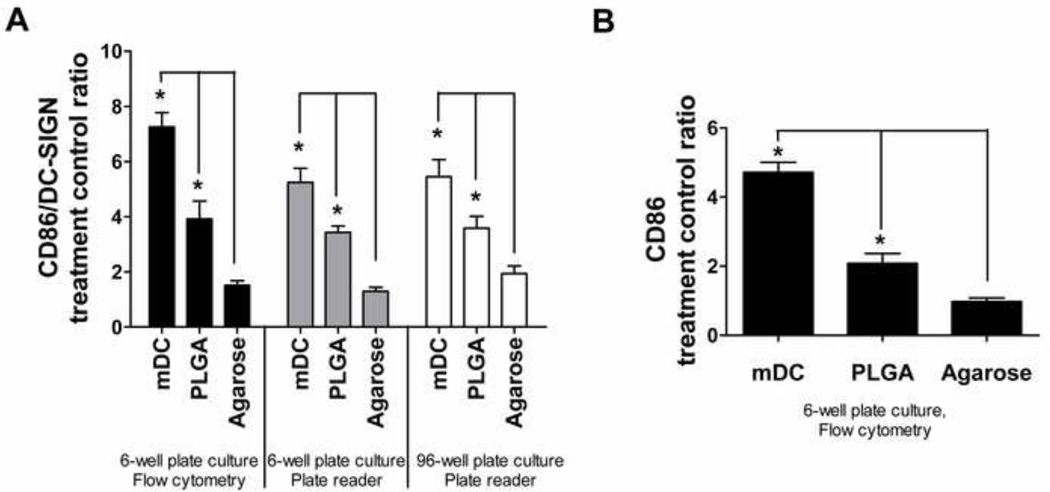
To validate the 96-well filter plate-based method as far as DC treatment with biomaterials in the 96-well plate format and analysis of levels of CD86 and DC-SIGN expression using the fluorescent plate reader, a biomaterial study using biomaterials with known effects on DCs phenotype, namely, PLGA or agarose films, was conducted. The results in Figure 6A showed that the trend for the fold change of ‘maturation factor’ of the cell samples, whether treated with biomaterials in a 96-well or a 6-well tissue-culture plate, were similar; hence, the use of a 96-well format for biomaterial treatment on DCs was appropriate. In addition, the samples from the 6-well plate were analyzed by the 96-well filter plate format using the fluorescence plate reader or by the conventional flow cytometric analysis to yield similar trends, further confirming previous results [20, 21], in which PLGA films induced DC maturation, but agarose films did not. The flow cytometric analysis of other maturation markers such as CD40, CD80, CD83, CD86, HLA-DQ, and HLA-DR also confirmed differential DC maturation in response to different biomaterials treated using the 6-well format (shown for CD86 in Figure 6B; data for other markers were similar to previously published results [20]). Collectively, this experiment validated the filter plate approach for assessing DC phenotype upon biomaterial contact. Furthermore, in such experimental setup, the signal/background (S/B) and signal/noise (S/N) ratios for FITC ranged from 2.3 to 3.3 and from 52.3 to 71.7, respectively. The corresponding ratios for PE ranged from 3.5 to 8.8 and from 53.2 to 113.8. Because of the large S/N ratios, signals could easily be distinguished from the background.
Figure 6.
Validation of the HTP methodology for assessing DC responses to biomaterials. A) Treatment/control ratios of ‘maturation factor’ (defined as CD86/DC-SIGN) for DCs treated with biomaterials or controls in the 6-well format and analyzed by flow cytometry (set of black bars), in the 6-well format and analyzed by fluorescent plate reader (set of grey bars), and in the 96-well format and analyzed by fluorescent plate reader (set of white bars). B) Treatment/control ratios of CD86 expression for DCs treated and analyzed using the conventional format of 6-well plates and flow cytometry for DCs treated with biomaterials or controls. Mean ± SEM; n=8 (6 donors). *: p<0.05, statistically different from iDCs and higher than iDCs. Brackets: p<0.05, statistically different between two biomaterial treatments or between biomaterial treatment and mDCs.
