Abstract
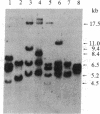
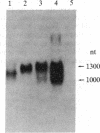
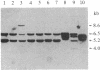
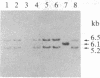
The functionally defined sets of T lymphocytes--helper T cells, cytotoxic T cells, and suppressor T cells--were examined for the possible involvement of a recently identified T-cell receptor beta gene locus in receptor formation. Since gene rearrangements are required for functional gene expression, cloned T-cell lines from each of the groups were surveyed for the expression of unique gene rearrangements. In addition, cell lines that showed gene rearrangements were further tested for the expression of the mature 1.2- to 1.3-kilobase mRNA transcribed from a productive gene rearrangement. The results of such experiments show that helper and cytotoxic T cells may use a common beta chain of the receptor, whereas suppressor cells do so rarely, if at all.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acuto O., Fabbi M., Smart J., Poole C. B., Protentis J., Royer H. D., Schlossman S. F., Reinherz E. L. Purification and NH2-terminal amino acid sequencing of the beta subunit of a human T-cell antigen receptor. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3851–3855. doi: 10.1073/pnas.81.12.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuto O., Hussey R. E., Fitzgerald K. A., Protentis J. P., Meuer S. C., Schlossman S. F., Reinherz E. L. The human T cell receptor: appearance in ontogeny and biochemical relationship of alpha and beta subunits on IL-2 dependent clones and T cell tumors. Cell. 1983 Oct;34(3):717–726. doi: 10.1016/0092-8674(83)90528-7. [DOI] [PubMed] [Google Scholar]
- Bevan M. J. The major histocompatibility complex determines susceptibility to cytotoxic T cells directed against minor histocompatibility antigens. J Exp Med. 1975 Dec 1;142(6):1349–1364. doi: 10.1084/jem.142.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y. H., Gascoigne N. R., Kavaler J., Lee N. E., Davis M. M. Somatic recombination in a murine T-cell receptor gene. Nature. 1984 May 24;309(5966):322–326. doi: 10.1038/309322a0. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Chien Y. H., Gascoigne N. R., Hedrick S. M. A murine T cell receptor gene complex: isolation, structure and rearrangement. Immunol Rev. 1984 Oct;81:235–258. doi: 10.1111/j.1600-065x.1984.tb01113.x. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Kim S. K., Hood L. E. DNA sequences mediating class switching in alpha-immunoglobulins. Science. 1980 Sep 19;209(4463):1360–1365. doi: 10.1126/science.6774415. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Gascoigne N. R., Chien Y., Becker D. M., Kavaler J., Davis M. M. Genomic organization and sequence of T-cell receptor beta-chain constant- and joining-region genes. Nature. 1984 Aug 2;310(5976):387–391. doi: 10.1038/310387a0. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Ju S. T., Kipps T. J., Benacerraf B., Dorf M. E. Shared idiotypic determinants on antibodies and T-cell-derived suppressor factor specific for the random terpolymer L-glutamic acid60-L-alanine30-L-tyrosine10. J Exp Med. 1979 Mar 1;149(3):613–622. doi: 10.1084/jem.149.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher L. H., Shevach E. M. Production of autoreactive I region-restricted T cell hybridomas. J Exp Med. 1982 Aug 1;156(2):640–645. doi: 10.1084/jem.156.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber-Katz E., Hansburg D., Schwartz R. H. The Ia molecule of the antigen-presenting cell plays a critical role in immune response gene regulation of T cell activation. J Mol Cell Immunol. 1983;1(1):3–18. [PubMed] [Google Scholar]
- Heber-Katz E., Schwartz R. H., Matis L. A., Hannum C., Fairwell T., Appella E., Hansburg D. Contribution of antigen-presenting cell major histocompatibility complex gene products to the specificity of antigen-induced T cell activation. J Exp Med. 1982 Apr 1;155(4):1086–1099. doi: 10.1084/jem.155.4.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Matis L. A., Hecht T. T., Samelson L. E., Longo D. L., Heber-Katz E., Schwartz R. H. The fine specificity of antigen and Ia determinant recognition by T cell hybridoma clones specific for pigeon cytochrome c. Cell. 1982 Aug;30(1):141–152. doi: 10.1016/0092-8674(82)90020-4. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Nielsen E. A., Kavaler J., Cohen D. I., Davis M. M. Sequence relationships between putative T-cell receptor polypeptides and immunoglobulins. Nature. 1984 Mar 8;308(5955):153–158. doi: 10.1038/308153a0. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Djeu J., Kay H. D., Ortaldo J. R., Riccardi C., Bonnard G. D., Holden H. T., Fagnani R., Santoni A., Puccetti P. Natural killer cells: characteristics and regulation of activity. Immunol Rev. 1979;44:43–70. doi: 10.1111/j.1600-065x.1979.tb00267.x. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Korsmeyer S. J., Waldmann T. A., Leder P. Human immunoglobulin kappa light-chain genes are deleted or rearranged in lambda-producing B cells. Nature. 1981 Apr 2;290(5805):368–372. doi: 10.1038/290368a0. [DOI] [PubMed] [Google Scholar]
