Abstract
The inappropriate programming of developing organ systems by exposure to excess native or environmental steroids, particularly the contamination of our environment and our food sources with synthetic endocrine disrupting chemicals that can interact with steroid receptors, is a major concern. Studies with native steroids have found that in utero exposure of sheep to excess testosterone, an estrogen precursor, results in low birth weight offspring and leads to an array of adult reproductive / metabolic deficits manifested as cycle defects, functional hyperandrogenism, neuroendocrine / ovarian defects, insulin resistance, and hypertension. Furthermore, the severity of reproductive dysfunction is amplified by excess postnatal weight gain. The constellation of adult reproductive and metabolic dysfunction in prenatal testosterone-treated sheep is similar to features seen in women with polycystic ovary syndrome. Prenatal dihydrotestosterone treatment failed to result in similar phenotype suggesting that many effects of prenatal testosterone excess are likely facilitated via aromatization to estradiol. Similarly, exposure to environmental steroid imposters such as bisphenol A (BPA) and methoxychlor (MXC) from days 30-90 of gestation had long-term but differential effects. Exposure of sheep to BPA, which resulted in maternal levels of 30-50 ng/ml BPA, culminated in low birth-weight offspring. These female offspring were hypergonadotropic during early postnatal life and characterized by severely dampened preovulatory LH surges. Prenatal MXC-treated females had normal birth weight and manifested delayed but normal amplitude LH surges. Importantly, the effects of BPA were evident at levels, which approximated twice the highest levels found in human maternal circulation of industrialized nations. These findings provide evidence in support of developmental origin of adult reproductive and metabolic diseases and highlight the risk posed by exposure to environmental endocrine disrupting chemicals.
Keywords: fetal programming, infertility, neuroendocrine, ovary, insulin resistance, endocrine disrupting chemicals, bisphenol A, methoxychlor, metabolic programming
Introduction
The developing fetus, in response to changes in the in utero environment develops compensatory strategies to overcome insults that they experience. Such compensations could be adaptive, if they support survival, or disruptive, if they compromise postnatal survival. Developmental plasticity, the ability of the developing fetus to change structure / function in response to physiological cues from the mother, underlies the developmental origin of disease or Barker hypothesis (Barker, 1994). The increased prevalence of some common diseases may be related to exposure during development to environmental pollutants, lifestyle choices of the mother, and medical interventions, all of which can adversely influence developmental trajectory of target tissue differentiation. This review addresses the reproductive and metabolic disruptions resulting from exposure to excess native steroids and environmental steroid receptor modulators with specific focus on those that signal through estrogen and androgen receptors.
Developmental programming of reproductive / metabolic dysfunction with native steroids
Steroid hormones play a major role during development in setting the trajectory of developing organ systems. Because, differentiation of organ systems depend upon precise exposure to steroid hormones at specific times during development, exposure to low doses of endocrine disrupting compounds (EDCs) that can signal through steroid receptors during these hormone sensitive, critical periods of development can lead to long term deleterious effects on the adult organism. It is well established that inappropriate exposure to excess testosterone (T) during fetal life leads to phenotypic virilization and behavioral masculinization in the female offspring (Jost et al. 1973; Gorski, 1986; Wood & Foster, 1998). The amount as well as the timing of T exposure dictates the degree of masculinization of external genitalia in the female (Wood & Foster, 1998). Inappropriate perinatal exposure to excess T during early development also disrupts reproductive cyclicity in several species (Abbott et al. 2006).
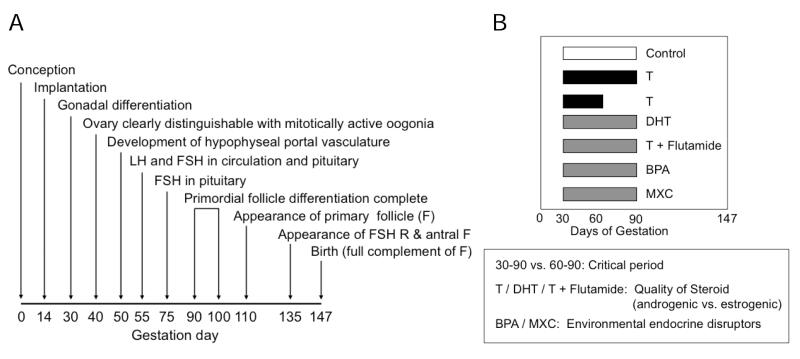
For the remainder of the review, focus is on the reproductive and metabolic disruptions resulting from inappropriate developmental exposure of sheep to native steroids or environmental steroid mimics. Sheep are exceptionally well suited for investigating developmental programming of adult disorders. They have long been used as model systems to study fetal physiology (Harding & Bloomfield, 2004). Their developmental time line (gestation length: 147 days, puberty in female: ~ 28 weeks) is ideally suited for integrative studies that address progression of reproductive / metabolic disruption from the initial developmental insult to manifestation of adult consequences, especially those that involve detailed hormonal profiling or sequential monitoring of ovarian follicular dynamics. Importantly, they can be studied in natural social settings thus reducing level of stress. From a reproductive perspective, ovarian differentiation in sheep is similar to humans with full follicular differentiation occurring by birth (Fig.1, panel A) (Padmanabhan et al. 2007). Neuroendocrine aspects of reproductive cyclicity are also similar to human (Goodman & Inskeep, 2006; McNeilly, 1991).
Fig. 1.
Panel A: Schematic showing the time of appearance of different classes of follicles in sheep, timing of establishment of hypophyseal portal vasculature to pituitary and timing of appearance of LH and FSH in circulation and pituitary during fetal life in sheep. Panel B: Schematic showing the timing and duration of the various steroid / EDC treatments used in studies discussed in this review.
Comparison of sheep treated with T (aromatizable androgen) from days 30 to 90 of gestation (T30-90 females) with those treated from days 60-90 of gestation (T60-90 females) has helped address critical period of programming of reproductive and metabolic disruptions. Comparison of prenatal testosterone (T), prenatal dihydrotestosterone (non aromatizable androgen, DHT) and T plus flutamide (an androgen antagonist) treatments has helped address the quality of steroid (androgen or estrogen) responsible for programming adult dysfunctions (Fig. 1, panel B). Earlier studies with the Dorset breed of sheep found T30-90 females showed progressive deterioration of cyclicity culminating in absent cycles during the second breeding season (Fig. 2, panel A) (Birch et al. 2003). Studies with other breed of sheep also found progressive loss of cyclicity (Clarke et al. 1977; Manikkam et al. 2006), the severity of which differing between breeds. In contrast, majority of the T60-90 females cycled during the second breeding season (Birch et al. 2003, Savabieasfahani et al. 2005).