The use of filter plate puts biomaterial-treated DCs in contact with the filter membrane (another material) and therefore fixation of DCs is required to prevent any DC maturation effects due to the filter membrane. Therefore, one issue was whether the level of surface molecule expression detected would be equivalent on the DCs with and without prior fixation. Another issue was whether paraformaldehyde fixation in the filter plate would cause undesired cell bonding to the filter membrane, thereby affecting subsequent surface marker staining. To address the first issue, it was demonstrated that the levels of CD86, DC-SIGN, and CD1c expression detected by flow cytometry were equivalent for DCs with and without fixation in an Eppendorf tube prior to staining with the monoclonal antibodies (results not shown). Furthermore, to address the second issue, equivalent levels of these markers were determined by flow cytometry for DCs fixed and stained in filter plate as compared to DCs stained in Eppendorf tubes without fixation (data not shown).
3.3. Biomaterial-induced cytotoxicity
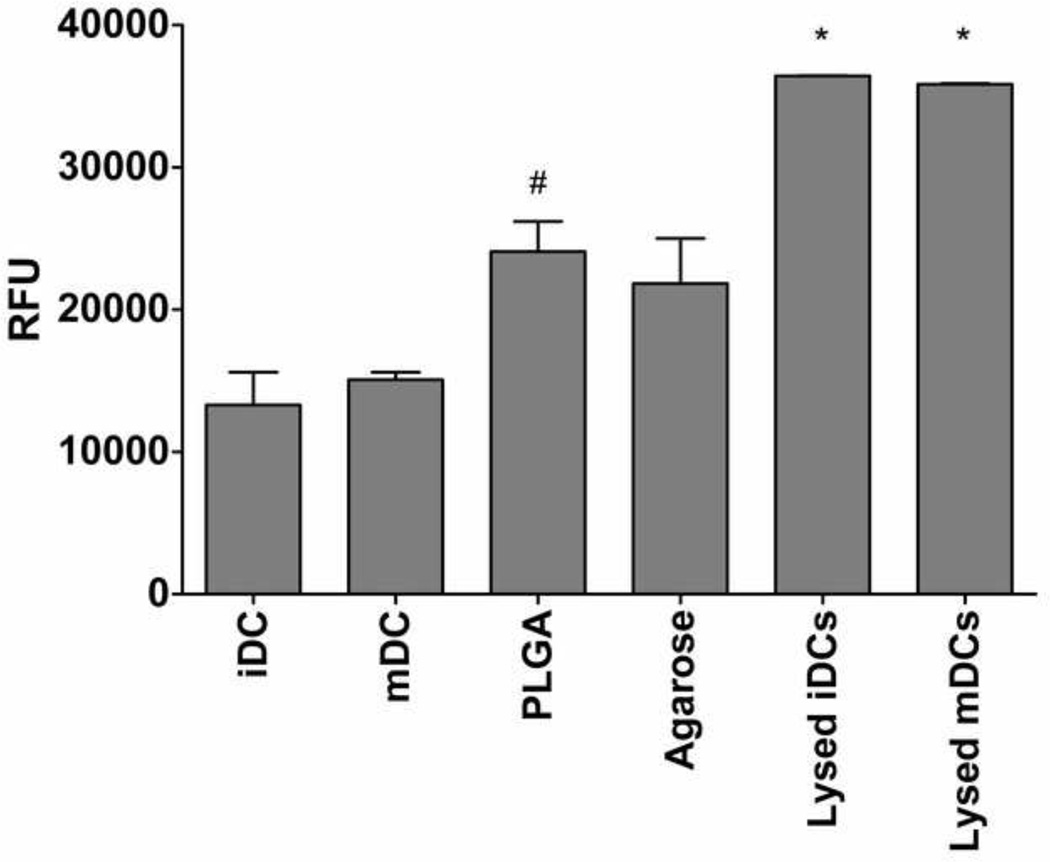
After 24 hr of treatment with biomaterials, cell supernatants were collected and assayed for the release of G6PD into the medium. DCs treatment with PLGA films was found to induce higher levels of released G6PD into the media as compared to iDC (Figure 7). This result is consistent with previous result in which DCs treated with PLGA induced higher annexin V staining than iDC but not mDC or agarose films (J. Park and J E. Babensee, unpublished observation). As expected, the lysed iDC and mDC samples induced very high G6PD release, and the fluorescence signal was saturated (Figure 7).
Figure 7.
Effect of PLGA and agarose on DC glucose-6-phosphate dehydrogenase (G6PD) release. Dendritic cells were cultured with or without biomaterials PLGA or agarose films in a 96-well format for 24 h. The supernatants were collected into a 96-well plate by centrifugation at 250 × g for 2 min and then measured for G6PD release using theVybrant Cytotoxicity Assay at 37°C. The fluorescence was measured at 30 min using a Tecan Infinite 500 microplate reader. Mean ± SEM; n =3. *: p<0.05 higher than all the treatment groups; #: p<0.05 higher than iDC and mDC.
