Abstract
Notch receptors regulate cell differentiation and function. Notch1 and Notch2 inactivation in osteoblasts and osteocytes increases cancellous bone volume, but the function of Notch signaling in osteoblast precursors is unknown. To inactivate Notch signaling in immature osteoblastic cells, mice homozygous for conditional Notch1 and Notch2 alleles (Notch1loxP/loxP;Notch2loxP/loxP) were crossed with mice where the osterix (Osx) promoter, regulated by a Tet-Off cassette, governs Cre expression (Osx-Cre). Notch1loxP/loxP;Notch2loxP/loxP control and Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ experimental littermate cohorts were obtained. To prevent the effects of embryonic Osx-Cre expression, doxycycline was administered to pregnant dams, but not to newborns. Recombination of conditional alleles was documented in calvarial DNA extracts from 1 month old mice. Notch1 and Notch2 inactivation did not affect femoral microarchitecture at 1 month of age. Cancellous bone volume was higher and structure model index was lower in 3 and 6 month old Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ mice than in control littermates and the effect was more pronounced in female mice. One month old Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ male mice transiently exhibited an increase in osteoblast number and a modest suppression in bone resorption. Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ female mice displayed a tendency toward increased bone formation at 3 months of age, although bone remodeling was suppressed in 6 month old Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ female mice. Notch1 and Notch2 inactivation increased porosity and reduced thickness of cortical bone. These effects were modest and more evident in 3 and 6 month old female than in male mice of the same age. In conclusion, Notch1 and Notch2 expression in osteoblast precursors regulates cancellous bone volume and microarchitecture.
Keywords: Notch1, Notch2, osteoblasts, cortical bone
1. INTRODUCTION
The microarchitecture of cancellous and cortical bone is determined by bone remodeling, the process of tissue renewal that is carried out by the concerted actions of osteoblasts, which form bone, and osteoclasts, the bone resorbing cells [1, 2]. Osteoblasts derive from mesenchymal stem cells residing in the bone marrow and the commitment of precursor cells to the osteoblastic lineage is governed by multiple factors, including molecules that regulate the activity of bone morphogenetic protein, Wnt and Notch receptors [3-6]. Expression of osterix (Osx) characterizes a population of osteoblast precursors that progresses to the mature osteoblastic phenotype and has the potential of further differentiation into endosteal lining cells and osteocytes [3, 7, 8]. Osteoclasts are multinucleated cells generated by the fusion of hematopoietic mononuclear precursors; interactions of the receptor of nuclear factor-κb ligand (Rankl) with the Rank receptor induce osteoclastogenesis and osteoclast activity [9].
Notch signaling regulates cell lineage commitment and contributes to the replacement of aging cells in multiple tissues, including bone [10-12]. Interactions of Notch receptors with cognate ligands result in the proteolytic cleavage of the Notch intracellular domain, which translocates to the nucleus to activate transcription [13, 14]. Notch target genes include those encoding for the transcriptional repressors hairy enhancer of split (Hes) and Hes-related with YRPW motif (Hey) [15].
Notch1 and Notch2 are expressed by skeletal cells and appear to mediate the effects of Notch signaling on bone microarchitecture and homeostasis, although skeletal cells also express low levels of Notch3 transcripts [16-22]. Work from our laboratory and from others has established that the effects of Notch signaling in osteoblastic cells are in part determined by the degree of cellular maturity. Activation of Notch in the early phases of osteoblastic differentiation and in osteoblasts reduces cancellous bone volume and causes deposition of disorganized woven bone [19, 23-25]. Inactivation of Notch signaling in osteoblasts increases trabecular bone volume due to enhanced osteoblastogenesis followed by increased osteoclast number and bone resorption [19]. Accordingly, induction of Notch signaling in osteoblasts and osteocytes increases bone mass by suppressing bone resorption [22, 25]. Studies of Notch misexpression in osteoblasts and osteocytes revealed that Notch regulates bone resorption by inducing expression of osteoprotegerin, which is a soluble Rankl decoy receptor [18, 21, 22, 26]. Recently, we demonstrated that Notch induction in cells of the osteoblastic lineage and in osteocytes results in cortical bone that is either absent, porous or assumes the appearance of trabecular bone, revealing a novel role of Notch signaling as a determinant of cortical bone structure [25].
Dual conditional inactivation of Notch1 and Notch2 in the limb bud causes a lengthening of the growth plate and an increase in cancellous bone volume [21]. Similarly, conditional inactivation of Hes1 in the developing limb increases trabecular bone volume [27]. However, interpretation of these findings is confounded by the consequences of Notch signaling inhibition during the early phases of skeletal development [28]. Inactivation of the Notch target Hey2 in osteoblast precursors phenocopies the effects of Notch inactivation in the developing limb, although the effect is mild, suggesting genetic compensation from other Hey genes [29].
To determine the consequences of Notch signaling inhibition in osteoblast precursors, Notch1 and Notch2 were inactivated in post-natal life in cells expressing Osx. To this end, the skeletal phenotype of male and female mice where Notch1 and Notch2 were inactivated in cells expressing Cre under the control of the Osx promoter was investigated by microcomputed tomography (μCT) and histomorphometric analysis of the femur.
2. EXPERIMENTAL PROCEDURES
2.1 Conditional Inactivation of Notch1 and Notch2 in Osteoblast Precursors
To express Cre recombinase in osteoblast precursors, we obtained C57BL/6 mice where the Cre coding sequence is cloned downstream of an Osterix (Osx) promoter (Osx-Cre) (Jackson Laboratory) [30]. In these mice, a Tet-Off cassette suppresses the activity of the Osx promoter in the presence of tetracycline [31]. For the conditional inactivation of Notch1, mice where the 3.5 kilobase upstream of the putative transcriptional start site and the first exon of the Notch1 locus are flanked by loxP sequences (Notch1loxP), were obtained from F. Radtke (Ludwig Institute for Cancer Research, University of Lausanne, Switzerland). In these mice, Cre recombination brings about the excision of DNA sequences that encode for the Notch1 signal peptide, precluding expression of a functional Notch receptor [32]. For the conditional inactivation of Notch2, mice where exon 3 of Notch2 is flanked by loxP sequences (Notch2loxP) were provided by T. Gridley (Jackson Laboratory, Bar Harbor, ME). In these mice, removal of the loxP –flanked DNA sequences by Cre recombination leads to a frame shift mutation and to the expression of a truncated and inactive Notch2 protein [33].