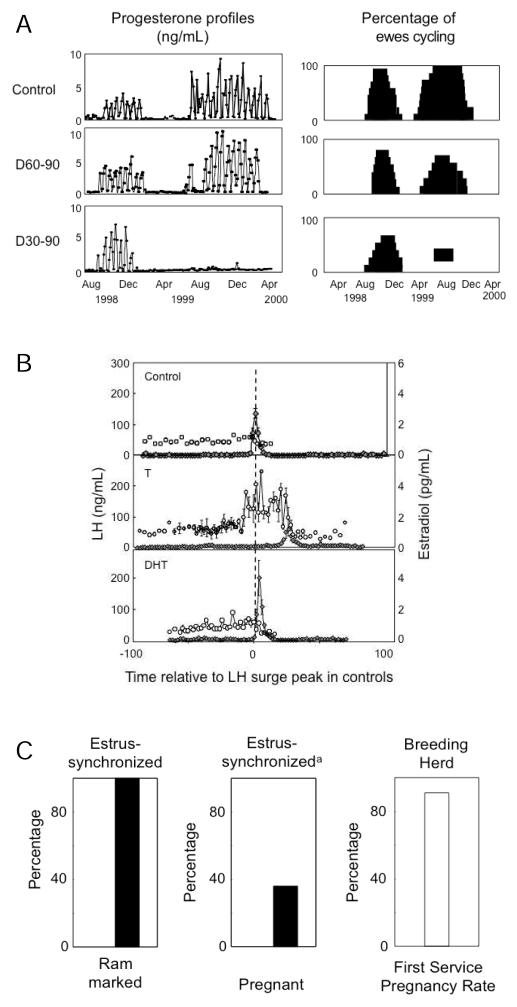
Fig. 2.
Panel A: Plasma progesterone profiles from representative control, T60-90, and T30-90 females during the first and second breeding seasons are shown on the left. On the right are shown percentages of sheep cycling during the first and second breeding seasons. (modified from Birch et al. 2003. Panel B: Patterns of LH (closed circles) and E (open circles) from control (top), T30-90 (middle), and DHT30-90 (bottom) following estrous synchronization with PGF2α (Veiga-Lopez et al. 2009). Panel C: Percentage of T60-90 females mated and becoming pregnant following estrous synchronization. Estrus was synchronized with two injections of PGF2α administered 11 days apart. Mating was determined by heavy rump markings left by a fertility-proven raddled ram (modified from Steckler et al. 2007b).
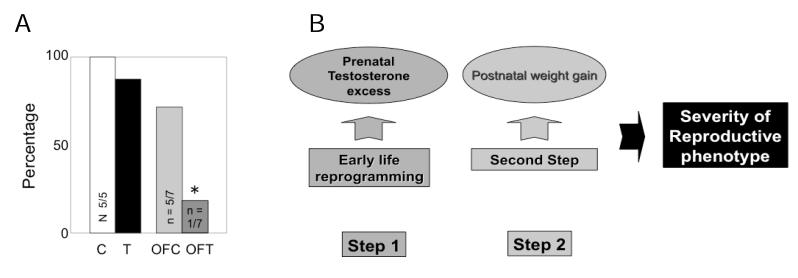
Comparison of cycle dynamics of T30-90 and DHT30-90 females during the estrous cycle found that T30-90 females were characterized by increased preovulatory levels of estradiol, as well as delayed and severely dampened LH surges (Fig. 2, panel B) (Veiga-Lopez et al. 2009). Detailed characterization of circulating LH dynamics during the follicular phase found T30-90 and T60-90 females were characterized by excess LH release (Manikkam et al. 2008; Savabieasfahani et al. 2005). Studies testing the fertility status of T60-90 females found that 100% of the T60-90 females (T30-90 females are phenotypically virilized and natural mating is not possible (Wood and Foster, 1998) were mated by the ram. However, fecundity was reduced with only 40% of those mated becoming pregnant as opposed to the 90% pregnancy rate in the control herd (Fig. 2, panel C) (Steckler et al. 2007b). Even more importantly, recent studies found that excess postnatal weight gain amplifies the reproductive disruptions in T30-90 females (Fig. 3, panel A) (Steckler et al. 2009). These findings are supportive of the two-step process (Tang et al. 2008), the first involving early life epigenetic reprogramming of susceptible organ systems and a later event influencing the severity of the pathologic phenotype (Fig. 3, panel B).
Fig. 3.
Panel A:Percent of control (C), over-fed control (OFC), T30-90 (T) and overfed T30-90 (OFT) females that showed a luteal progesterone increase following estrus synchronization with progesterone. Note that almost all of the overfed T30-90 females were anovulatory (modified from Steckler et al. 2009). Panel B: Schematic showing the two step model of programming severity of reproductive dysfunction with the first insult occurring from prenatal T excess during fetal life and the second metabolic insult stemming from overfeeding.
