INTRODUCTION
HIV research has broadened to include prevention strategies, latent virus eradication approaches, co-infection treatment, new drug development and novel formulations of approved drugs. This broadened agenda has transitioned the global clinical pharmacology effort and underscores the need for a coordinated quality assurance. Current status of a comprehensive program developed by the University at Buffalo HIV Clinical Pharmacology Quality Assurance and Quality Control program is described in this report.
BACKGROUND
The global HIV epidemic continues to grow leading to a broadened research agenda, including clinical pharmacology, underscoring the need for a coordinated quality assurance approach [1–4]. Data are often obtained from diverse clinical sites and laboratories and integrated into a dataset that addresses research objectives and regional HIV treatment issues. Quality assurance approaches should facilitate pharmacology-specific training to ensure that the conduct of multicenter protocols includes uniform study conduct, data collection, sampling collection, processing and storage across diverse university-based clinical trial unit (CTU) sites. Quality assurance of clinical pharmacology laboratories (CPL) that analyze samples is essential. CPL assays should yield accurate and reproducible results prior to and during bioanalytical testing and compliant with regulations of clinical trials.
The CPQA was formed at a time when existing CPLs were providing expertise to the networks and new laboratories were added for emerging research areas. Annual site assessments were prioritized according to the type of CPL research, the emphasis on implementation of international CPLs, and the CTU participation in pharmacology research. Site assessments focused on areas of operation, human resources, facilities and resource management, policies and standard procedures, pre-, during- and post-analysis operations, and quality systems. The CPQA was initiated with one international laboratory in Thailand with additional CPLs added in South Africa and Zimbabwe. Preliminary program interactions have occurred in Nigeria, Uganda, Botswana, Kenya, Tanzania and India with an emphasis on “pre-developmental” laboratory status and a plan for further implementation.
PROGRAM DEVELOPMENT
A comprehensive program was designed that integrated quality initiatives for CTUs and CPLs supporting NIH HIV Research Networks. The quality initiatives are delivered by multiple programs and include peer review for bioanalytical methods, proficiency testing, pharmacology-specific training for CPL and CTU staff, and on-site laboratory assessments. An international assessment component was included for developmental laboratories. These program activities are monitored to provide insight for remediation and quality improvements within and across HIV Research Networks. All programs are governed by CPQA policies and standard procedures.
Located at the University at Buffalo (UB), the CPQA utilizes subcontractors for data management and proficiency testing (PT) sample production. An external Advisory Board consists of HIV-specific NIAID representatives, CPL representatives and non-CPL experts. The CPQA is interactively coupled to the NIH Research Networks through Cross-Network Laboratory Groups (CNLGs). The scientific CNLG is comprised of CPL directors and is charged with review of research priorities and advances that impact CPQA activities. The second CNLG includes CPL directors, supervisors and senior technologists. This group discusses priorities for standardized quality CPL operations, ongoing CPQA activities, and changes in CPQA policies and procedures.
The quality and acceptability of the drug assay methods used by the CPLs is assured by a blinded peer review program. Online submission collects information about the method for the purpose of network or protocol reference. Guidelines for acceptance utilize the FDA bioanalysis guidance. With the expansion of research into areas that include latent viral reservoirs, distributional pharmacokinetics and compartment penetration, and prevention research, non-plasma biomatrix assays are now an important component of NIH clinical pharmacology research [5]. The CPQA has refined a specific guidance for tissue and biofluid sample assays used for clinical trial specimens often collected in prevention and eradication studies.
Proficiency testing (PT) is accomplished on a bi-annual schedule with analytes including antiretrovirals (NRTIs, NNRTIs, PIs, CCR5 antagonists and integrase inhibitors). Results are compiled in a laboratory data management system and stored at the data center. Reports are developed for each PT round and accessed through the CPQA website. Suboptimal performance by a CPL leads to submission of a site-specific remediation plan outlining deficiencies and the recommended remediation actions. The CPL is “at risk” and performance is monitored to assure the return to adequate performance. CTU and CPL training facilitate the development and implementation of quality pharmacology research. An on-line tutorial was designed and made available at the CPQA website. A certificate is issued following completion of the tutorial, and records are maintained in a database for reporting. The CPQA also leads interactive sessions at NIAID HIV Research Network meetings. Individual CPLs may request general CPL training at UB in bioanalytical method validation, application and remediation, or data management. The assessment program developed by CPQA investigates the quality of CPL development, implementation or operations by investigating the CPL human resources, facilities, equipment and other areas) on-site at the CPL.