Notch1 and Notch2 conditional mice, both provided in a 129SvJ/C57BL/6 background, were crossed to generate dual Notch1loxP/loxP and Notch2loxP/loxP mice (Notch1loxP/loxP;Notch2loxP/loxP). Osx-Cre transgenics were crossed with Notch1loxP/loxP;Notch2loxP/loxP mice to create Osx-Cre+/−;Notch1loxP/wt;Notch2loxP/wt mice, which were bred with Notch1loxP/loxP;Notch2loxP/loxP to obtain Osx-Cre+/−;Notch1loxP/loxP;Notch2loxP/loxP mice. The latter were crossed with Notch1loxP/loxP;Notch2loxP/loxP to generate an experimental cohort, in which Cre excises the loxP-flanked sequences from the Notch1loxP and Notch2loxP alleles (Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ), and littermate controls (Notch1loxP/loxP;Notch2loxP/loxP), not carrying the Osx-Cre transgene. In parallel studies, we compared Osx-Cre+/− male mice to wild type littermate controls of the same sex. To suppress Osx-Cre expression during embryonic development, pregnant dams were administered chow containing approximately 0.55 g/kg doxycycline (Harlan Laboratories, Indianapolis, IN) from the time of conception to delivery, resulting in an estimated daily doxycycline dose of 2 to 3 mg. All animal experiments were approved by the Animal Care and Use Committee of Saint Francis Hospital and Medical Center.
2.2 Genotyping, Recombination of Conditional Alleles, Body Weight and Femoral Length
The presence of the Osx-Cre transgene and of the Notch1loxP and Notch2loxP alleles was determined by polymerase chain reaction (PCR) in tail DNA extracts from adult mice, and primers specific for fatty acid-binding protein 1 (Fabp1) were used as positive controls in the PCR reactions (Table 1). Recombination of conditional alleles was assessed by PCR in DNA extracts from the parietal bone of 1 month old mice, using primers specific for the Notch1 and Notch2 deletion (Table 1). Body weight was determined at the time of sacrifice. Femurs were dissected from surrounding tissues and fixed in 70 % ethanol, and images obtained on a μCT 40 scanner (Scanco Medical AG, Bassersdorf, Switzerland) were used to measure femoral length.
Table 1.
Primers used for allele identification by PCR.
| Genotyping | |||
|---|---|---|---|
| Allele | Strand | Sequence 5′- to −3′ | Amplicon Size (base pairs) |
| Fabp1 | Forward | TGGACAGGACTGGACCTCTGCTTTCC | 200 |
| Reverse | TAGAGCTTTGCCACATCACAGGTCAT | ||
| Notch1loxP | Forward | CTGACTTAGTAGGGGGAAAAC |
Notch1wt = 300 Notch1loxP = 350 |
| Reverse | AGTGGTCCAGGGTGTGAGTGT | ||
| Notch2loxP | Forward | GCTCAGCTAGAGTGTTGTTCTTG |
Notch2wt = 400 Notch2loxP = 500 |
| Reverse | TTTGTGGCCGTAACTTTCTCATG | ||
| Osx-Cre | Forward | GCGGTCTGGCAGTAAAAACTATC | 100 Notch2loxP = 500 |
| Reverse | GTGAAACAGCATTGCTGTCACTT | ||
| Recombination | |||
| Notch1Δ | Forward | CTGACTTAGTAGGGGGAAAAC | 370 |
| Reverse | TAAAAAGAGACAGCTGCGGAG | ||
| Notch2Δ | Forward | GCTCAGCTAGAGTGTTGTTCTTG | 450 |
| Reverse | ATAACGCTAAACGTGCACTGGAG | ||
2.3 Microcomputed Tomography
Femurs were scanned in 70 % ethanol at an energy level of 55 kVp, an intensity of 145 μA, and an integration time of 200 ms on a μCT 40 scanner. Trabecular bone volume fraction and microarchitecture were evaluated starting approximately 1.0 mm proximal to the femoral condyles. A total of 160 consecutive slices at a thickness of 6 μm acquired at an isotropic voxel size of 216 μm3, were chosen for analysis. Contours were manually drawn every 10 slices a few voxels away from the endocortical boundary to define the region of interest for analysis, whereas the contours of the remaining slices were iterated automatically. Bone volume fraction, trabecular thickness, number and separation, connectivity density, and structure model index (SMI) were measured in the trabecular region using a Gaussian filter (σ = 0.8, support = 1) and a user defined threshold [34]. For the cortical region, a total of 100 slices at a thickness of 6 μm were measured at the femoral mid-diaphysis with an isotropic voxel size of 216 μm3. For determination of cortical microarchitecture, contours were iterated across all slices along the cortical shell and the bone marrow cavity was excluded. Cortical bone volume fraction, porosity and thickness, total area, bone area, periosteal and endocortical perimeters and density of material were determined using a Gaussian filter (σ = 0.8, support = 1) and a user defined threshold. Terminology and units used were those suggested by the Journal of Bone and Mineral Research [34].
2.4 Bone Histomorphometry
Cancellous bone at the distal femur was analyzed by static and dynamic histomorphometry after injection with calcein 20 mg/kg, and demeclocycline 50 mg/kg, at an interval of 2 days for 1 month old, and 7 days for 3 and 6 month old mice. Euthanasia was carried out by CO2 inhalation 2 days after administration of demeclocycline. Femurs were sectioned on a microtome at a thickness of 5 μm (Microm, Richards-Allan Scientific, Kalamazoo, MI) and stained with 0.1 % toluidine blue. Static parameters of bone formation and resorption were measured with an OsteoMeasure morphometry system (Osteometrics, Atlanta, GA) in a defined area between 360 μm and 2160 μm from the proximal end of the distal growth plate [35]. Mineralizing surface per bone surface and mineral apposition rate were measured on unstained sections under ultraviolet light, using a triple diamino-2-phenylindole fluorescein set long pass filter, and bone formation rate was calculated. Terminology and units used were those recommended in the 2012 update of the American Society for Bone and Mineral Research Histomorphometry Nomenclature Committee [36].
2.5 Statistical Analysis
Data are expressed as means ± SEM. Statistical differences were determined by Student’s t-test [37].