4. Discussion
A 96-well filter plate-based HTP methodology has been optimized and validated for the assessment of DC responses to biomaterials. In this methodology, after treatment with biomaterials, DCs were transferred to a black 96-well filter plate, supernatant collected for analysis of soluble mediators/indicators of cell viability and remnant cells analyzed for expression of the ‘maturation factor’, CD86/DC-SIGN, using a fluorescent plate reader. Using this methodology, DC responses to the biomaterials, PLGA or agarose films, were consistent with results obtained using conventional flow cytometry analysis. Specifically, DCs treated with biomaterials in a 96-well or a 6-well tissue-culture plate format yielded similar trends of maturation; therefore, a 96-well format was appropriate for DC treatment with biomaterials. In addition, the DC samples from the 6-well plate (conventional method) were analyzed in a 96-well filter plate format using the fluorescence plate reader or by the standard flow cytometric analysis and yielded similar trends, further confirming previous results, in which PLGA films induced DC maturation but agarose films did not. Analysis of biomaterial-induced DC cytotoxicity by measuring release of G6PD into supernatants by damaged cells showed that PLGA-treated DCs showed higher annexin V staining than iDCs consistent with independent experiments (J. Park and J.E. Babensee, unpublished observations). Since unstained PLGA-treated DCs showed similar autofluorescence as iDCs, the observed PLGA-induced maturation and the resulting higher fluorescence signal were not due to the autofluorescence from apoptotic DCs or associated apoptotic bodies. DC culture media supernatants can also be stored for cytokine profiling experiments using Multiplex technology.
The HTP methodology developed herein offers several benefits for analyzing non-adherent or loosely-adherent DC responses to biomaterials as compared to other approaches. Although flow cytometry is a powerful analytical tool, both sample preparation and data analysis are time-consuming, especially when a large number of samples are analyzed. Automated sample loaders for 96-well plate or tubes for flow cytometry are available commercially (e.g. Guava Technologies) to address this issue, but these systems are usually very expensive, poorly accessible to most laboratories, and have long sampling times (approximately 1.5 to 2 hours for automated sampling a 96-well plate) for a large number of samples. The latter characteristic is prohibitive for living cells (particularly responsive leukocytes). Although high-throughput biomaterial arrays exist for cell studies such as the nanoliter-scale biomaterial combinatorial arrays developed by Anderson et al. [34, 35], these arrays are only applicable for adherent cell types and the cellular response to biomaterial differences was measured by immuno-detection or ELISA-based methods which was possible because the cells were localized to a ‘spot’. For DCs used herein, a filter plate-based method was necessary instead of a traditional cell-based ELISA due to the loosely-adherent or non-adherent nature of the cells, which would result in expected cell loss during washing steps using centrifugation and aspiration in a regular microplate during the ELISA analysis. Furthermore, supernatant collection from a regular microplate is time-consuming and inaccurate due to the aspiration of supernatant from individual well with a pipette, while supernatants from all the samples in a filter plate can be simultaneously collected into a collection plate without any cells being collected. An efficient means of defining biomaterial ‘hits’ in a combinatorial library of test polymers can be defined herein for this HTP methodology as a significant (p<0.05) increase or decrease in the value of the ‘maturation factor’ as compared to iDCs. Furthermore, the S/N and S/B ratios were sufficient for the analysis.
There were however complexities in the development of this HTP methodology for the analysis of DC responses to biomaterials which required attention. The first complexity was the non-homogeneous cellular population i.e. presence of B cells. As such, total DNA could not be used to normalize the fluorescent signal which was further justified by the vastly varied DC:B-cell ratios in the culture and the lack of a compatible DNA stain. To consider whether DC responses to biomaterials could be assessed using purified DCs (no B cells) which would clearly make analysis easier, resident B cells in the culture were removed using positive selection and subsequent negative selection of DCs by magnetic sorting. However, the purification of DCs significantly decreased their responsiveness to LPS, which may be explained by the important role of B cells in modulating DC maturation and function, possibly due to the release of cytokines or natural antibodies from B cells (even in the absence of T cells) in the culture system [30]. Although the results herein appear to be contrary to some reports that purified DCs respond well to LPS, it is important to note that the response of purified DCs may be highly dependent on the purification protocol. For example, Jefford et al. reported that DCs differentiated from purified CD14+ monocytes or from CD1c+ peripheral blood DCs responded very differently to maturation stimuli [36]. Therefore, the DC types and culture methods should be considered for particular clinical applications. DCs differentiated from purified CD14+ monocytes or from CD1c+ peripheral blood DCs, or from CD34+ cells from cord blood [37], are the most commonly employed purified DC types. Herein, iDCs were purified (removal of resident B cells) on day 5 of culture after iDCs differentiation had been fully completed. Purifying iDCs at this later stage, rather than performing the purification step at the beginning of the culture for precursor cells, may render the purified iDCs less responsive to maturation stimuli. The DC culture system in this study represents one of the most widely used and well-characterized DC culture systems [28], and without the DC purification step, less stress is exerted on the sensitive DCs.
