Abstract
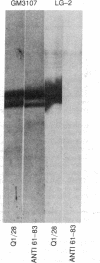
A chemically synthesized peptide (sequence in text) homologous to residues 61-83 of the HLA-B7 heavy chain, induced antibodies that specifically recognized the HLA heavy chain-beta 2-microglobulin complex and the free heavy chain of the HLA-B7 antigen. These antibodies specifically immunoprecipitated the HLA-B7 beta 2-microglobulin complex solubilized from human lymphoblastoid cells by nonionic detergents and reacted with free HLA-B7 heavy chains in blots on nitrocellulose. These observations suggest that the antigenic conformation of this region of the HLA-B7 molecule is independent of the presence of beta 2-microglobulin and that amino acid residues 61-83 mimic an alloreactive site expressed by the HLA-B7 antigen.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J. P., Ferrone S., Walker L. E., Pellegrino M. A., Silver J., Reisfeld R. A. Partial amino acid sequence of HLA-A9 antigen purified with a specific xenoantiserum. Transplantation. 1978 Dec;26(6):451–454. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Callewaert D. M., Smeekens S. P., Mahle N. H. Improved quantification of cellular cytotoxicity reactions: determination of kinetic parameters for natural cytotoxicity by a distribution-free procedure. J Immunol Methods. 1982;49(1):25–37. doi: 10.1016/0022-1759(82)90363-5. [DOI] [PubMed] [Google Scholar]
- Church W. R., Walker L. E., Houghten R. A., Reisfeld R. A. Anti-HLA antibodies of predetermined specificity: a chemically synthesized peptide induces antibodies specific for HLA-A,B heavy chain. Proc Natl Acad Sci U S A. 1983 Jan;80(1):255–258. doi: 10.1073/pnas.80.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghten R. A., Chang W. C., Li C. H. Human beta-endorphin: synthesis and characterization of analogs iodinated and tritiated at tyrosine residues 1 and 27. Int J Pept Protein Res. 1980 Oct;16(4):311–320. doi: 10.1111/j.1399-3011.1980.tb02592.x. [DOI] [PubMed] [Google Scholar]
- Jordan B. R., Bregegere F., Kourilsky P. Human HLA gene segment isolated by hybridization with mouse H-2 cDNA probes. Nature. 1981 Apr 9;290(5806):521–523. doi: 10.1038/290521a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu F. T., Zinnecker M., Hamaoka T., Katz D. H. New procedures for preparation and isolation of conjugates of proteins and a synthetic copolymer of D-amino acids and immunochemical characterization of such conjugates. Biochemistry. 1979 Feb 20;18(4):690–693. doi: 10.1021/bi00571a022. [DOI] [PubMed] [Google Scholar]
- López de Castro J. A., Strominger J. L., Strong D. M., Orr H. T. Structure of crossreactive human histocompatibility antigens HLA-A28 and HLA-A2: possible implications for the generation of HLA polymorphism. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3813–3817. doi: 10.1073/pnas.79.12.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López de Castro J., Bragado R., Strong D. M., Strominger J. L. Primary structure of papain-solubilized human histocompatibility antigen HLA-B40 (-Bw60). An outline of alloantigenic determinants. Biochemistry. 1983 Aug 2;22(16):3961–3969. doi: 10.1021/bi00285a036. [DOI] [PubMed] [Google Scholar]
- Orr H. T., López de Castro J. A., Lancet D., Strominger J. L. Complete amino acid sequence of a papain-solubilized human histocompatibility antigen, HLA-B7. 2. Sequence determination and search for homologies. Biochemistry. 1979 Dec 11;18(25):5711–5720. doi: 10.1021/bi00592a030. [DOI] [PubMed] [Google Scholar]
- Parham P., Barnstable C. J., Bodmer W. F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979 Jul;123(1):342–349. [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Molecular cloning of a human histocompatibility antigen cDNA fragment. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6081–6085. doi: 10.1073/pnas.77.10.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaranta V., Walker L. E., Ruberto G., Pellegrino M. A., Ferrone S. The free and the beta 2-microglobulin-associated heavy chains of HLA-A, B alloantigens share the antigenic determinant recognized by the monoclonal antibody Q1/28. Immunogenetics. 1981;13(4):285–295. doi: 10.1007/BF00364494. [DOI] [PubMed] [Google Scholar]
- Rose G. D. Prediction of chain turns in globular proteins on a hydrophobic basis. Nature. 1978 Apr 13;272(5654):586–590. doi: 10.1038/272586a0. [DOI] [PubMed] [Google Scholar]
- Schulz G., Bumol T. F., Reisfeld R. A. Monoclonal antibody-directed effector cells selectively lyse human melanoma cells in vitro and in vivo. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5407–5411. doi: 10.1073/pnas.80.17.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodoyer R., Damotte M., Delovitch T. L., Trucy J., Jordan B. R., Strachan T. Complete nucleotide sequence of a gene encoding a functional human class I histocompatibility antigen (HLA-CW3). EMBO J. 1984 Apr;3(4):879–885. doi: 10.1002/j.1460-2075.1984.tb01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood A. K., Pereira D., Weissman S. M. Isolation and partial nucleotide sequence of a cDNA clone for human histocompatibility antigen HLA-B by use of an oligodeoxynucleotide primer. Proc Natl Acad Sci U S A. 1981 Jan;78(1):616–620. doi: 10.1073/pnas.78.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]