3. RESULTS
3.1 General Characteristics of Mice Harboring Notch1 and Notch2 Inactivation in Osteoblast Precursors
To inactivate Notch1 and Notch2 in osteoblast precursors, female Notch1loxP/loxP;Notch2loxP/loxP mice were bred with male Osx-Cre+/−;Notch1loxP/loxP;Notch2loxP/loxP mice, so that the progeny was evenly distributed between Notch1loxP/loxP;Notch2loxP/loxP control and Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ experimental cohorts. In previous studies, we established that Notch1loxP/loxP;Notch2loxP/loxP do not display a skeletal phenotype or changes in weight and general appearance when compared to wild-type C57BL/6 mice [22]. Expression of Osx-Cre during embryonic development is reported to cause a modest and transient thinning of cortical bone and reduced body weight at 8 weeks of age [30, 38-40]. To ensure that the Osx-Cre transgene did not cause a skeletal phenotype, pregnant dams were administered doxycycline to suppress expression of Cre, and the phenotype of 1 month old Osx-Cre+/− male mice and wild type littermates of the same sex was characterized. Body weight, femoral length and microarchitecture of cancellous bone were not different between Osx-Cre+/− male mice and controls. The Osx-Cre transgene caused a modest reduction in total area, but not in cortical thickness or cortical area (Table 2). These findings confirm that in the context of doxycycline exposure during embryonic development the Osx-Cre transgene does not cause an obvious skeletal phenotype [25].
Table 2.
Body weight, femoral length and μCT analysis of femoral microarchitecture of 1 month old Osx-Cre+/− male mice or wild type littermate controls of the same sex.
| Wild type | Osx-Cre+/− | |
|---|---|---|
| Body weight (g) | 16.3 ± 0.7 | 14.5 ± 0.7 |
| Femoral length (mm) | 12.3 ± 0.3 | 11.7 ± 0.2 |
| Distal femoral cancellous bone | Wild type | Osx-Cre+/− |
| Bone Volume/Total Volume (%) | 2.8 ± 0.3 | 3.4 ± 0.4 |
| Trabecular Separation (μm) | 290 ± 8 | 270 ± 12 |
| Trabecular Number (mm−1) | 3.48 ± 0.10 | 3.79 ± 0.17 |
| Trabecular Thickness (μm) | 23 ± 1 | 22 ± 1 |
| Connectivity Density (mm−3) | 29 ± 17 | 80 ± 22 |
| Structure Model Index (SMI) | 3.23 ± 0.10 | 3.10 ± 0.08 |
| Cortical bone at the femoral midshaft | Wild type | Osx-Cre+/− |
| Bone Volume/Total Volume (%) | 89.2 ± 0.6 | 89.2 ± 0.5 |
| Cortical Porosity (%) | 10.8 ± 0.6 | 10.8 ± 0.5 |
| Cortical Thickness (mm) | 98 ± 3 | 93 ± 3 |
| Total Area (mm2) | 1.61 ± 0.05 | 1.42 ± 0.04* |
| Bone Area (mm2) | 0.50 ± 0.02 | 0.43 ± 0.02 |
| Periosteal Perimeter (mm) | 4.50 ± 0.07 | 4.22 ± 0.06* |
| Endocortical Perimeter (mm) | 3.73 ± 0.06 | 3.52 ± 0.04* |
| Density of Material (mg HA/cm3) | 931 ± 9 | 918 ± 11 |
Values are means ± SEM; n = 5 - 10.
Significantly different between wild type and Osx-Cre+/−, p < 0.05.
To test for recombination of the Notch1 and Notch2 conditional alleles, DNA was extracted from parietal bones of 1 month old Notch1loxP/loxP;Notch2loxP/loxP and Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ male and female mice. PCR products for the Notch1 and Notch2 recombined alleles were observed in DNA from Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ mice and not from Notch1loxP/loxP;Notch2loxP/loxP mice (Figure 1A). Body weight increased as mice matured, but weight gain was less pronounced in Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ male mice, so that at 6 months of age experimental male mice were smaller than control male littermates. Femoral length increased with age but the effect was less pronounced in Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ female mice, so that femurs of 3 and 6 month old Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ female mice were shorter than femurs of female littermate controls (Figure 1B).
Figure 1. Notch1 and Notch2 inactivation in osteoblast precursors.
One to 6 month old male or female Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ mice (Notch1/2 Null, empty circles) or littermate Notch1loxP/loxP;Notch2loxP/loxP controls of the same sex (Control, full circles), were studied. In panel A, the presence of the Notch1Δ and Notch2Δ alleles was determined by PCR in DNA extracted from parietal bones of 1 month old mice. Control reactions conducted in DNA from Notch1loxP/loxP;Notch2loxP/loxP mice, or in water, were performed to confirm specificity of the primer pairs. Amplified DNA was separated by electrophoresis, and results from representative mice are shown. In panel B, body weight was determined at sacrifice (left) and length of dissected femurs was measured in radiographic images obtained by μCT (right). Values are means ± SEM, n = 5-10. * Significantly different between Notch1/2 Null and Control, p < 0.05.
3.2 Notch1 and Notch2 Inactivation in Osteoblast Precursors Increases Cancellous Bone Volume
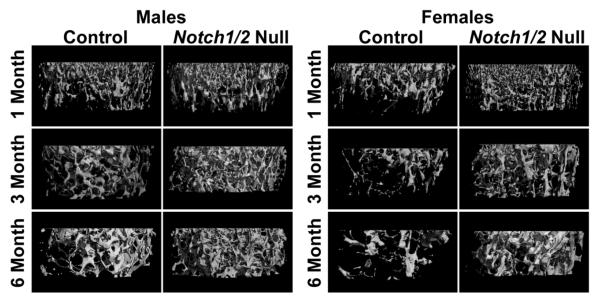
Inactivation of Notch1 and Notch2 in osteoblast precursors did not affect cancellous bone microarchitecture in male mice at 1 month of age but caused an increase in trabecular bone volume in 3 month old mice. This effect was associated with an increase in trabecular number and in connectivity density, although trabeculae were thinner. A less pronounced phenotype was observed in 6 month old Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ male mice (Table 3, Figure 2). One month old conditional Notch1 and Notch2 null male mice exhibited a higher number of osteoblasts and a greater osteoid surface coupled to a lower number of osteoclasts and decreased eroded surface than Notch1loxP/loxP;Notch2loxP/loxP littermates of the same sex. Despite a modest suppression in mineral apposition rate, bone formation was not affected by the inactivation of Notch1 and Notch2. Although these effects may explain the increased bone volume observed in older animals, they were transient and Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ male mice had no cellular phenotype at 3 and 6 months of age (Table 3).
Table 3.