Neuroendocrine disruptions
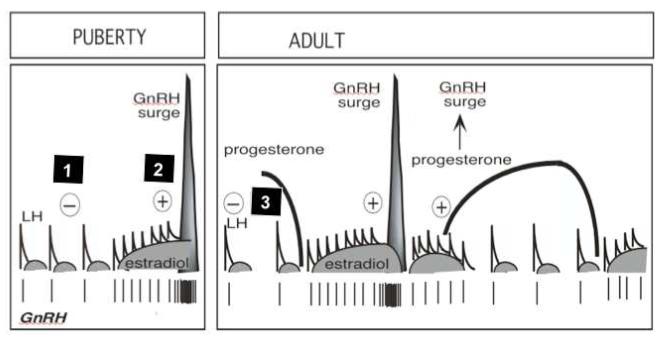
At the neuroendocrine level, prenatal T treatment reduces hypothalamic sensitivity to all three major feedback systems involved in the control of cyclic changes in GnRH / gonadotropin secretion; estradiol (E) negative feedback (Wood & Foster, 1998; Sarma et al. 2005), E positive feedback (Wood & Foster, 1998; Sharma et al. 2002, Unsworth et al. 2005) and progesterone negative feedback (Robinson et al. 1999; Veiga-Lopez et al. 2009) (Fig. 4). Further investigations have pointed to disruptions of E negative feedback being programmed by androgenic action of T, with both, T and DHT and not T + flutamide reducing sensitivity to E (Wood & Foster, 1998, Veiga-Lopez et al. 2009, Jackson et al. 2008). E positive feedback disruptions were found in T30-90 but not DHT30-90 females suggesting that this disruption is likely programmed via estrogenic actions of prenatal T (Wood & Foster, 1998, Veiga-Lopez et al. 2009). Studies testing pituitary sensitivity also found that both T30-90 and DHT30-90 females have enhanced sensitivity to GnRH suggesting that this aspect is programmed likely via androgenic actions of T (Manikkam et al. 2008).
Fig. 4.
Neuroendocrine feedback systems involved in the control of GnRH / LH secretion that are reprogrammed by prenatal T excess. GnRH / LH release is under the control of negative feedback action of estradiol (E) which is predominant during the prepubertal and anestrus period (feedback 1), stimulatory feedback action of E responsible for generation of the preovulatory LH surge (feedback 2) and negative feedback action of progesterone, operational during the luteal phase (feedback 3) (modified from Foster et al. 2007).
Ovarian disruptions
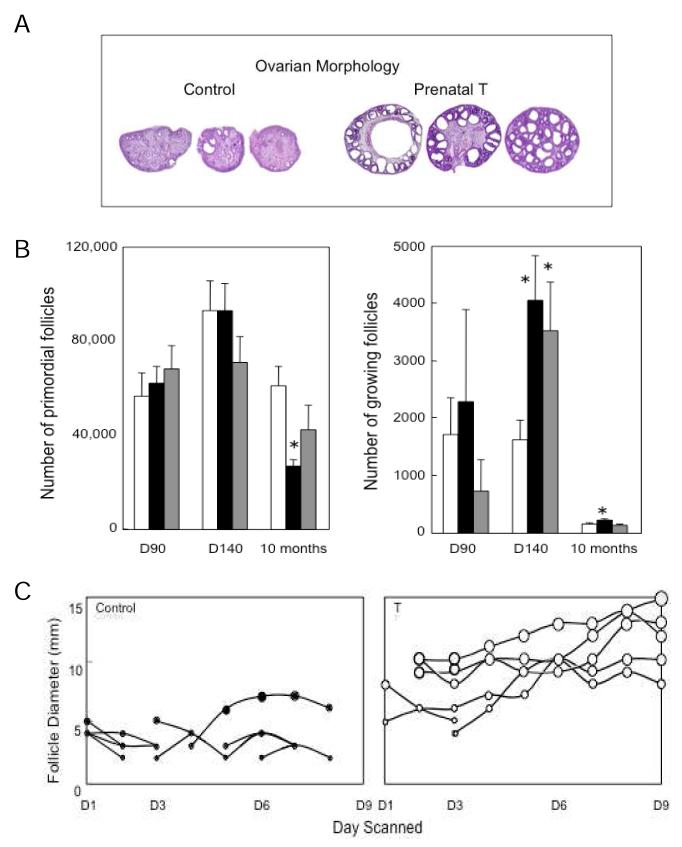
In addition to reproductive neuroendocrine disruptions, prenatal T treatment resulted in larger ovaries with a multifollicular morphology (Fig. 5, panel A) (West et al. 2001). These effects appear not to be facilitated by the androgenic actions of T as prenatal DHT treatment failed to create a multifollicular ovarian phenotype (West et al. 2001; Steckler et al. 2007a). Detailed morphometric analyses found prenatal T and DHT treatment enhanced follicular recruitment with only prenatal T treatment reducing ovarian follicular reserve to ~ 50% by the end of the first breeding season (Smith et al. 2009) (Fig. 5, panel B). Similarly, detailed daily ultrasonographic evaluation found that follicles persist longer in prenatal T-treated female (Manikkam et al. 2006) (Fig. 5, panel C) and this appears to be programmed by estrogenic actions of prenatal T (Steckler et al. 2007a). As such, the multifollicular phenotype of prenatal T females appears to be the consequence of both enhanced follicular recruitment and failure to regress. Immunohistochemical studies found that prenatal T treatment increases androgen receptor expression in the stroma and granulosa cells during fetal life and culminates in increased granulosa cell androgen receptor expression in antral follicles of adult females (Ortega et al. 2009). Taken together these studies document that excess exposure to T disrupts the ovarian trajectory with some aspects programmed by androgenic and others estrogenic actions of T.
Fig. 5.
Panel A: Follicular morphology of ovary from control and T30-90 females. Note the disrupted nature of follicular development in T30-90 sheep (from West et al. 2001). Panel B: Mean (± SEM) number of primordial and growing follicles on fetal days 90 and 140 and 10 months of age in control (open bars), T30-90 (closed bars) and DHT30-90 (gray bars) ovaries (from Smith et al. 2009). Panel C: Ovarian follicular dynamics determined by ultrasonography for 8 days in both ovaries control and T30-90 sheep during the first breeding season (from Manikkam et al. 2006). Each line represents only one follicle and follicles from both ovaries are shown in the same panel. Only follicles that reached a size of 3 mm and persisted for at least 2 days are shown. Note the increase in maximum size and duration of the largest follicles on the ovary in T30-90 sheep compared to controls.