- Hünig T., Bevan M. J. Specificity of T-cell cones illustrates altered self hypothesis. Nature. 1981 Dec 3;294(5840):460–462. doi: 10.1038/294460a0. [DOI] [PubMed] [Google Scholar]
- Kapp J. A., Araneo B. A., Clevinger B. L. Suppression of antibody and T cell proliferative responses to L-glutamic acid60-L-alanine30-L-tyrosine10 by a specific monoclonal T cell factor. J Exp Med. 1980 Jul 1;152(1):235–240. doi: 10.1084/jem.152.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Marrack P. C. Helper T cells recognise antigen and macrophage surface components simultaneously. Nature. 1976 Aug 26;262(5571):797–799. doi: 10.1038/262797a0. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J., Kubo R., Haskins K., White J., Marrack P. The mouse T cell receptor: comparison of MHC-restricted receptors on two T cell hybridomas. Cell. 1983 Oct;34(3):727–737. doi: 10.1016/0092-8674(83)90529-9. [DOI] [PubMed] [Google Scholar]
- Kaufmann Y., Berke G., Eshhar Z. Cytotoxic T lymphocyte hybridomas that mediate specific tumor-cell lysis in vitro. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2502–2506. doi: 10.1073/pnas.78.4.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann Y., Berke G. Monoclonal cytotoxic T lymphocyte hybridomas capable of specific killing activity, antigenic responsiveness, and inducible interleukin secretion. J Immunol. 1983 Jul;131(1):50–56. [PubMed] [Google Scholar]
- Kaye J., Porcelli S., Tite J., Jones B., Janeway C. A., Jr Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med. 1983 Sep 1;158(3):836–856. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori J. A., Winoto A., McNicholas J., Hood L. Molecular characterization of the recombination region of six murine major histocompatibility complex (MHC) I-region recombinants. J Mol Cell Immunol. 1984;1(2):125–135. [PubMed] [Google Scholar]
- Kraig E., Kronenberg M., Kapp J. A., Pierce C. W., Abruzzini A. F., Sorensen C. M., Samelson L. E., Schwartz R. H., Hood L. E. T and B cells that recognize the same antigen do not transcribe similar heavy chain variable region gene segments. J Exp Med. 1983 Jul 1;158(1):192–209. doi: 10.1084/jem.158.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz D. M., Sherman D. H., Sitkovsky M. V., Pasternack M. S., Eisen H. N. Immunoprecipitation of cell surface structures of cloned cytotoxic T lymphocytes by clone-specific antisera. Proc Natl Acad Sci U S A. 1984 Jan;81(2):573–577. doi: 10.1073/pnas.81.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M., Steinmetz M., Kobori J., Kraig E., Kapp J. A., Pierce C. W., Sorensen C. M., Suzuki G., Tada T., Hood L. RNA transcripts for I-J polypeptides are apparently not encoded between the I-A and I-E subregions of the murine major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5704–5708. doi: 10.1073/pnas.80.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N. E., D'Eustachio P., Pravtcheva D., Ruddle F. H., Hedrick S. M., Davis M. M. Murine T cell receptor beta chain is encoded on chromosome 6. J Exp Med. 1984 Sep 1;160(3):905–913. doi: 10.1084/jem.160.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matis L. A., Longo D. L., Hedrick S. M., Hannum C., Margoliash E., Schwartz R. H. Clonal analysis of the major histocompatibility complex restriction and the fine specificity of antigen recognition in the T cell proliferative response to cytochrome C. J Immunol. 1983 Apr;130(4):1527–1535. [PubMed] [Google Scholar]
- McIntyre B. W., Allison J. P. The mouse T cell receptor: structural heterogeneity of molecules of normal T cells defined by xenoantiserum. Cell. 1983 Oct;34(3):739–746. doi: 10.1016/0092-8674(83)90530-5. [DOI] [PubMed] [Google Scholar]
- Melchers F., Zeuthen J., Gerhard W. Influenza virus-specific murine T cell hybridomas which recognize virus hemagglutinin in conjunction with H-2d and display helper functions for B cells. Curr Top Microbiol Immunol. 1982;100:153–163. doi: 10.1007/978-3-642-68586-6_18. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Acuto O., Hussey R. E., Hodgdon J. C., Fitzgerald K. A., Schlossman S. F., Reinherz E. L. Evidence for the T3-associated 90K heterodimer as the T-cell antigen receptor. Nature. 1983 Jun 30;303(5920):808–810. doi: 10.1038/303808a0. [DOI] [PubMed] [Google Scholar]