μCT and histomorphometry of distal femoral cancellous bone. One, 3 and 6 month old Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ male mice (Notch1/2 Null) or littermate Notch1loxP/loxP;Notch2loxP/loxP controls of the same sex (Control).
| Male Mice | 1 Month | 3 Month | 6 Month | |||
|---|---|---|---|---|---|---|
| μCT | Control | Notch1/2 Null | Control | Notch1/2 Null | Control | Notch1/2 Null |
| Bone Volume/Total Volume (%) | 4.6 ± 0.7 | 4.4 ± 0.5 | 4.6 ± 0.8 | 6.7 ± 0.3* | 5.5 ± 0.8 | 7.0 ± 0.5 |
| Trabecular Separation (μm) | 236 ± 12 | 239 ± 9 | 262 ± 6 | 190 ± 2* | 266 ± 9 | 235 ± 13 |
| Trabecular Number (mm−1) | 4.31 ± 0.22 | 4.26 ± 0.17 | 3.85 ± 0.87 | 5.25 ± 0.54* | 3.75 ± 0.11 | 4.32 ± 0.21* |
| Trabecular Thickness (μm) | 23.9 ± 0.8 | 25.1 ± 1.4 | 32.4 ± 1.5 | 28.4 ± 1.0* | 33.3 ± 1.4 | 30.3 ± 2.9 |
| Connectivity Density (mm−3) | 137 ± 53 | 147 ± 26 | 86 ± 26 | 209 ± 18* | 90 ± 15 | 231 ± 30* |
| Structure Model Index (SMI) | 3.06 ± 0.10 | 3.08 ± 0.07 | 2.86 ± 0.13 | 2.64 ± 0.06 | 2.52 ± 0.15 | 2.17 ± 0.10+ |
| Histomorphometry | Control | Notch1/2 Null | Control | Notch1/2 Null | Control | Notch1/2 Null |
| Osteoblast Surface/Bone Surface (%) | 19.7 ± 2.1 | 30.9 ± 1.5* | 19.3 ± 1.9 | 23.7 ± 1.7 | 19.1 ± 1.9 | 17.8 ± 2.3 |
| Osteoblasts/Bone Perimeter (mm−1) | 16.7 ± 1.7 | 24.4 ± 0.9* | 16.6 ± 1.7 | 19.7 ± 1.3 | 18.1 ± 1.7 | 16.2 ± 1.8 |
| Osteoid Surface/Bone Surface (%) | 3.0 ± 0.5 | 5.1 ± 0.6* | 3.8 ± 0.7 | 3.3 ± 0.3 | 2.5 ± 0.6 | 2.6 ± 0.7 |
| Osteoclast Surface/Bone Surface (%) | 16.9 ± 1.0 | 13.9 ± 0.9* | 13.5 ± 0.8 | 15.8 ± 1.0 | 11.0 ± 1.1 | 11.0 ± 0.5 |
| Osteoclasts/Bone Perimeter (mm−1) | 8.9 ± 0.5 | 7.3 ± 0.5* | 5.8 ± 0.4 | 6.8 ± 0.5 | 5.6 ± 0.8 | 5.3 ± 0.3 |
| Eroded Surface/Bone Surface (%) | 21.5 ± 1.2 | 18.2 ± 0.8* | 13.6 ± 0.4 | 15.2 ± 0.9 | 12.0 ± 2.0 | 11.5 ± 0.8 |
| Mineral Apposition Rate (μm day−1) | 2.68 ± 0.10 | 2.24 ± 0.06* | 1.85 ± 0.16 | 1.58 ± 0.08 | 1.32 ± 0.16 | 1.52 ± 0.12 |
| Mineralizing Surface/Bone Surface (%) | 8.6 ± 1.9 | 10.3 ± 1.2 | 6.8 ± 1.3 | 6.2 ± 0.9 | 2.9 ± 0.7 | 4.9 ± 0.8 |
| Bone Formation Rate (μm3 μm−2day−1) | 0.23 ± 0.05 | 0.23 ± 0.03 | 0.13 ± 0.03 | 0.10 ± 0.02 | 0.04 ± 0.01 | 0.08 ± 0.01 |
Values are means ± SEM; n = 4 - 10.
Significantly different between Notch1/2 Null and Control, p < 0.05.
Figure 2. Notch1 and Notch2 inactivation in osteoblast precursors increases cancellous bone volume.
μCT images of proximal trabecular bone of femurs from representative 1 to 6 month old male or female Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ mice (Notch1/2 Null) or littermate Notch1loxP/loxP;Notch2loxP/loxP controls of the same sex (Control).
Trabecular microarchitecture was not different between 1 month old Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ female mice and Notch1loxP/loxP;Notch2loxP/loxP littermates of the same sex. At 3 and 6 months of age, trabecular bone volume, number and connectivity density were higher, although trabeculae were thinner in Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ female mice than in Notch1loxP/loxP;Notch2loxP/loxP female littermates (Table 4, Figure 2). SMI was lower in Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ female than in Notch1loxP/loxP;Notch2loxP/loxP female littermates at 3 and 6 months of age, indicating a higher ratio of plate-like over rod-like trabeculae (Table 4). Notch1 and Notch2 inactivation in female mice had no effect on osteoblast and osteoclast number or on bone formation and resorption at 1 month of age, although a tendency toward increased mineralizing surface was noticed at 3 months. At 6 months of age, the phenotype evolved and rates of bone formation and resorption were lower in Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ female mice than in control female littermates (Table 4).
Table 4.
μCT and histomorphometry of distal femoral cancellous bone. One, 3 and 6 month old Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ female mice (Notch1/2 Null) or littermate Notch1loxP/loxP;Notch2loxP/loxP controls of the same sex (Control).