The second complexity was that given that the presence of B cells in the DC culture can be beneficial for DC function (as presented below) their contribution to the measured CD86 level needed to be minimal to none (as compared to the DCs). B cells were found to release cytokine(s) (e.g. IL-16) and natural antibodies (e.g. CD40-reactive natural antibody) (in the absence of T cells) that aid in monocyte-derived DC migration, differentiation, and maturation [29, 31], indicating that B cells can support DC function without T cell activation. Furthermore, the presence of B cells in the culture system may better represent the physiological multicellular host response to biomaterials in vivo and presumably provides insight into how DCs specifically respond to biomaterials. For the DC culture system used herein, macrophages were not present even though their cultures start with a common monocytic precursor [28], due to the presence of the cytokine IL-4, which induces DC differentiation but inhibits macrophage differentiation [38]. Thus, there would be no contribution from macrophages to the CD86 expression level in this study. Of note, the B cells in our culture system expressed only less than 5% of the CD86 that is expressed on iDCs and less than 2% on mDCs. Thus, the B cells in the culture negligibly contributed to the CD86 fluorescence signal. In addition, previous research indicated that DCs are much more potent in stimulating T cells compared with B cells [39]. Therefore, the presence of B cells is not expected to confound the analysis of DC maturation in this assay. Results presented here indicated that although 9.4% and 7.3% B cells were CD86+ in the iDC and mDC culture, respectively, B cells expressed minimal level of CD86 as compared to DCs. In addition, the expression of CD86 on B cells in the iDC and mDC culture was not different. On the contrary, blood peripheral B cells have been widely reported to express CD86 at low level (7% CD86+ of total B cells) in the resting population and at high level (30.3% CD86+ of total B cells) in the activated population [32]. In addition, B cells have also been reported to upregulate their CD86 expression in response to LPS stimulation [40]. Human B cells are often isolated by FACS cell sorting or magnetic isolation, and then cultured in complete medium as used for DCs in the study herein but without the cytokines IL-4 and GM-CSF [29]. As such, although IL-4 induces CD86 expression on tonsillar B cells [41], presumably the presence of GM-CSF results in the low CD86 expression on B lymphocytes and their unresponsiveness to LPS in the DC culture system herein. However, side-by-side comparison of CD86 expression by DCs and B cells has not been reported in the literature.
Given that total DNA was not a suitable normalization factor for CD86 expression the third complexity was identifying a suitable normalization marker. Of the possible choices, CD1c functions to initiate adaptive immune responses against self or microbial lipid antigens [42–44] and is a characteristic of human DC populations [45]. In addition to DC populations, CD1c has been reported to be expressed on subsets of B cells [45]. Nonetheless, results here showed only a negligible level of expression of CD1c on B cells as compared to DCs (data not shown). Furthermore, some studies reported that CD1c expression only slightly increased during DC maturation [33] and therefore was initially considered as a normalization factor. However, our results showed that CD1c expression increased significantly upon DC maturation (Figure 5), which may offset the increase in CD86 expression. Therefore, CD1c is not suitable as a normalization factor.
Another possible normalization factor considered was the DC-specific cell surface molecule, C-type lectin DC-SIGN. DC-SIGN is a DC-specific adhesion and endocytic receptor [46] that is highly expressed on immature human monocyte-derived DCs [13, 47]. Ideally, an invariant DC marker that is DC-specific and does not change upon DC maturation would be preferred as a normalization factor. However, since no such marker was found, we defined the parameter ‘maturation factor.’ Our rationale of using DC-SIGN were the following: 1) DC-SIGN is expressed on only the monocyte-derived DCs used in our system; therefore, the fluorescent signal measured is specific to the DCs in our culture; 2) The nature that DC-SIGN down-regulates upon DC maturation [13] causes the ratio of CD86/DC-SIGN to further increase, which may in fact give rise to a more sensitive assay for the assessment of DC maturation; 3) If a biomaterial changes DC-SIGN expression significantly while keeping CD86 expression unaltered, such material also becomes a ‘hit’ (a false positive in the context of CD86 expression) and may be further studied due to the importance of DC-SIGN in immunity. Thus, DC-SIGN serves as an additional marker for DC response to stimuli and was selected for defining the DC number-independent parameter – ‘maturation factor’, CD86/DC-SIGN.