IMPLEMENTATION
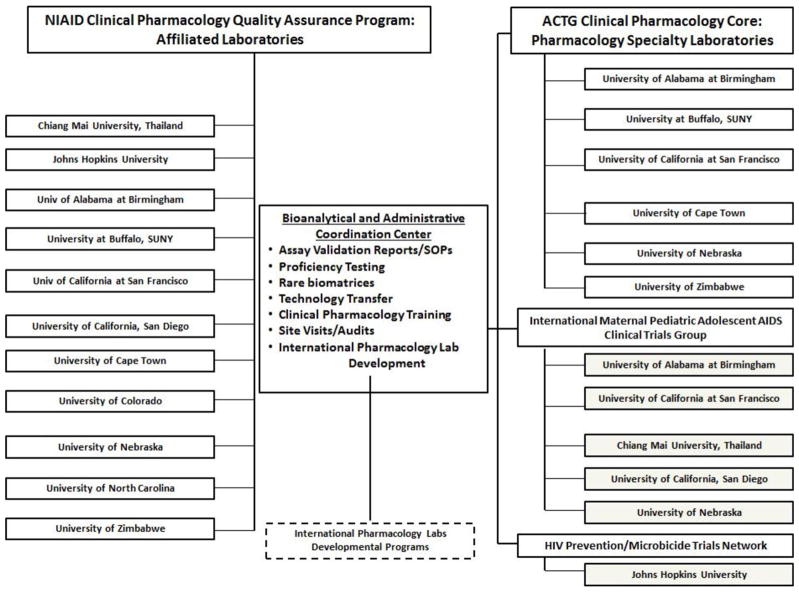
Figure 1 summarizes the research programs, CPLs and NIH groups within the CPQA. Conference calls, meetings and web-based informatics tools facilitate activities and routine monitoring and evaluation.
Figure 1.
NIAID Clinical Pharmacology Quality Assurance Program (CPQA) Bioanalytical and Administrative Center and Participating Clinical Pharmacology Laboratories.
The bioanalytical method review program has performed 123 reviews from 12 laboratories with 44 approved methods from 60 submissions. Most have been plasma methods and eight are for tissue or biofluids. Non-CPLs have also submitted at the request of protocol teams. Details for samples, analysis and performance of all CPQA-approved methods are collated in a searchable utility for network leadership, protocol teams, committees and supporting staff.
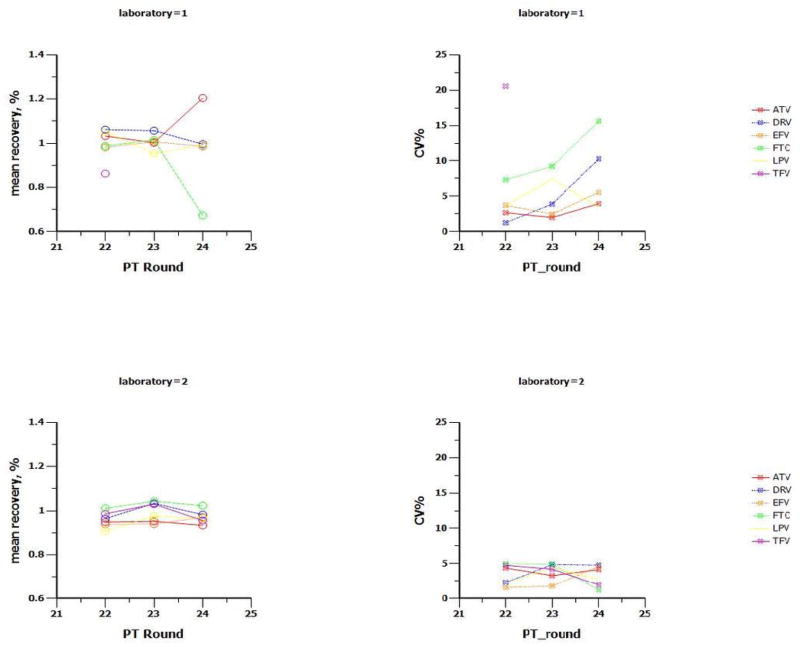
The PT program has emerged as an international initiative that ensures high quality clinical pharmacology research that includes the accurate analysis of ARVs. The CPQA has developed an informatics support structure for: PT analyte selection by CPL, data management and statistical analysis. Nine PT rounds have been conducted including 21 ARVs at multiple concentrations. Eleven CPLs have maintained CLIA-proficiency for the ARVs tested. Figure 2 provides representative PT results for two CPLs across three rounds.