| Female Mice | 1 Month | 3 Month | 6 Month | |||
|---|---|---|---|---|---|---|
| μCT | Control | Notch1/2 Null | Control | Notch1/2 Null | Control | Notch1/2 Null |
| Bone Volume/Total Volume (%) | 3.4 ± 0.3 | 3.2 ± 0.2 | 1.8 ± 0.2 | 4.5 ± 0.4* | 2.0 ± 0.3 | 5.5 ± 0.8* |
| Trabecular Separation (μm) | 289 ± 11 | 266 ± 10 | 379 ± 23 | 242 ± 15* | 411 ± 21 | 291 ± 14* |
| Trabecular Number (mm−1) | 3.52 ± 0.13 | 3.80 ± 0.14 | 2.70 ± 0.17 | 4.20 ± 0.21* | 2.46 ± 0.12 | 3.48 ± 0.17* |
| Trabecular Thickness (μm) | 23.8 ± 0.7 | 22.2 ± 0.3 | 33.8 ± 0.7 | 29.0 ± 1.5* | 36.7 ± 0.2 | 29.0 ± 1.0* |
| Connectivity Density (mm−3) | 66 ± 18 | 61 ± 6 | 21 ± 3 | 94 ± 13* | 20 ± 8 | 175 ± 39* |
| Structure Model Index (SMI) | 3.00 ± 0.07 | 3.19 ± 0.13* | 3.56 ± 0.05 | 2.79 ± 0.06* | 3.30 ± 0.16 | 2.06 ± 0.17* |
| Histomorphometry | Control | Notch1/2 Null | Control | Notch1/2 Null | Control | Notch1/2 Null |
| Osteoblast Surface/Bone Surface (%) | 27.3 ± 1.7 | 30.9 ± 1.8 | 33.7 ± 3.6 | 29.2 ± 3.6 | 22.5 ± 1.4 | 18.3 ± 2.4 |
| Osteoblasts/Bone Perimeter (mm−1) | 22.4 ± 1.3 | 25.8 ± 1.8 | 25.4 ± 2.6 | 23.6 ± 3.3 | 25.7 ± 1.5 | 20.7 ± 2.6 |
| Osteoid Surface/Bone Surface (%) | 5.7 ± 0.6 | 4.2 ± 0.7 | 4.5 ± 2.3 | 2.5 ± 0.7 | 5.0 ± 1.4 | 4.6 ± 1.0 |
| Osteoclast Surface/Bone Surface (%) | 16.5 ± 0.8 | 17.0 ± 1.1 | 13.8 ± 1.1 | 14.5 ± 0.7 | 11.6 ± 0.8 | 8.3 ± 1.0* |
| Osteoclasts/Bone Perimeter (mm−1) | 8.2 ± 0.4 | 8.5 ± 0.5 | 6.0 ± 0.5 | 6.4 ± 0.3 | 7.9 ± 0.5 | 5.4 ± 0.6* |
| Eroded Surface/Bone Surface (%) | 21.8 ± 1.0 | 21.2 ± 1.3 | 14.1 ± 1.2 | 14.8 ± 0.8 | 18.5 ± 1.2 | 14.5 ± 1.6 |
| Mineral Apposition Rate (μm day−1) | 2.42 ± 0.11 | 2.17 ± 0.09 | 2.41 ± 0.39 | 2.35 ± 0.12 | 1.57 ± 0.08 | 1.43 ± 0.11 |
| Mineralizing Surface/Bone Surface (%) | 8.6 ± 0.8 | 6.8 ± 0.5 | 3.2 ± 0.9 | 5.6 ± 0.9+ | 23.5 ± 3.0 | 13.9 ± 1.0* |
| Bone Formation Rate (μm3 μm−2day−1) | 0.21 ± 0.03 | 0.15 ± 0.01 | 0.08 ± 0.04 | 0.13 ± 0.02 | 0.37 ± 0.06 | 0.20 ± 0.03* |
Values are means ± SEM; n = 4 - 8.
Significantly different between Notch1/2 Null and Control, p < 0.05.
3.3 Notch1 and Notch2 Inactivation in Osteoblast Precursors Affects Cortical Bone Structure
In previous work, we reported that cortical bone is absent in the context of Notch activation in osteoblast precursors, and we tested whether conditional inactivation of Notch1 and Notch2 in these cells has an impact on cortical bone microarchitecture [25]. Three and 6 month old Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ male mice exhibited higher cortical porosity than Notch1loxP/loxP;Notch2loxP/loxP male littermates of the same age, although the effect was modest. Cortical bone was thinner in 1 month old Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ male mice than in control male littermates, but not in 3 and 6 month old male mice. Notch1 and Notch2 inactivation caused an increase in total and bone area at 6 months of age (Table 5, Figure 3).
Table 5.
μCT of cortical bone at the femoral midshaft. One, 3 and 6 month old Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ mice (Notch1/2 Null) or littermate Notch1loxP/loxP;Notch2loxP/loxP controls of the same sex (Control).
| 1 Month | 3 Month | 6 Month | ||||
|---|---|---|---|---|---|---|
| Male Mice | Control | Notch1/2 Null | Control | Notch1/2 Null | Control | Notch1/2 Null |
| Bone Volume/Total Volume (%) | 89.3 ± 0.4 | 88.0 ± 0.5 | 93.0 ± 0.3 | 92.1 ± 0.3* | 93.1 ± 0.4 | 91.3 ± 0.7 |
| Cortical Porosity (%) | 10.7 ± 0.4 | 12.0 ± 0.5 | 7.0 ± 0.3 | 7.9 ± 0.3* | 6.9 ± 0.4 | 8.8 ± 0.7 |
| Cortical Thickness (mm) | 106 ± 2 | 99 ± 2* | 167 ± 7 | 154 ± 4 | 174 ± 6 | 154 ± 11 |
| Total Area (mm2) | 1.53 ± 0.02 | 1.49 ± 0.06 | 1.77 ± 0.14 | 1.96 ± 0.07 | 1.75 ± 0.06 | 2.00 ± 0.11 |
| Bone Area (mm2) | 0.52 ± 0.01 | 0.50 ± 0.02 | 0.80 ± 0.05 | 0.86 ± 0.04 | 0.82 ± 0.03 | 0.95 ± 0.05* |
| Periosteal Perimeter (mm) | 4.39 ± 0.03 | 4.33 ± 0.08 | 4.70 ± 0.18 | 4.95 ± 0.09 | 4.68 ± 0.08 | 5.01 ± 0.13 |
| Endocortical Perimeter (mm) | 3.57 ± 0.03 | 3.53 ± 0.07 | 3.48 ± 0.16 | 3.71 ± 0.07 | 3.41 ± 0.08 | 3.62 ± 0.15 |
| Density of Material (mg HA/cm3) | 956 ± 6 | 947 ± 6 | 1108 ± 8 | 1086 ± 7 | 1159 ± 10 | 1146 ± 14 |
| Female Mice | Control | Notch1/2 Null | Control | Notch1/2 Null | Control | Notch1/2 Null |
| Bone Volume/Total Volume (%) | 87.9 ± 0.2 | 86.9 ± 0.4 | 92.8 ± 0.1 | 91.8 ± 0.3* | 93.8 ± 0.2 | 92.8 ± 0.1* |
| Cortical Porosity (%) | 12.1 ± 0.2 | 13.1 ± 0.4 | 7.2 ± 0.1 | 8.2 ± 0.3* | 6.2 ± 0.2 | 7.2 ± 0.1* |
| Cortical Thickness (mm) | 97 ± 2 | 92 ± 4 | 160 ± 3 | 146 ± 4* | 183 ± 7 | 168 ± 2 |
| Total Area (mm2) | 1.42 ± 0.04 | 1.53 ± 0.06* | 1.50 ± 0.06 | 1.61 ± 0.06 | 1.42 ± 0.06 | 1.66 ± 0.05* |
| Bone Area (mm2) | 0.47 ± 0.01 | 0.51 ± 0.02 | 0.67 ± 0.02 | 0.69 ± 0.02 | 0.73 ± 0.03 | 0.83 ± 0.04 |
| Periosteal Perimeter (mm) | 4.22 ± 0.05 | 4.37 ± 0.08 | 4.34 ± 0.08 | 4.49 ± 0.08 | 4.21 ± 0.08 | 4.56 ± 0.07* |
| Endocortical Perimeter (mm) | 3.45 ± 0.05 | 3.56 ± 0.08 | 3.19 ± 0.07 | 3.38 ± 0.08 | 2.93 ± 0.07 | 3.24 ± 0.04* |
| Density of Material (mg HA/cm3) | 934 ± 5 | 927 ± 7 | 1122 ± 9 | 1097 ± 14* | 1205 ± 8 | 1168 ± 6* |
Values are means ± SEM; n = 5 - 10.