To our knowledge, this is the first report describing a microliter plate-based HTP analysis of DC maturation which takes into account the non-adherent nature of these cells, the inherent heterogeneity of this culture, and the sensitivity of these cells. Table 1 compares the HTP filter plate and the flow cytometric methods. The HTP 96-well plate format is superior to the 6-well format because it offers a number of advantages: 1) it provides much higher throughput in the assessment of DC response to biomaterials within the same experimental time frame; 2) this format allows for the simple collection of cell culture supernatants from the DC samples and their storage for multiple cytokine profiling using Multiplex technology; 3) the HTP assay significantly reduces the quantity of biomaterial samples and the time for sample preparation and measurement; 4) data acquisition requires a microplate reader, which is much less expensive and easier to maintain than a flow cytometer. However, the HTP assay also has a few obvious disadvantages: 1) it only provides an average maturation signal from the well but not a distribution or histogram of the cells (more precisely, the events) provided by flow cytometry, so it is impossible to deduce the population or percentage of DCs that are actually affected by the presence of the biomaterials; 2) the HTP assay requires highly expressed and movable markers, while flow cytometer can measure markers of much lower expression level. Despite these disadvantages, the value of the HTP assay lies in its ability to allow for the screening of a large number of biomaterials in a combinatorial biomaterial array. The biomaterial ‘hits’ can be selected and their effects on DCs can then be further probed using conventional assays such as flow cytometry, mixed lymphocyte reactions, endocytosis assay, cytokine profiling, which have been used and continue to be used to analyze DC responses to biomaterials [21, 48].
Table 1.
The comparison between the conventional flow cytometric method and the filter plate-based HTP method.
| Flow Cytometry | Filter Plate | |
|---|---|---|
| Plate format | 6-well plate | 96-well plate |
| Fixation step | No generally | Yes |
| Equipment | Flow cytometer | Microplate reader |
| Experimental time | 3 hours (1 hr incubation) | 2.5 – 3 hours (1.5 hr incubation) for 3 plates |
| # of materials | 5 | Up to 279 (or 135 if duplicate) in 3 plates |
| Size of biomaterial in well | 9.5 cm2 | 0.32 cm2 |
| Data acquisition time | 30 – 60 min | 30 – 60 second per plate, 2 fluorophores |
| Data | Expression of 6 surface markers with histograms and dot plots | A ratio from the average expression of a limited set of surface markers |
| Marker expression level | Very low to high (for markers with any expression level) | Relatively high and movable (appropriate for markers with high expression level and huge fold change upon response) |
The discovery of compounds for new drugs has been dramatically changed with the advent of combinatory chemistry [49]. Similarly, combinatorial arrays of well-controlled and characterized biomaterials are expected to enable the discovery of biomaterials that alter cell behavior. A number of studies have used combinatorial libraries to study cellular response to biomaterial properties. For example, Anderson et al. developed an impressive nanoliter-scale synthesis of biomaterial combinatorial array of different compositions and observed distinct differentiation of human embryonic stem cells (hESCs) on those materials [34, 35]. Meredith et al. created a system with continual changes of topography on glass slide using blends of poly(D,L-lactide) (PDLA) and poly(ε -caprolactone) (PCL) and identified preferred microstructural feature sizes for the attachment, spreading, and proliferation of UMR-106 (rat osteoblastic cell line) and MC3T3-E1 (mouse osteoblastic cell line) cells [50, 51]. Mei et al. created surfaces with well-defined biocompatible polymer brush nanostructures by using varying graft density of poly(2-hydroxyethyl methacrylate) (PHEMA) and showed decreased fibroblast spreading and attachment with increased grafting density [52]. Brocchini et al. developed a polyarylate-based combinatorial array and showed that fibroblast proliferation was more sensitive to chemical structure than contact angle [53]. However, most of these studies investigated basic cell functions such as adhesion or proliferation to well-defined combinatorial polymer libraries with the exception that Anderson’s system investigated biomaterial effects on the differentiation of hESCs. Furthermore, none of these HTP systems allow for the screening of loosely- or non-adherent cells. In contrast, the HTP assay developed and validated herein will be used to study the maturation of a highly sensitive and loosely- and non-adherent cell type, DCs. More importantly, correlations between DC phenotype and material properties can be drawn from the HTP assays using well-characterized combinatorial arrays. Such correlations are highly advantageous due to their potential as a guide for immunomodulatory biomaterial design for both tissue engineering and vaccine delivery applications. Clearly, thorough characterization of the members in the combinatorial array is important in the derivation of such correlations. However, very few good HTP methodologies exist for polymer characterization [54]. As a result, the number of polymers in a well-characterized combinatorial array may be tens to a few hundred, which is well within the capability of the 96-well plate-based methodology developed and validated in this study. Importantly, the success in such a HTP assay relies heavily on the sensitivity of the microplate reader. As a final note, the sensitivity of this methodology may potentially be further improved by using time-resolved fluorescence or miniaturized to a 384-well format with properly optimized conditions and fluorescent dyes to accommodate a larger combinatorial biomaterial library.