- Minami M., Aoki I., Honji N., Waltenbaugh C. R., Dorf M. E. The role of I-J and Igh determinants on F1-derived suppressor factor in controlling restriction specificity. J Exp Med. 1983 Nov 1;158(5):1428–1443. doi: 10.1084/jem.158.5.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K., Sugimura K., Yaoita Y., Maeda K., Kashiwamura S., Honjo T., Kishimoto T. A T15-idiotype-positive T suppressor hybridoma does not use the T15 VH gene segment. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6984–6988. doi: 10.1073/pnas.79.22.6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Minami M., Furusawa M., Dorf M. E. Analysis of T cell hybridomas. II. Comparisons among three distinct types of monoclonal suppressor factors. J Exp Med. 1981 Dec 1;154(6):1838–1851. doi: 10.1084/jem.154.6.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Minami M., Sherr D. H., Dorf M. E. Hapten-specific T cell responses to 4-hydroxy-3-nitrophenyl acetyl. XI. Pseudogenetic restrictions of hybridoma suppressor factors. J Exp Med. 1981 Aug 1;154(2):468–479. doi: 10.1084/jem.154.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternack M. S., Bevan M. J., Klein J. R. Release of discrete interferons by cytotoxic T lymphocytes in response to immune and nonimmune stimuli. J Immunol. 1984 Jul;133(1):277–280. [PubMed] [Google Scholar]
- Paul W. E., Benacerraf B. Functional specificity of thymus- dependent lymphocytes. Science. 1977 Mar 25;195(4284):1293–1300. doi: 10.1126/science.320663. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Schwartz R. H. The use of antisera and monoclonal antibodies to identify the antigen-specific T cell receptor from pigeon cytochrome c-specific T cell hybrids. Immunol Rev. 1983;76:59–78. doi: 10.1111/j.1600-065x.1983.tb01097.x. [DOI] [PubMed] [Google Scholar]
- Sorensen C. M., Pierce C. W. Antigen-specific suppression in genetic responder mice to L-glutamic acid60-L-alanine30-L-tyrosine10 (GAT). Characterization of conventional and hybridoma-derived factors produced by suppressor T cells from mice injected as neonates with syngeneic GAT macrophages. J Exp Med. 1982 Dec 1;156(6):1691–1710. doi: 10.1084/jem.156.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen C. M., Pierce C. W. Haplotype-specific suppression of antibody responses in vitro. II. Suppressor factor produced by T cells and T cell hybridomas from mice treated as neonates with semiallogeneic spleen cells. J Exp Med. 1981 Jul 1;154(1):48–59. doi: 10.1084/jem.154.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sprent J. Restricted helper function of F1 hybrid T cells positively selected to heterologous erythrocytes in irradiated parental strain mice. I. Failure to collaborate with B cells of the opposite parental strain not associated with active suppression. J Exp Med. 1978 Apr 1;147(4):1142–1158. doi: 10.1084/jem.147.4.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staerz U. D., Pasternack M. S., Klein J. R., Benedetto J. D., Bevan M. J. Monoclonal antibodies specific for a murine cytotoxic T-lymphocyte clone. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1799–1803. doi: 10.1073/pnas.81.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Minard K., Horvath S., McNicholas J., Srelinger J., Wake C., Long E., Mach B., Hood L. A molecular map of the immune response region from the major histocompatibility complex of the mouse. Nature. 1982 Nov 4;300(5887):35–42. doi: 10.1038/300035a0. [DOI] [PubMed] [Google Scholar]
- Taniguchi M., Tokuhisa T., Itoh T., Kanno M. Functional roles of two polypeptide chains that compose an antigen-specific suppressor T cell factor. J Exp Med. 1984 Apr 1;159(4):1096–1104. doi: 10.1084/jem.159.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K., Chao N., Murphy D. B., Gershon R. K. Molecular composition of an antigen-specific, Ly-1 T suppressor inducer factor. One molecule binds antigen and is I-J-; another is I-J+, does not bind antigen, and imparts an Igh-variable region-linked restriction. J Exp Med. 1982 Mar 1;155(3):655–665. doi: 10.1084/jem.155.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi Y., Yoshikai Y., Leggett K., Clark S. P., Aleksander I., Mak T. W. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984 Mar 8;308(5955):145–149. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. H-2 compatability requirement for T-cell-mediated lysis of target cells infected with lymphocytic choriomeningitis virus. Different cytotoxic T-cell specificities are associated with structures coded for in H-2K or H-2D;. J Exp Med. 1975 Jun 1;141(6):1427–1436. doi: 10.1084/jem.141.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]