Metabolic dysfunctions
In addition to the neuroendocrine and ovarian disruptions, prenatal T treatment leads to intrauterine growth restriction (IUGR), low birth weight and postnatal catch-up growth (Manikkam et al. 2004), risk factors for adult well being (Boney et al. 2005; Dulloo, 2008). Developmental changes in the insulin-like growth factor (IGF) / IGF binding protein (IGFBP) system in the prenatal T-treated sheep were consistent with changes in growth trajectory with a reduction in IGF bioavailability evident during IUGR and an increase during postnatal catch-up growth (Crespi et al. 2006, Manikkam et al. 2004). Prenatal T-treatment also culminated in insulin resistance (DeHaan et al. 1990; Hansen et al. 1995; Recabarren et al. 2005; Padmanabhan et al. 2009) with programming of insulin resistance facilitated via androgenic actions of prenatal T (Padmanabhan et al. 2009). Importantly, the window of susceptibility for developing insulin resistance was found to be confined to a shorter programming window, namely 60-90 days of gestation (Padmanabhan et al. 2009). Recent studies assessing the impact of prenatal T excess revealed tissue specific regulation of members of the insulin-signaling cascade (Nada et al., 2009). At the hepatic level, there was a general downregulation of many members of the insulin signaling cascade consistent with liver being insulin resistant. In contrast, prenatal T excess upregulated many members of the insulin signaling cascade at the level of the adipose tissue supportive of increased insulin sensitivity (Nada et al., 2009). Our unpublished observations also indicate increased visceral adiposity in the prenatal T-treated females. Radiotelemetric studies found that the T30-90 females are also hypertensive (King et al. 2007). Metabolic disruptions have also been reported in other prenatal T treated animal models (Abbott et al. 2006, Demissie et al. 2008).
Male reproduction
In contrast to several studies addressing the impact of prenatal T excess in female sheep, limited information is available addressing the impact of prenatal T excess on male reproduction / metabolism in sheep. Prenatal T treatment increased ano-genital distance in the male offspring compared to controls (Manikkam et al. 2004), altered the developmental trajectory of gonadal responsiveness to GnRH in prepubertal males (Recabarren et al. 2007) and culminated in reduced sperm count and motility (Recabarren et al. 2008). Prenatal T treatment also increased the volume of the sexually dimorphic nucleus in the males (Roselli, 2007), a complex of aromatase-expressing neurons, whose size has been correlated with sexual attraction in rams. Exposure to an aromatase inhibitor prenatally (days 50-80 of gestation) has also been correlated with decreased adult mounting behavior (Roselli, 2006). Information is lacking as to whether prenatal T excess disrupts the metabolic axis in the ovine male.
Translational significance
The reproductive phenotype of T30-90 sheep parallels features seen in women with polycystic ovarian disease (PCOS) (Table 1). PCOS is one of the most common reproductive disorder affecting >100 million women worldwide with the economic burden exceeding several billion dollars annually in the U.S. women with PCOS are characterized by oligo- / anovulation, hyperandrogenism, polycystic ovaries, LH hypersecretion and reduced fecundity with most manifesting insulin resistance (Franks, 1995). Some view PCOS as a clinical phenotype of the metabolic syndrome (Essah & Nestler 2006; Sam & Dunaif, 2003). Because the reproductive and metabolic phenotype of prenatal T-treated sheep recapitulates characteristics of women with PCOS, they provide a valuable cost-effective resource for addressing the mechanisms underlying the etiology of development of PCOS phenotype. The constellation of reduced insulin sensitivity, hypertension and visceral adiposity found in prenatal T treatment suggest that these animals may also be suitable for understanding the developmental origin of the metabolic syndrome phenotype (Mikhail, 2009).
Table 1.
Characteristics of women with PCOS vs. prenatal T-treated sheep
| Attributes | Women with PCOS |
Prenatal T-treated
sheep |
|---|---|---|
| Anovulation | Yes | Yes |
| Hyperandrogenism | Yes (functional) | Yes |
| Hypergonadotropism | Yes | Yes |
| Reduced sensitivity to steroids | Yes | Yes |
| Multifollicular ovaries | Yes | Yes |
| Increased follicular recruitment | Yes | Yes |
| Altered insulin sensitivity | Yes | Yes |
| Insulin resistance | Yes | Yes |
| Fetal growth retardation | YesA | Yes |
| Altered behavior | Yes | Yes |
| Hypertension | YesB | Yes |
| Visceral adiposity | Yes | Yes (observational) |
| Obesity amplification | Yes | Yes |
Spanish cohort
Risk factor in PCOS
Developmental programming by endocrine disruption chemicals
The inappropriate exposure to steroids is becoming a major concern in the context of development of adult pathologies. The fetus is exposed to exogenous steroids via failed contraception, use of anabolic steroids or inadvertent exposure to environmental compounds with estrogenic or anti-androgenic activity. Public concern has been mounting over harmful effects of environmental EDC, which can interfere with hormone signaling by acting as agonists or antagonists (Damstra et al. 2002; Hotchkiss et al. 2008). Of particular concern is the contamination of our environment and our food sources with the synthetic androgenic and estrogenic EDCs, which have the potential to disrupt normal androgen and estrogen signaling. This review focuses predominantly on two such EDCs namely, bisphenol-A (BPA) a widely used industrial plasticizer and methoxychlor (MXC), a pesticide. BPA is widely used in the manufacture of epoxy resins and polycarbonate plastics and accounts for most estrogenic activity in landfill leachates (Vandenberg et al. 2009; Ranjit et al. 2009). It has been detected in river water and sediments, and more recently, in indoor air and dust (Vandenberg et al. 2009; Ranjit et al. 2009). MXC, was used to control pests in agricultural, dairy, and domestic settings and found to persist in the environment (National Research Council, 1999). Both these EDCs have been shown to possess estrogenic and anti-androgenic properties (Vandenberg et al. 2009, Staub et al. 2002).