5. Conclusion
In this study, a 96-well filter plate-based HTP methodology was developed and validated for the assessment of DC response to biomaterials. This methodology was shown to reproducibly yield similar results of DC maturation in response to biomaterial treatment as compared to the conventional flow cytometric method upon DC treatment in 6-well plates. In addition, the supernatants from each treatment can be easily collected for cytotoxicity using G6PD-based assay and cytokine profiling using multiplex technology. In other words, the 96-well filter plate-based methodology can generate three outcomes from one single cell culture: 1) maturation marker expression, 2) cytotoxicity, and 3) cytokine profile. For future work, this methodology will serve as a HTP screening platform to study the effects of combinatorial biomaterial libraries on DC phenotype. The correlations between biomaterial properties and DC phenotype derived from these studies will provide criteria for biomaterial scientist to design biomaterials with appropriate immunomodulatory properties for specific applications.
Acknowledgement
The authors thank Drs. Philip Santangelo, Andrés J. García, and Barbara Boyan for helpful discussions. The project was supported by a Wallace H. Coulter GT/Emory-PKU BME Collaborative Research Seed Grant and by the National Institutes of Health grant 1RO1 EB004633-01A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 3.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 4.Ouaaz F, Arron J, Zheng Y, Choi YW, Beg AA. Dendritic cell development and survival require distinct NF-kappa B subunits. Immunity. 2002;16:257–270. doi: 10.1016/s1074-7613(02)00272-8. [DOI] [PubMed] [Google Scholar]
- 5.Pulendran B, Palucka K, Banchereau J. Sensing pathogens and tuning immune responses. Science. 2001;293:253–256. doi: 10.1126/science.1062060. [DOI] [PubMed] [Google Scholar]
- 6.Janeway CA, Medzhitov R. Innate immunity: Lipoproteins take their Toll on the host. Curr Biol. 1999;9:R879–R882. doi: 10.1016/s0960-9822(00)80073-1. [DOI] [PubMed] [Google Scholar]
- 7.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 8.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 9.Banchereau J, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 10.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defense. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 11.West MA, et al. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305:1153–1157. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 12.Kalinski P, Hilkens CMU, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 13.van Kooyk Y, Geijtenbeek TBH. DC-sign: Escape mechanism for pathogens. Nat Rev Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- 14.Kwissa M, Kasturi SP, Pulendran B. The science of adjuvants. Expert Rev Vaccines. 2007;6:673–684. doi: 10.1586/14760584.6.5.673. [DOI] [PubMed] [Google Scholar]
- 15.Gupta RK, Siber GR. Adjuvants for human vaccines - current status, problems and future prospects. Vaccine. 1995;13:1263–1276. doi: 10.1016/0264-410x(95)00011-o. [DOI] [PubMed] [Google Scholar]
- 16.Singh M, O'Hagan D. Advances in vaccine adjuvants. Nat Biotechnol. 1999;17:1075–1081. doi: 10.1038/15058. [DOI] [PubMed] [Google Scholar]
- 17.Alving CR. Design and selection of vaccine adjuvants: animal models and human trials. Vaccine. 2002;20:S56–S64. doi: 10.1016/s0264-410x(02)00174-3. [DOI] [PubMed] [Google Scholar]
- 18.Bennewitz NL, Babensee JE. The effect of the physical form of poly(lactic-co-glycolic acid) carriers on the humoral immune response to co-delivered antigen. Biomaterials. 2005;26:2991–2999. doi: 10.1016/j.biomaterials.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Matzelle MM, Babensee JE. Humoral immune responses to model antigen co-delivered with biomaterials used in tissue engineering. Biomaterials. 2004;25:295–304. doi: 10.1016/s0142-9612(03)00531-3. [DOI] [PubMed] [Google Scholar]