Significantly different between Notch1/2 Null and Control, p < 0.05.
Figure 3. Notch1 and Notch2 inactivation in osteoblast precursors reduces cortical thickness and increases cortical porosity.
μCT images of cortical bone at the femoral midshaft from representative 1 to 6 month old male or female Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ mice (Notch1/2 Null) or Notch1loxP/loxP;Notch2loxP/loxP littermate controls of the same sex (Control).
Three and 6 month old Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ female mice exhibited higher cortical porosity than Notch1loxP/loxP;Notch2loxP/loxP female littermates. Cortical bone was not affected by Notch1 and Notch2 inactivation in 1 month old female mice but was thinner in 3 month old Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ female mice than in control littermates of the same sex. Total area was higher in the context of Notch1 and Notch2 inactivation in female mice, although at 3 months of age the effect was not statistically significant only at 1 and 6 months of age. Periosteal and endocortical perimeters were greater in 6 month old Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ female mice than in control female littermates. A modest but statistically significant reduction of density of material was observed in 3 and 6 month old Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ female mice in comparison to littermate female controls (Table 5, Figure 3).
4. DISCUSSION
In the present study, we report that inactivation of Notch1 and Notch2 in osteoblast precursors increases cancellous bone volume, providing further support to the notion that Notch1 and Notch2 are responsible for the effects of Notch in osteoblastic cells [6]. Presence of the Osx-Cre transgene in the male progeny of pregnant dams administered doxycycline did not modify trabecular microarchitecture and caused a small decrease in cross sectional area without affecting thickness of cortical bone. These changes were modest and do not phenocopy the effects of Notch1 and Notch2 inactivation in osteoblast precursors, indicating that the skeletal phenotype of Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ male mice is secondary to inactivation of Notch1 and Notch2 and not due to the Osx-Cre transgene [30, 38-40]. Osx-Cre+/− female mice were not studied and it cannot be excluded with certainty that the transgene contributed to aspects of the skeletal phenotype of conditional Notch1 and Notch2 null female mice. Hes1 and Hey2 are induced by Notch in skeletal cells and the phenotype of Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ mice was reminiscent of the one observed in the context of Hes1 inactivation in osteoblasts and of Hey2 inactivation in osteoblast precursors of male mice [25, 27, 29]. However, the skeletal consequences of the conditional Notch1 and Notch2 inactivation in osteoblast precursors were more pronounced and observed in mice of both sexes, and inactivation of Notch did not affect expression of Hes1 or Hey2 in parietal bones of mice of both sexes (data not shown). These findings could suggest that Hes1 and Hey2 are not required for the effects of Notch signaling in osteoblast precursors, or that other cellular signals contribute to their expression [41, 42].
Conditional inactivation of Notch1 and Notch2 was associated with reduced body weight in male mice and shorter femurs in female mice, although systemic effects secondary to the inhibition of Notch signaling in osteoblastic cells were not reported in previous studies [6, 22]. Notch signaling is required for elongation of the hypertrophic chondrocyte zone, a process that determines the length of long bones and contributes to body size [43]. The Osx-Cre transgene is expressed in differentiating chondrocytes and post-natal inactivation of Notch1 and Notch2 in these cells might affect elongation of long bones and explain the reduced growth of Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ mice [44].
In male mice, Notch inactivation increased the number of osteoblasts at 1 month of age, confirming that Notch1 and Notch2 mediate the inhibitory effect of Notch on osteoblast differentiation [19, 21, 23]. A decrease in the activity of the Osx-Cre transgene as male mice matured might explain why the cellular phenotype was transient. Notch inactivation did not result in a cellular phenotype in 1 month old female mice, although in agreement with the inhibitory effects of Notch activation on osteoblastogenesis, Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ female mice exhibited a transient increase in mineralizing surface at 3 months of age [25]. This would indicate that a larger proportion of osteoblasts were actively making bone. Although the cellular phenotype was modest, Notch1 and Notch2 inactivation caused a sustained increase in cancellous bone in 3 and 6 month old female mice. Bone remodeling was suppressed in 6 month old Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ female mice, possibly contributing to the sustained increase in cancellous bone. These findings are consistent with previous reports indicating that the effects of Notch misexpression in osteoblastic cells depend on the stage of cell maturation [19, 25].
Recent work from our laboratory demonstrated that osteoblast surface and number are higher in C57BL/6 female mice than in male littermates at 1 month of age [45, 46]. In the present manuscript, Notch1 and Notch2 inactivation in osteoblast precursors increased osteoblast surface and number in male but not in female mice, so that differences between sexes were no longer detectable, suggesting that Notch1 and Notch2 could mediate the effects of sex in osteoblasts. Notch signaling suppresses osteoblastogenesis and might be less active in female than in male mice, so that Notch1 and Notch2 inactivation in osteoblast precursors could affect male to a greater extent than female mice, providing a possible explanation for the sexually dimorphic phenotype at 1 month of age [21, 23, 25]. However, there is no apparent explanation for the sexually dimorphic effects of Notch1 and Notch2 inactivation on osteoclast number and bone resorption. Sex might have an impact on the function of Notch1 and Notch2, and this would be consistent with previous studies, which reported differences related to sex in the cellular phenotype of mice where components of the Notch signaling pathway were inactivated in skeletal cells [27, 29]. The results reported in this study further illustrate the importance of performing analysis of the effects of gene misexpression in mice of both sexes.
