Abstract
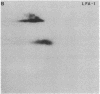
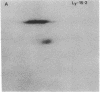
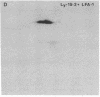
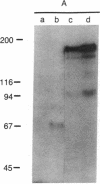
Serological and biochemical studies using monoclonal antibodies have demonstrated that the Ly-15 cell membrane alloantigens are polymorphic sites on the lymphocyte function-associated antigen-1 (LFA-1) molecule. Ly-15.2 and LFA-1 show identical tissue distributions, being present on all thymocytes, lymphocytes, and neutrophils, and flow cytofluorometric analysis indicated identical cell surface expression of these molecules. Identity of Ly-15.2 and LFA-1 was confirmed by immunochemical analysis. The Ly-15.2 and LFA-1 molecules have an identical heterodimeric structure of Mr 180,000 alpha chain and Mr 94,000 beta chain, which coelectrophorese on two-dimensional NaDodSO4/PAGE. Furthermore, anti-Ly-15.2 and anti-LFA-1 antibodies coprecipitate the same molecule from thymocyte lysates, and peptide mapping studies show that the Ly-15.2 and LFA-1 alpha chains are identical, as are the beta chains.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beller D. I., Springer T. A., Schreiber R. D. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982 Oct 1;156(4):1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorney M., Shen F. W., Michaelson J., Boyse E. A. Monoclonal antibody to an alloantigenic determinant on beta2-microglobulin (beta 2M) of the mouse. Immunogenetics. 1982;16(1):91–93. doi: 10.1007/BF00364446. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Davignon D., Martz E., Reynolds T., Kürzinger K., Springer T. A. Monoclonal antibody to a novel lymphocyte function-associated antigen (LFA-1): mechanism of blockade of T lymphocyte-mediated killing and effects on other T and B lymphocyte functions. J Immunol. 1981 Aug;127(2):590–595. [PubMed] [Google Scholar]
- Dialynas D. P., Loken M. R., Glasebrook A. L., Fitch F. W. Lyt-2-/Lyt 3- variants of a cloned cytolytic T cell line lack an antigen receptor functional in cytolysis. J Exp Med. 1981 Mar 1;153(3):595–604. doi: 10.1084/jem.153.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Shykind B., Seidman J. G., Ozato K. Exon shuffling: mapping polymorphic determinants on hybrid mouse transplantation antigens. Nature. 1982 Dec 23;300(5894):755–757. doi: 10.1038/300755a0. [DOI] [PubMed] [Google Scholar]
- Gutman G. A. Genetic and structural studies on rat kappa chain allotypes. Transplant Proc. 1981 Jun;13(2):1483–1488. [PubMed] [Google Scholar]
- Hogarth P. M., Edwards J., McKenzie I. F., Goding J. W., Liew F. Y. Monoclonal antibodies to the murine Ly-2.1 cell surface antigen. Immunology. 1982 May;46(1):135–144. [PMC free article] [PubMed] [Google Scholar]
- Hogarth P. M., McKenzie I. F., Lanier L., Bailey D. W., Taylor B. A. Location of Ly-7 on mouse chromosome 12. Immunogenetics. 1984;19(6):539–543. doi: 10.1007/BF00403445. [DOI] [PubMed] [Google Scholar]
- Hogarth P. M., Walker I. D., Rigby A. J., McKenzie I. F. The H-2dm1 mutation and Qa antigens. Immunogenetics. 1983;18(6):617–624. doi: 10.1007/BF00345969. [DOI] [PubMed] [Google Scholar]
- Kappler J., Kubo R., Haskins K., White J., Marrack P. The mouse T cell receptor: comparison of MHC-restricted receptors on two T cell hybridomas. Cell. 1983 Oct;34(3):727–737. doi: 10.1016/0092-8674(83)90529-9. [DOI] [PubMed] [Google Scholar]