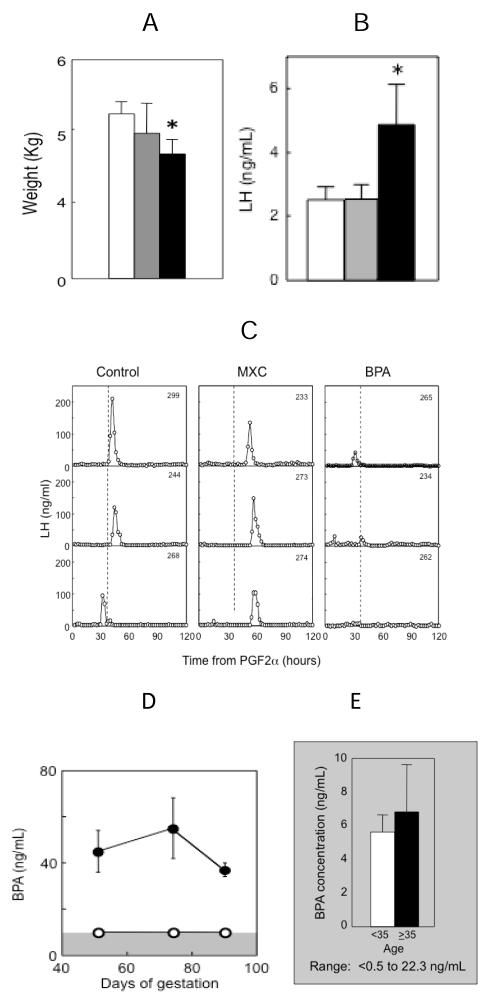
Targeting critical periods established by treating with native steroids, our recent studies found that prenatal BPA and MXC treatment had differential effects on the reproductive axis (Savabieasfahani et al. 2006). Prenatal BPA treatment, like prenatal T treatment, resulted in low birth weight offspring, early hypergonadotropism and severely dampened or absent preovulatory LH surges (Fig. 6, panels A, B, C). In contrast, MXC had no effect on somatic growth (Fig. 6, panel A) but delayed the onset of LH surges (Fig. 5, panel C). The levels of BPA achieved in maternal circulation following administration of 5 mg / kg body weight of BPA (Fig. 6, panel D) were 2-3 fold higher than the highest levels observed in the maternal circulation of U.S. women (Fig. 6, panel E) (Padmanabhan et al. 2008) and other industrialized nations (Vandenberg et al. 2009, Schonfelder et al. 2002). MXC levels in abdominal fat (Savabieasfahani et al. 2006) were several-fold higher than that found in human population (Botella et al. 2004). Comparison of reproductive defects in BPA and MXC treated females with prenatal T-treated model reveal considerable similarities between prenatal BPA and T treated models (Table 2). Both groups of animals showed reduced birth weight, LH excess and severely dampened preovulatory LH surges (Savabieasfahani et al. 2006). Others studies in sheep found administration from days110 to 115 days of gestation of octylphenol, a alkylphenol polyethoxylate used in detergents and pesticides with estrogenic properties, suppressed FSH levels in both female and male offspring (Sweeney et al. 2000). Administration of octylphenol starting from day 70 of gestation to birth advanced the time of puberty in female offspring (Wright et al. 2000).
Fig. 6.
Panel A: Birth weight of control (open bars), prenatal MXC (gray bars) and BPA-treated (closed bars) female offspring (Savabieasfahani et al. 2006). Panel B: Mean circulating levels of LH in prepubertal control (open bars), prenatal MXC (gray bars) and BPA-treated (closed bars) female offspring (Savabieasfahani et al. 2006). Panel C: Circulating patterns of LH from 3 control, 3 prenatal MXC- and 3 BPA-treated females taken at 2 hourly intervals for 120 h, after induction of luteolysis with 2 injections of PGF2α 11 days apart (Savabieasfahani et al. 2006). Panel D: Levels of circulating BPA achieved in control (open circles) and BPA treated (closed circles) pregnant sheep on day 50, 70 and 90 of gestation (days 20, 40 and 60 of treatment) following administration of 5 mg / kg / daily administration of BPA s.c. (Savabieasfahani et al. 2006). Panel E: Maternal levels of BPA (mean ± SEM) in Southeastern Michigan relative to maternal age (Padmanabhan et al. 2008).
Table 2.
Characteristics of prenatal T, BPA and MXC treated sheep
| Attributes |
Prenatal
T-treated |
Prenatal
BPA-treated |
Prenatal
MXC-treated |
|---|---|---|---|
| Hypergonadotropism | Yes | Yes | No |
| Cycle disruption | Yes | Yes | Yes |
| Dampened LH surge | Yes | Yes | No |
| Increased amplitude of E2 | Yes | Yes | No |
| Delayed LH surge onset | Yes | Yes | No |
| Fetal growth retardation | Yes | Yes | No |
Studies testing the effects of BPA and MXC in male sheep are not available. The only available information testing effects of EDC in male sheep comes from studies testing the effects of octylphenol. Prenatal octylphenol treatment from days 70 of gestation to birth reduced testis weight and Sertoli cell number in newborns (Sweeney et al. 2000) but not semen volume / concentration and motility in adult males (Sweeney et al. 2007). As opposed to the limited information available in sheep, a large volume of literature already exists relative to impact of BPA on the male offspring using rodent models. These rodent studies provide evidence that prenatal BPA exposure leads to disruptions in the male reproductive system, which include constricted urethra and prostate hyperplasia and cancer (Talsness et al. 2009; Diamanti-Kandarakis et al. 2009). A recent study found exposure to BPA from gestational day 12 to postnatal day 21 reduced sperm count and motility leading to subfertility in the offspring with effects persisting in F2 and F3 generations (Salian et al. 2009). Similarly exposure to MXC orvinclozolin during gestation resulted in reduced spermatogenic capacity and increasedincidence of male infertility with effects transferred to subsequent generations (Anway et al. 2005).