Work from our laboratory and others demonstrated that Notch inactivation in osteoblasts and osteocytes causes an increase in osteoclast number and activity [21, 22]. Conversely, Notch1 and Notch2 inactivation in osteoblast precursors did not cause an increase in bone resorption. It is possible that when Notch is inactivated during the early stages of osteoblast differentiation, the effects on osteoblast number and function prevail over the effects on osteoclast number and bone resorption. These observations provide further support to the notion that the function of Notch signaling in osteoblastic cells is determined by the cellular context [25].
We reported that Osx-Cre+/−;Notch1Δ/Δ;Notch2Δ/Δ mice exhibit a modest degree of cortical porosity and thinning of cortical bone, an effect that is similar to the one observed following the inactivation of Notch1 and Notch2 in osteocytes [22, 25]. Selected cells expressing Osx differentiate into osteocytes and it is plausible that osteocytes derived from osteoblast precursors harboring the Notch1 and Notch2 deletion are responsible for the cortical bone phenotype observed [8]. In previous work we demonstrated that the effects of Notch induction under the control of the Osx promoter on cortical bone microarchitecture are similar to those of Notch1 and Notch2 inactivation governed by the same promoter [25]. These observations indicate that basal expression and activity of Notch receptors in osteoblast precursors is required for cortical bone integrity and that either an excess or a deficit of Notch signaling have an impact cortical bone structure.
In conclusion, Notch1 and Notch2 expression in osteoblast precursors regulates trabecular bone microarchitecture and osteoblastic differentiation and function.
Highlights.
Inactivation of Notch1 and Notch2 in osteoblast precursors increases cancellous bone volume
Notch1 and Notch2 mediate the effects of Notch signaling in osteoblastic cells
Inactivation of Notch1 and Notch2 in osteoblast precursors increases porosity and reduces thickness of cortical bone
ACKNOWLEDGMENTS
The authors thank Drs. F. Radtke for Notch1loxP/loxP and T. Gridley for Notch2loxP/loxP mice. The authors thank A. Kent and L. Kranz for technical support and M. Yurczak for secretarial assistance.
This work was supported by grant AR063049 from the National Institute of Arthritis and Musculoskeletal and Skin Disorders (E.C.). Sponsors of this study had no role in the study design, collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
The abbreviations used are
- Fabp
fatty acid-binding protein
- Hes
Hairy Enhancer of Split
- Hey
Hes-related with YRPW motif
- HeyL
Hey-like
- kb
kilobase
- μCT
microcomputed tomography
- Osx
osterix
- Rank
receptor activator of NF-kappa-B
- Rankl
Rank ligand
- SEM
standard error of the mean
- SMI
structure model index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. REFERENCE LIST
- [1].Parfitt AM. Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J Cell Biochem. 1994;55:273–86. doi: 10.1002/jcb.240550303. [DOI] [PubMed] [Google Scholar]
- [2].Canalis E, Giustina A, Bilezikian JP. Mechanisms of Anabolic Therapies for Osteoporosis. N Engl J Med. 2007;357:905–16. doi: 10.1056/NEJMra067395. [DOI] [PubMed] [Google Scholar]
- [3].Bianco P, Gehron RP. Marrow stromal stem cells. J Clin Invest. 2000;105:1663–8. doi: 10.1172/JCI10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218–35. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- [5].Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- [6].Zanotti S, Canalis E. Notch signaling in skeletal health and disease. Eur J Endocrinol. 2013;168:R95–R103. doi: 10.1530/EJE-13-0115. EJE-13-0115 [pii];10.1530/EJE-13-0115 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Canalis E. The fate of circulating osteoblasts. N Engl J Med. 2005;352:2014–6. doi: 10.1056/NEJMe058080. [DOI] [PubMed] [Google Scholar]
- [8].Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL, et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012;10:259–72. doi: 10.1016/j.stem.2012.02.003. S1934-5909(12)00061-6 [pii];10.1016/j.stem.2012.02.003 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am J Pathol. 2007;170:427–35. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009;16:633–47. doi: 10.1016/j.devcel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- [11].Zanotti S, Canalis E. Notch and the Skeleton. Mol Cell Biol. 2010;30:886–96. doi: 10.1128/MCB.01285-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Borggrefe T, Liefke R. Fine-tuning of the intracellular canonical Notch signaling pathway. Cell Cycle. 2012;11:264–76. doi: 10.4161/cc.11.2.18995. 18995 [pii];10.4161/cc.11.2.18995 [doi] [DOI] [PubMed] [Google Scholar]
- [13].Kovall RA. More complicated than it looks: assembly of Notch pathway transcription complexes. Oncogene. 2008;27:5099–109. doi: 10.1038/onc.2008.223. [DOI] [PubMed] [Google Scholar]
- [14].Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–33. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194:237–55. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- [16].Dallas DJ, Genever PG, Patton AJ, Millichip MI, McKie N, Skerry TM. Localization of ADAM10 and Notch receptors in bone. Bone. 1999;25:9–15. doi: 10.1016/s8756-3282(99)00099-x. [DOI] [PubMed] [Google Scholar]
- [17].Pereira RM, Delany AM, Durant D, Canalis E. Cortisol regulates the expression of Notch in osteoblasts. J Cell Biochem. 2002;85:252–8. doi: 10.1002/jcb.10125. [DOI] [PubMed] [Google Scholar]
- [18].Bai S, Kopan R, Zou W, Hilton MJ, Ong CT, Long F, et al. NOTCH1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells. J Biol Chem. 2008;283:6509–18. doi: 10.1074/jbc.M707000200. [DOI] [PubMed] [Google Scholar]