- Kürzinger K., Ho M. K., Springer T. A. Structural homology of a macrophage differentiation antigen and an antigen involved in T-cell-mediated killing. Nature. 1982 Apr 15;296(5858):668–670. doi: 10.1038/296668a0. [DOI] [PubMed] [Google Scholar]
- Kürzinger K., Reynolds T., Germain R. N., Davignon D., Martz E., Springer T. A. A novel lymphocyte function-associated antigen (LFA-1): cellular distribution, quantitative expression, and structure. J Immunol. 1981 Aug;127(2):596–602. [PubMed] [Google Scholar]
- Lafuse W. P., McCormick J. F., Corser P. S., David C. S. Hybrid (combinatorial) Ia specificities: gene complementations to generate Ia.22. Immunogenetics. 1981;13(1-2):115–125. doi: 10.1007/BF00524609. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Seaman W. E., Tsu T. T., Herzenberg L. A. Lyt-2 and lyt-3 antigens are on two different polypeptide subunits linked by disulfide bonds. Relationship of subunits to T cell cytolytic activity. J Exp Med. 1981 Jun 1;153(6):1503–1516. doi: 10.1084/jem.153.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Glasebrook A. L., Bron C., Kelso A., Cerottini J. C. Clonal heterogeneity in the functional requirement for Lyt-2/3 molecules on cytolytic T lymphocytes (CTL): possible implications for the affinity of CTL antigen receptors. Immunol Rev. 1982;68:89–115. doi: 10.1111/j.1600-065x.1982.tb01061.x. [DOI] [PubMed] [Google Scholar]
- Parham P., Barnstable C. J., Bodmer W. F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979 Jul;123(1):342–349. [PubMed] [Google Scholar]
- Parish C. R., McKenzie I. F. A sensitive rosetting method for detecting subpopulations of lymphocytes which react with alloantisera. J Immunol Methods. 1978;20:173–183. doi: 10.1016/0022-1759(78)90254-5. [DOI] [PubMed] [Google Scholar]
- Potter T. A., Hogarth P. M., McKenzie I. F. Ly-15: a new murine lymphocyte alloantigenic locus. Transplantation. 1981 May;31(5):339–342. [PubMed] [Google Scholar]
- Reinherz E. L., Meuer S. C., Schlossman S. F. The human T cell receptor: analysis with cytotoxic T cell clones. Immunol Rev. 1983;74:83–112. doi: 10.1111/j.1600-065x.1983.tb01085.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A., Davignon D., Ho M. K., Kürzinger K., Martz E., Sanchez-Madrid F. LFA-1 and Lyt-2,3, molecules associated with T lymphocyte-mediated killing; and Mac-1, an LFA-1 homologue associated with complement receptor function. Immunol Rev. 1982;68:171–195. doi: 10.1111/j.1600-065x.1982.tb01064.x. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Sutton V. R., Hogarth P. M., McKenzie I. F. Description of a new Qa antigenic specificity, "Qa-m9," whose expression is under complex genetic control. J Immunol. 1983 Sep;131(3):1363–1367. [PubMed] [Google Scholar]
- Swain S. L., Dialynas D. P., Fitch F. W., English M. Monoclonal antibody to L3T4 blocks the function of T cells specific for class 2 major histocompatibility complex antigens. J Immunol. 1984 Mar;132(3):1118–1123. [PubMed] [Google Scholar]
- Trowbridge I. S., Lesley J., Schulte R., Hyman R., Trotter J. Biochemical characterization and cellular distribution of a polymorphic, murine cell-surface glycoprotein expressed on lymphoid tissues. Immunogenetics. 1982 Mar;15(3):299–312. doi: 10.1007/BF00364338. [DOI] [PubMed] [Google Scholar]
- Ware C. F., Sanchez-Madrid F., Krensky A. M., Burakoff S. J., Strominger J. L., Springer T. A. Human lymphocyte function associated antigen-1 (LFA-1): identification of multiple antigenic epitopes and their relationship to CTL-mediated cytotoxicity. J Immunol. 1983 Sep;131(3):1182–1188. [PubMed] [Google Scholar]