Conclusions
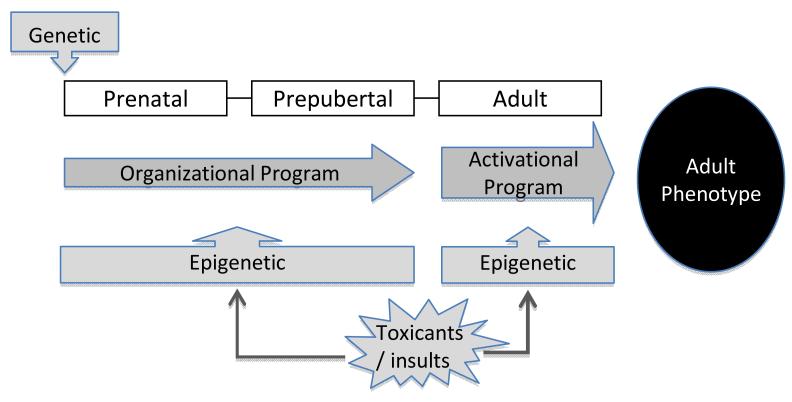
Studies discussed in this review centering on sheep as a model system enforce that the organizational program involved in establishing the adult phenotype is the result of the interplay between genetic susceptibility and developmental insults (Fig. 7). These findings reinforce the concern that inappropriate exposure to steroid hormones / steroid mimics pose to the well being of the developing offspring. The pathology programmed in sheep by BPA and MXC provides further support for the deleterious effects of EDCs on developing organ systems. Clearly, an understanding of mechanisms underlying developmental reprogramming following exposure to EDCs is essential for developing interventions to prevent development or reduce severity of pathology in adults. Several recent studies point to restoration of function via methylation by dietary supplements (Waterland, 2006; Pennisi, 2005; Burdge et al. 2009; Dolinoy et al. 2007), providing hope that dietary interventions may be beneficial in improving human health. Environmental exposures are modifiable risk factors and can be effectively regulated at the personal, behavioral as well as the regulatory policy level. For instance, exposure to BPA through sources such as over consumption of fast food and canned food and overuse of baby bottles, can be addressed through public health education campaigns and by health care providers, including physicians, nurses, social workers, and dentists. At the policy level, environmental justice advocates can mobilize efforts to protect poor neighborhoods from exposures to EDCs.
Fig. 7.
Schematic showing organizational palette of adult phenotype as influenced by genetic and epigenetic interactions.
References
- Abbott DH, Dumesic DA, Levine JE, Dunaif A, Padmanabhan V. Animal models and fetal programming of PCOS. In: Azziz R, Nestler JE, Dewailly D, editors. Contemporary Endocrinology: Androgen Excess Disorders in Women: Polycystic Ovary Syndrome and Other Disorders. Humana Press Inc; Totowa, New Jersey: 2006. pp. 259–272. [Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP. Mothers, babies, and disease in later life. BMJ Publishing Group; 1994. Programming the baby; pp. 14–36. [Google Scholar]
- Birch RA, Padmanabhan V, Foster DL, Robinson JE. Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology. 2003;144:1426–1434. doi: 10.1210/en.2002-220965. [DOI] [PubMed] [Google Scholar]
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- Botella B, Crespo J, Rivas A, Cerrillo I, Olea-Serrano MF, Olea N. Exposure of women to organochlorine pesticides in Southern Spain. Environmental Research. 2004;96:34–40. doi: 10.1016/j.envres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Burdge GC, Lillycrop KA, Phillips ES, Slater-Jefferies JL, Jackson AA, Hanson MA. Folic acid supplementation during the juvenile-pubertal period in rats modifies the phenotype and epigenotype induced by prenatal nutrition. Journal of Nutrition. 2009;139:1054–1060. doi: 10.3945/jn.109.104653. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Scaramuzzi RJ, Short RV. Ovulation in prenatally androgenized ewes. Journal of Endocrinology. 1977;73:385–389. doi: 10.1677/joe.0.0730385. [DOI] [PubMed] [Google Scholar]
- Crespi EJ, Steckler TL, Mohankumar PS, Padmanabhan V. Prenatal exposure to excess testosterone modifies the developmental trajectory of the insulin-like growth factor system in female sheep. Journal of Physiology. 2006;572:119–30. doi: 10.1113/jphysiol.2005.103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damstra T, Barlow S, Bergman A, Kavlock R, Van der Kraak G. Global assessment of the state-of-the-science of endocrine disruptors. International Programme on Chemical Safety (IPCS), World Health organization; Geneva, Switzerland: 2002. [Google Scholar]
- DeHaan KC, Berger LL, Bechtel PJ, Kesler DJ, McKeith FK, Thomas DL. Effect of prenatal testosterone treatment on nitrogen utilization and endocrine status of ewe lambs. Journal of Animal Science. 1990;68:4100–4108. doi: 10.2527/1990.68124100x. [DOI] [PubMed] [Google Scholar]
- Demissie M, Lazic M, Foecking EM, Aird F, Dunaif A, Levine JE. Transient prenatal androgen exposure produces metabolic syndrome in adult female rats. American Journal of Physiology Endocrinology and Metabolism. 2008;295:E262–E268. doi: 10.1152/ajpendo.90208.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocrine Reviews. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulloo AG. Thrifty energy metabolism in catch-up growth trajectories to insulin and leptin resistance. Best Practices in Research in Clinical Endocrinology and Metabolism. 2008;22:155–171. doi: 10.1016/j.beem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Essah PA, Nestler JE. The metabolic syndrome in polycystic ovary syndrome. Journal of Endocrinological Investigation. 2006;29:270–280. doi: 10.1007/BF03345554. [DOI] [PubMed] [Google Scholar]
- Franks S. Polycystic ovary syndrome. New England Journal of Medicine. 1995;333:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- Foster DL, Jackson LM, Padmanabhan V. Novel concepts about normal sexual differentiation of reproductive neuroendocrine function and the developmental origins of female reproductive dysfunction: the sheep model. Society for Reproduction and Fertility Supplement. 2007;64:83–107. doi: 10.5661/rdr-vi-83. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Inskeep EI. Neuroendocrine Control of the Ovarian Cycle of the Sheep. In: Knobil, Neill, editors. Physiology of Reproduction. Third Edition Academic Press; New York: 2006. pp. 2389–2447. [Google Scholar]
- Gorski RA. Sexual differentiation of the brain: A model for drug—induced alterations of the reproductive system. Environmental Health Perspectives. 1986;70:163–175. doi: 10.1289/ehp.8670163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen LR, Drackley JK, Berger LL, Grum DE. Prenatal androgenization of lambs: I. Alterations of growth, carcass characteristics, and metabolites in blood. Journal of Animal Science. 1995;73:1694–1700. doi: 10.2527/1995.7361694x. [DOI] [PubMed] [Google Scholar]