Figure 2.
Proficiency testing performance for two laboratories over first three rounds for most commonly tested antiretrovirals (ARV)
Top Row: laboratory 1 recovery (left) and variability (CV%) of recovery for five PT samples of various concentrations per round per ARV(right)
Bottom Row: laboratory 2 recovery (left) and variability (CV%) of recovery for five PT samples of various concentrations per round per ARV (right)
Recovery = reported concentration divided by targeted concentration; CV% = standard deviation of recovery values divided by mean recovery
ARV: ATV= atazanavir, DRV= darunavir, EFV = efavirenz, FTC = emtricitabine, LPV = lopinavir, TFV = tenofovir
The CPQA has hosted multiple domestic CPL staff for short duration training and a large number of international students and staff. The CPQA tutorial has been well received by investigators and research staff, and is an important mechanism for staff to gain conceptual understanding and practical application of clinical pharmacology research. Nearly 1500 clinical tutorial certifications have been issued to domestic and international CTU staff.
The CPQA has assessed domestic and international CPLs for in-study conduct, general operations, implementation and development. Each assessment provides recommendations for quality improvements, quality-based planning, and minor adjustments to laboratory policies and operations. CPLs submit plans to meet the recommendations and self-monitor the effectiveness of the plan. Each assessment requires interactive CPQA-CPL efforts leading to a final report. For developmental planning, the CPQA provides longitudinal mentoring and monitors progress until implementation plans are finalized.
PROGRESS
The UB CPQA program has implemented a comprehensive quality assurance program to foster the use of best practices in the field of HIV clinical pharmacology research. The CPQA is a multifaceted program that includes training CTU staff responsible for collecting, processing and shipping the samples that are shipped CPLs and assayed. NIAID-designated CPLs must document active regulatory compliance with CPQA method validation and application guidelines, and have demonstrated PT outcomes to be designated as an approved laboratory for completing protocol assays. The rigor that has been traditionally associated with industry-supported, bioanalytical laboratories has been adopted by CPQA for application to a network of university-based CPLs. The CPQA is transitioning to include bioanalytical method approvals and PT for tuberculosis and viral hepatitis. In addition, the CPQA will evolve based on the FDA draft revised guidance for bioanalysis that is being finalized.
The HIV epidemic provides a challenging research environment in that many patients in protocols are located in resource limited countries (RLCs) in an environment that is developing the infrastructure to provide care and conduct research. The CPQA and its affiliated CTUs and CPLs provide an excellent mechanism to bridge the experience that was gained in the HIV/AIDS epidemic since the mid-1980s The current capacity building efforts, through initiatives such as the NIH Fogarty International Center, require that the clinical pharmacology community contribute to technology transfer and extensive mentoring. This assures that new generations of investigators in RLCs gain the needed guidance and training in quality systems to conduct research at their sites is of the highest quality and guide individual government decisions regarding bioequivalence, regional ART pharmacology, drug interactions and pharmacovigilance efforts.
Acknowledgments
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract number HHSN272200800019C.
The authors acknowledge the contributions of the faculty and staff at the Translational Pharmacology Research Core, University at Buffalo, New York State Center of Excellence in Bioinformatics and Life Sciences and the University at Buffalo, School of Pharmacy and Pharmaceutical Sciences. The authors acknowledge the contributions of the HIV AIDS Network Coordination (HANC) staff for their assistance with cross-network call coordination.
Footnotes
CONFLICT OF INTEREST/DISCLOSURE
The authors have no disclosures or conflicts of interest.
References
- 1.Morse GD, Maartens G, Maponga CC, Ma Q. Global HIV/AIDS Clinical and Translational Pharmacology. AIDS Res Treat. 2012;2012:973627. doi: 10.1155/2012/973627. Epub 2012 Jul 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MS, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson PL, Kiser JJ, Gardner EM, Rower JE, Meditz A, Grant RM. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J Antimicrob Chemother. 2011 Feb;66(2):240–250. doi: 10.1093/jac/dkq447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendrix CW, Cao YJ, Fuchs EJ. Topical microbicides to prevent HIV: clinical drug development challenges. Annu Rev Pharmacol Toxicol. 2009;49:349–75. doi: 10.1146/annurev.pharmtox.48.113006.094906. [DOI] [PubMed] [Google Scholar]
- 5.DiFrancesco R, Maduke G, Patel R, Taylor CR, Morse GD. A Review of Antiretroviral Bioanalysis Methods of Tissues and Body Biofluids. Bioanalysis. doi: 10.4155/bio.12.319. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]