- [19].Engin F, Yao Z, Yang T, Zhou G, Bertin T, Jiang MM, et al. Dimorphic effects of Notch signaling in bone homeostasis. Nat Med. 2008;14:299–305. doi: 10.1038/nm1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fukushima H, Nakao A, Okamoto F, Shin M, Kajiya H, Sakano S, et al. The association of Notch2 and NF-kappaB accelerates RANKL-induced osteoclastogenesis. Mol Cell Biol. 2008;28:6402–12. doi: 10.1128/MCB.00299-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hilton MJ, Tu X, Wu X, Bai S, Zhao H, Kobayashi T, et al. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med. 2008;14:306–14. doi: 10.1038/nm1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Canalis E, Adams DJ, Boskey A, Parker K, Kranz L, Zanotti S. Notch Signaling in Osteocytes Differentially Regulates Cancellous and Cortical Bone Remodeling. J Biol Chem. 2013;288:25614–25. doi: 10.1074/jbc.M113.470492. M113.470492 [pii];10.1074/jbc.M113.470492 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zanotti S, Smerdel-Ramoya A, Stadmeyer L, Durant D, Radtke F, Canalis E. Notch Inhibits Osteoblast Differentiation And Causes Osteopenia. Endocrinology. 2008;149:3890–9. doi: 10.1210/en.2008-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tao J, Chen S, Yang T, Dawson B, Munivez E, Bertin T, et al. Osteosclerosis owing to Notch gain of function is solely Rbpj-dependent. J Bone Miner Res. 2010;25:2175–83. doi: 10.1002/jbmr.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Canalis E, Parker K, Feng JQ, Zanotti S. Osteoblast Lineage-specific Effects of Notch Activation in the Skeleton. Endocrinology. 2013;154:623–34. doi: 10.1210/en.2012-1732. en.2012-1732 [pii];10.1210/en.2012-1732 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yamada T, Yamazaki H, Yamane T, Yoshino M, Okuyama H, Tsuneto M, et al. Regulation of osteoclast development by Notch signaling directed to osteoclast precursors and through stromal cells. Blood. 2003;101:2227–34. doi: 10.1182/blood-2002-06-1740. [DOI] [PubMed] [Google Scholar]
- [27].Zanotti S, Smerdel-Ramoya A, Canalis E. Hairy and enhancer of split (HES)1 is a determinant of bone mass. J Biol Chem. 2011;286:2648–57. doi: 10.1074/jbc.M110.183038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33:77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- [29].Zanotti S, Canalis E. Hairy and Enhancer of Split-related with YRPW Motif (HEY)2 Regulates Bone Remodeling in Mice. J Biol Chem. 2013;288:21547–57. doi: 10.1074/jbc.M113.489435. M113.489435 [pii];10.1074/jbc.M113.489435 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–44. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- [31].Furth PA, St OL, Boger H, Gruss P, Gossen M, Kistner A, et al. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc Natl Acad Sci U S A. 1994;91:9302–6. doi: 10.1073/pnas.91.20.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Radtke F, Wilson A, Stark G, Bauer M, van MJ, MacDonald HR, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–58. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- [33].McCright B, Lozier J, Gridley T. Generation of new Notch2 mutant alleles. Genesis. 2006;44:29–33. doi: 10.1002/gene.20181. [DOI] [PubMed] [Google Scholar]
- [34].Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- [35].Gazzerro E, Pereira RC, Jorgetti V, Olson S, Economides AN, Canalis E. Skeletal overexpression of gremlin impairs bone formation and causes osteopenia. Endocrinology. 2005;146:655–65. doi: 10.1210/en.2004-0766. [DOI] [PubMed] [Google Scholar]
- [36].Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17. doi: 10.1002/jbmr.1805. 10.1002/jbmr.1805 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sokal RR, Rohlf FJ. Biometry. 2nd Edition. W. H. Freeman; San Francisco, CA: 1981. Biometry. 2nd Edition. [Google Scholar]
- [38].Berman SD, Calo E, Landman AS, Danielian PS, Miller ES, West JC, et al. Metastatic osteosarcoma induced by inactivation of Rb and p53 in the osteoblast lineage. Proc Natl Acad Sci U S A. 2008;105:11851–6. doi: 10.1073/pnas.0805462105. 0805462105 [pii];10.1073/pnas.0805462105 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Walkley CR, Qudsi R, Sankaran VG, Perry JA, Gostissa M, Roth SI, et al. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev. 2008;22:1662–76. doi: 10.1101/gad.1656808. 22/12/1662 [pii];10.1101/gad.1656808 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Davey RA, Clarke MV, Sastra S, Skinner JP, Chiang C, Anderson PH, et al. Decreased body weight in young Osterix-Cre transgenic mice results in delayed cortical bone expansion and accrual. Transgenic Res. 2012;21:885–93. doi: 10.1007/s11248-011-9581-z. 10.1007/s11248-011-9581-z [doi] [DOI] [PubMed] [Google Scholar]
- [41].Zamurovic N, Cappellen D, Rohner D, Susa M. Coordinated activation of notch, Wnt, and transforming growth factor-beta signaling pathways in bone morphogenic protein 2-induced osteogenesis. Notch target gene Hey1 inhibits mineralization and Runx2 transcriptional activity. J Biol Chem. 2004;279:37704–15. doi: 10.1074/jbc.M403813200. [DOI] [PubMed] [Google Scholar]
- [42].Ricard N, Ciais D, Levet S, Subileau M, Mallet C, Zimmers TA, et al. BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. Blood. 2012;119:6162–71. doi: 10.1182/blood-2012-01-407593. blood-2012-01-407593 [pii];10.1182/blood-2012-01-407593 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kohn A, Dong Y, Mirando AJ, Jesse AM, Honjo T, Zuscik MJ, et al. Cartilage-specific RBPjkappa-dependent and -independent Notch signals regulate cartilage and bone development. Development. 2012;139:1198–212. doi: 10.1242/dev.070649. 139/6/1198 [pii];10.1242/dev.070649 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329–44. doi: 10.1016/j.devcel.2010.07.010. S1534-5807(10)00338-2 [pii];10.1016/j.devcel.2010.07.010 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-Related Changes in Trabecular Architecture Differ in Female and Male C57BL/6J Mice. J Bone Miner Res. 2007;22:1197–207. doi: 10.1359/jbmr.070507. [DOI] [PubMed] [Google Scholar]
- [46].Zanotti S, Kalajzic I, Aguila HL, Canalis E. Sex and Genetic Factors Determine Osteoblastic Differentiation Potential of Murine Bone Marrow Stromal Cells. PLoS One. 2014 doi: 10.1371/journal.pone.0086757. doi: 10.1371/journal.pone.0086757 (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]