- Harding JE, Bloomfield FH. Prenatal treatment of intrauterine growth restriction: Lessons from the sheep model. Pediatric Endocrinology Reviews. 2004;2:182–192. [PubMed] [Google Scholar]
- Hotchkiss AK, Rider CV, Blystone CR, Wilson VS, Hartig PC, Ankley GT, Foster PM, Gray CL, Gray LE. Fifteen years after “Wingspread”-environmental endocrine disrupters and human and wildlife health: where we are today and where we need to go. Toxicological Sciences. 2008;105:235–259. doi: 10.1093/toxsci/kfn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LM, Timmer KM, Foster D. Sexual differentiation of the external genitalia and the timing of puberty in the presence of an antiandrogen in sheep. Endocrinology. 2008;149:4200–4208. doi: 10.1210/en.2007-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost A, Vigier B, Prepin J, Perchellet J. Studies on sex differentiation in mammals. Recent Progress in Hormone Research. 1973;29:1–41. doi: 10.1016/b978-0-12-571129-6.50004-x. [DOI] [PubMed] [Google Scholar]
- King AJ, Olivier NB, MohanKumar PS, Lee JS, Padmanabhan V, Fink GD. CHBR: Hypertension caused by prenatal testosterone excess in female sheep. American Journal of Physiology Endocrinology and Metabolism. 2007;292:E1837–1841. doi: 10.1152/ajpendo.00668.2006. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal Programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–798. doi: 10.1210/en.2003-0478. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Steckler TS, Welch KB, Inskeep EK, Padmanabhan V. Fetal Programming: prenatal testosterone treatment leads to follicular persistence / luteal defects; partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology. 2006;147:1997–2007. doi: 10.1210/en.2005-1338. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Thompson RC, Herkimer C, Welch KB, Flak J, Karsch FJ, Padmanabhan V. Developmental Programming: impact of prenatal testosterone excess on pre- and postnatal gonadotropin regulation in sheep. Biology of Reproduction. 2008;78:648–660. doi: 10.1095/biolreprod.107.063347. [DOI] [PubMed] [Google Scholar]
- McNeilly A,S. The ovarian follicle and fertility. Journal of Steroid Biochemistry and Molecular Biology. 1991;40:29–33. doi: 10.1016/0960-0760(91)90164-z. [DOI] [PubMed] [Google Scholar]
- Mikhail N. The metabolic syndrome: insulin resistance. Current Hypertension Reports. 2009;11:156–158. doi: 10.1007/s11906-009-0027-4. [DOI] [PubMed] [Google Scholar]
- Nada S, Thompson RC, Padmanabhan V. Developmental programming: Differential effects of prenatal testosterone excess on insulin target tissues; Endocrinology Meeting 2009; Washington, D.C.. June 10-13, 2009; 2009. pp. P1–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . Hormonally active agents in the environment. National Academy Press; Washington D.C.: 1999. pp. 1–430. [PubMed] [Google Scholar]
- Ortega HH, Salvetti NR, Padmanabhan V. Developmental programming: prenatal androgen excess disrupts ovarian steroid receptor balance. Reproduction. 2009;137:865–877. doi: 10.1530/REP-08-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga Lopez, Abbott DH, Dumesic DA. Developmental programming of ovarian disruption. In: Gonzalez-Bulnes A, editor. Novel concepts in ovarian endocrinology. Research Signpost; India: 2007. pp. 329–352. [Google Scholar]
- Padmanabhan V, Siefert K, Ransom S, Johnson T, Pinkerton J, Anderson L, Tao L, Kannan K. Maternal bisphenol-A levels at delivery: a looming problem? Journal of Perinatology. 2008;28:258–263. doi: 10.1038/sj.jp.7211913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A, Abbott DH, Recabarren S, Herkimer C. Developmental programming: Impact of prenatal testosterone excess and postnatal weight gain on insulin sensitivity index and transfer of traits to offspring of overweight females. Endocrinology. doi: 10.1210/en.2009-1015. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi E. Environmental epigenomics meeting. Supplements restore gene function via methylation. Science. 2005;310:1761. doi: 10.1126/science.310.5755.1761. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Siefert K, Padmanabhan V. Bisphenol-A and disparities in birth outcomes: a review and directions for future research. Journal of Perinatology. 2009 doi: 10.1038/jp.2009.90. (Epub ahead of print) http://www.nature.com/jp/journal/vaop/ncurrent/abs/jp200990a.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recabarren SE, Padmanabhan V, Codner E, Lobos A, Durán C, Vidal M, Foster DL, Sir-Petermann T. Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. American Journal of Physiology Endocrinology and Metabolism. 2005;289:E801–806. doi: 10.1152/ajpendo.00107.2005. [DOI] [PubMed] [Google Scholar]
- Recabarren SE, Lobos A, Figueroa Y, Padmanabhan V, Foster DL, Sir-Petermann T. Prenatal testosterone treatment alters LH and testosterone responsiveness to GnRH agonist in male sheep. Biological Research. 2007;40:329–38. [PubMed] [Google Scholar]
- Recabarren SE, Rojas-García PP, Recabarren MP, Alfaro VH, Smith R, Padmanabhan V, Sir-Petermann T. Prenatal testosterone excess reduces sperm count and motility. Endocrinology. 2008;149:6444–6448. doi: 10.1210/en.2008-0785. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Forsdike RA, Taylor JA. In utero exposure of female lambs to testosterone reduces the sensitivity of the gonadotropin-releasing hormone neuronal network to inhibition by progesterone. Endocrinology. 1999;140:5797–5805. doi: 10.1210/endo.140.12.7205. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Schrunk JM, Stadelman HL, Resko JA, Stormshak F. The effect of aromatase inhibition on the sexual differentiation of the sheep brain. Endocrine. 2006;29:501–511. doi: 10.1385/ENDO:29:3:501. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Stadelman H, Reeve R, Bishop CV, Stormshak F. The ovine sexually dimorphic nucleus of the medial preoptic area is organized prenatally by testosterone. Endocrinology. 2007;148:4450–4457. doi: 10.1210/en.2007-0454. [DOI] [PubMed] [Google Scholar]