- 20.Babensee JE, Paranjpe A. Differential levels of dendritic cell maturation on different biomaterials used in combination products. J Biomed Mater Res Part A. 2005;74A:503–510. doi: 10.1002/jbm.a.30429. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida M, Babensee JE. Differential effects of agarose and poly(lactic-co-glycolic acid) on dendritic cell maturation. J Biomed Mater Res Part A. 2006;79A:393–408. doi: 10.1002/jbm.a.30798. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida M, Babensee JE. Poly(lactic-co-glycolic acid) enhances maturation of human monocyte-derived dendritic cells. J Biomed Mater Res A. 2004;71A:45–54. doi: 10.1002/jbm.a.30131. [DOI] [PubMed] [Google Scholar]
- 23.Selkirk JV, Nottebaum LM, Ford IC, Santos M, Malany S, Foster AC, Lechner SM. A novel cell-based assay for G-protein-coupled receptor-mediated cyclic adenosine monophosphate response element binding protein phosphorylation. J Biomol Screening. 2006;11:351–358. doi: 10.1177/1087057106286608. [DOI] [PubMed] [Google Scholar]
- 24.Greenwalt DE, Szabo J, Manchel I. High throughput cell-based assay of hematopoietic progenitor differentiation. J Biomol Screening. 2001;6:383–392. doi: 10.1177/108705710100600604. [DOI] [PubMed] [Google Scholar]
- 25.Romani N, et al. Proliferating Dendritic Cell Progenitors in Human Blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jotwani R, Pulendran B, Agrawal S, Cutler CW. Human dendritic cells respond to Porphyromonas gingivalis LPS by promoting a Th2 effector response in vitro. Eur J Immunol. 2003;33:2980–2986. doi: 10.1002/eji.200324392. [DOI] [PubMed] [Google Scholar]
- 27.Lundholt BK, Scudder KM, Pagliaro L. A simple technique for reducing edge effect in cell-based assays. J Biomol Screening. 2003;8:566–570. doi: 10.1177/1087057103256465. [DOI] [PubMed] [Google Scholar]
- 28.Shankar SP, Babensee JE. Comparative Characterization of Cultures Of Primary Human Macropages Or Dendritic Cells For Biomaterials Studies Journal of Biomedical Materials Research. doi: 10.1002/jbm.a.32406. (Accepted). [DOI] [PubMed] [Google Scholar]
- 29.Kaser A, et al. B lymphocyte-derived IL-16 attracts dendritic cells and Th cells. J Immunol. 2000;165:2474–2480. doi: 10.4049/jimmunol.165.5.2474. [DOI] [PubMed] [Google Scholar]
- 30.Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Hermine O, Tough DF, Kaveri SV. Modulation of dendritic cell maturation and function by B lymphocytes. J Immunol. 2005;175:15–20. doi: 10.4049/jimmunol.175.1.15. [DOI] [PubMed] [Google Scholar]
- 31.Bayry J, et al. Natural antibodies sustain differentiation and maturation of human dendritic cells. Proc Natl Acad Sci U S A. 2004;101:14210–14215. doi: 10.1073/pnas.0402183101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folzenlogen D, Hofer MF, Leung DY, Freed JH, Newell MK. Analysis of CD80 and CD86 expression on peripheral blood B lymphocytes reveals increased expression of CD86 in lupus patients. Clin Immunol and Immunopathol. 1997;83:199–204. doi: 10.1006/clin.1997.4353. [DOI] [PubMed] [Google Scholar]
- 33.Cao XC, Sugita M, van der Wel N, Lai J, Rogers RA, Peters PJ, Brenner MB. CD1 molecules efficiently present antigen in immature dendritic cells and traffic independently of MHC class II during dendritic cell maturation. J Immunol. 2002;169:4770–4777. doi: 10.4049/jimmunol.169.9.4770. [DOI] [PubMed] [Google Scholar]
- 34.Anderson DG, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat Biotechnol. 2004;22:863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 35.Anderson DG, Putnam D, Lavik EB, Mahmood TA, Langer R. Biomaterial microarrays: rapid, microscale screening of polymer-cell interaction. Biomaterials. 2005;26:4892–4897. doi: 10.1016/j.biomaterials.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 36.Jefford M, et al. Functional comparison of DCs generated in vivo with Flt3 ligand or in vitro from blood monocytes: differential regulation of function by specific classes of physiologic stimuli. Blood. 2003;102:1753–1763. doi: 10.1182/blood-2002-12-3854. [DOI] [PubMed] [Google Scholar]