- Salian S, Doshi T, Vanage G. Perinatal exposure of rats to Bisphenol A affects the fertility of male offspring. Life Science. 2009 doi: 10.1016/j.lfs.2009.10.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Sam S, Dunaif A. Polycystic ovary syndrome: syndrome XX? Trends in Endocrinology and Metabolism. 2003;14:365–370. doi: 10.1016/j.tem.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Sarma HN, Manikkam M, Herkimer C, Dell’Orco J, Foster DL, Padmanabhan V. Fetal programming: excess prenatal testosterone reduces postnatal LH, but not FSH responsiveness to estradiol negative feedback in the female. Endocrinology. 2005;146:4281–4291. doi: 10.1210/en.2005-0322. [DOI] [PubMed] [Google Scholar]
- Savabieasfahani M, Kannan K, Astapova O, Evans N, Padmanabhan V. Developmental programming: differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function. Endocrinology. 2006;147:5956–5966. doi: 10.1210/en.2006-0805. [DOI] [PubMed] [Google Scholar]
- Savabieasfahani M, Lee JS, Herkimer C, Sharma TP, Foster DL, Padmanabhan V. Fetal Programming: Testosterone exposure of the female sheep during mid-gestation disrupts the dynamics of its adult gonadotropin secretion during the periovulatory period. Biology of Reproduction. 2005;72:221–229. doi: 10.1095/biolreprod.104.031070. [DOI] [PubMed] [Google Scholar]
- Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environmental Health Perspectives. 2002;110:A703–707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma TP, Herkimer C, West C, Ye W, Birch R, Robinson J, Foster DL, Padmanabhan V. Fetal programming: prenatal androgen excess disrupts positive feedback actions of estradiol but has no effect on timing of onset of puberty in female sheep. Biology of Reproduction. 2002;66:924–933. doi: 10.1095/biolreprod66.4.924. [DOI] [PubMed] [Google Scholar]
- Smith P, Steckler TL, Veiga-Lopez A, Padmanabhan V. Developmental programming: differential effects of prenatal testosterone and dihydrotestosterone on follicular recruitment and depletion of follicular reserve. Biology of Reproduction. 2009;80:726–736. doi: 10.1095/biolreprod.108.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub C, Hardy VB, Chapin RE, Harris MW, Johnson L. The hidden effect of estrogenic/antiandrogenic methoxychlor on spermatogenesis. Toxicology and Applied Pharmacology. 2002;180:129–135. doi: 10.1006/taap.2002.9369. [DOI] [PubMed] [Google Scholar]
- Steckler T, Manikkam M, Inskeep EK, Padmanabhan V. Developmental Programming: Follicular persistence in prenatal testosterone-treated sheep is not programmed by androgenic actions of testosterone. Endocrinology. 2007a;148:3532–3540. doi: 10.1210/en.2007-0339. [DOI] [PubMed] [Google Scholar]
- Steckler TL, Roberts EK, Doop DD, Lee TM, Padmanabhan V. Developmental programming in sheep: Administration of testosterone during 60 to 90 days of pregnancy reduces breeding success and pregnancy outcome. Theriogenology. 2007b;67:459–467. doi: 10.1016/j.theriogenology.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Steckler TL, Herkimer C, Dumesic DA, Padmanabhan V. Developmental Programming: excess weight gain amplifies the effects of prenatal testosterone excess on reproductive cyclicity-implication to PCOS. Endocrinology. 2009;150:1456–1465. doi: 10.1210/en.2008-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney T, Nicol L, Roche JF, Brooks AN. Maternal exposure to octylphenol suppresses ovine fetal follicle-stimulating hormone secretion, testis size, and sertoli cell number. Endocrinology. 2000;141:2667–2673. doi: 10.1210/endo.141.7.7552. [DOI] [PubMed] [Google Scholar]
- Sweeney T, Foxa J, Robertson L, Kelly G, Duffy P, Lonergan P, O’Doherty J, Roche JF, Evans NP. Postnatal exposure to octylphenol decreases semen quality in the adult ram. Theriogenology. 2007;67:1068–1075. doi: 10.1016/j.theriogenology.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Talsness CE, Andrade AJ, Kuriyama SN, Taylor JA, vom Saal FS. Components of plastic: experimental studies in animals and relevance for human health. Philosophical Transactions of the Royal Society London B: Biological Sciences. 2009;364:2079–2096. doi: 10.1098/rstb.2008.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WY, Newbold R, Mardilovich K, Jefferson W, Cheng RY, Medvedovic M, Ho SM. Persistent hypomethylation in the promoter of nucleosomal binding protein 1 (Nsbp1) correlates with overexpression of Nsbp1 in mouse uteri neonatally exposed to diethylstilbestrol or genistein. Endocrinology. 2008;149:5922–5931. doi: 10.1210/en.2008-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth WP, Taylor JA, Robinson JE. Prenatal programming of reproductive neuroendocrine function: the effect of prenatal androgens on the development of estrogen positive feedback and ovarian cycles in the ewe. Biology of Reproduction. 2005;72:619–627. doi: 10.1095/biolreprod.104.035691. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocrine Reviews. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Astapova OI, Aizenberg E, Lee JS, Padmanabhan V. Developmental programming: contribution of prenatal androgen and estrogen in organizing estradiol feedback systems and periovulatory hormonal dynamics in sheep. Biology of Reproduction. 2009;80:718–725. doi: 10.1095/biolreprod.108.074781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterland RA. Assessing the effects of high methionine intake on DNA methylation. Journal of Nutrition. 2006;136(6 Suppl):1706S–1710S. doi: 10.1093/jn/136.6.1706S. [DOI] [PubMed] [Google Scholar]
- West C, Foster DL, Evans NP, Robinson J, Padmanabhan V. Intra follicular activin availability is altered in prenatally-androgenized lambs. Molecular Cellular Endocrinology. 2001;185:51–59. doi: 10.1016/s0303-7207(01)00632-3. [DOI] [PubMed] [Google Scholar]
- Wright C, Evans AC, Evans NP, Duffy P, Fox J, Boland MP, Roche JF, Sweeney T. Effect of maternal exposure to the environmental estrogen, octylphenol, during fetal and/or postnatal life on onset of puberty, endocrine status, and ovarian follicular dynamics in ewe lambs. Biology of Reproduction. 2002;67:1734–1740. doi: 10.1095/biolreprod.101.002006. [DOI] [PubMed] [Google Scholar]
- Wood RI, Foster DL. Sexual differentiation of reproductive neuroendocrine function in sheep. Reviews in Reproduction. 1998;3:130–140. doi: 10.1530/ror.0.0030130. [DOI] [PubMed] [Google Scholar]