- 37.Sozzani S, et al. Human monocyte-derived and CD34(+) cell-derived dendritic cells express functional receptors for platelet activating factor. FEBS Lett. 1997;418:98–100. doi: 10.1016/s0014-5793(97)01358-6. [DOI] [PubMed] [Google Scholar]
- 38.Jansen JH, Wientjens G, Fibbe WE, Willemze R, Kluinnelemans HC. Inhibition of human macrophage colony formation by interleukin-4. J Exp Med. 1989;170:577–582. doi: 10.1084/jem.170.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santambrogio L, Sato AK, Carven GJ, Belyanskaya SL, Strominger JL, Stern LJ. Extracellular antigen processing and presentation by immature dendritic cells. Proc Natl Acad Sci U S A. 1999;96:15056–15061. doi: 10.1073/pnas.96.26.15056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 41.Jeannin P, Delneste Y, LecoanetHenchoz S, Gauchat JF, Ellis J, Bonnefoy JY. CD86 (B7-2) on human B cells - A functional role in proliferation and selective differentiation into IgE- and IgG4-producing cells. J Biol Chem. 1997;272:15613–15619. doi: 10.1074/jbc.272.25.15613. [DOI] [PubMed] [Google Scholar]
- 42.Beckman EM, et al. CD1c restricts responses of mycobacteria-specific T cells - Evidence for antigen presentation by a second member of the human CD1 family. J Immunol. 1996;157:2795–2803. [PubMed] [Google Scholar]
- 43.Briken V, Jackman RM, Watts GFM, Rogers RA, Porcelli SA. Human CD1b and CD1c isoforms survey different intracellular compartments for the presentation of microbial lipid antigens. J Exp Med. 2000;192:281–287. doi: 10.1084/jem.192.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Im JS, et al. Direct measurement of antigen binding properties of CD1 proteins using fluorescent lipid probes. J Biol Chem. 2004;279:299–310. doi: 10.1074/jbc.M308803200. [DOI] [PubMed] [Google Scholar]
- 45.Brigl M, Brenner MB. CD1: Antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 46.Geijtenbeek TBH, Torensma R, van Vliet SJ, van Duijnhoven GCF, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 47.Geijtenbeek TBH, van Vliet SJ, Engering A, t Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu Rev Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- 48.Woltman AM, Schlagwein N, van der Kooij SW, van Kooten C. The novel cyclophilin-binding drug sanglifehrin A specifically affects antigen uptake receptor expression and endocytic capacity of human dendritic cells. J Immunol. 2004;172:6482–6489. doi: 10.4049/jimmunol.172.10.6482. [DOI] [PubMed] [Google Scholar]
- 49.Gordon EM, Gallop MA, Patel DV. Strategy and tactics in combinatorial organic synthesis. Applications to drug discovery. Acc Chem Res. 1996;29:144–154. [Google Scholar]
- 50.Meredith JC, Sormana JL, Keselowsky BG, Garcia AJ, Tona A, Karim A, Amis EJ. Combinatorial characterization of cell interactions with polymer surfaces. J Biomed Mater Res Part A. 2003;66A:483–490. doi: 10.1002/jbm.a.10004. [DOI] [PubMed] [Google Scholar]
- 51.Zapata P, Su J, Garcia AJ, Meredith JC. Quantitative high-throughput screening of osteoblast attachment, spreading, and proliferation on demixed polymer blend micropatterns. Biomacromolecules. 2007;8:1907–1917. doi: 10.1021/bm061134t. [DOI] [PubMed] [Google Scholar]
- 52.Mei Y, et al. Gradient substrate assembly for quantifying cellular response to biomaterials. J Biomed Mater Res Part A. 2006;79A:974–988. doi: 10.1002/jbm.a.30883. [DOI] [PubMed] [Google Scholar]
- 53.Brocchini S, James K, Tangpasuthadol V, Kohn J. Structure-property correlations in a combinatorial library of degradable biomaterials. J Biomed Mater Res. 1998;42:66–75. doi: 10.1002/(sici)1097-4636(199810)42:1<66::aid-jbm9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 54.Kohn J. New approaches to biomaterials design. Nat Mater. 2004;3:745–747. doi: 10.1038/nmat1249. [DOI] [PubMed] [Google Scholar]