Abstract
Biotinidase deficiency is an autosomal recessively inherited disorder characterized by neurological and cutaneous abnormalities. We have developed a transgenic knock-out mouse with biotinidase deficiency to better understand aspects of pathophysiology and natural history of the disorder in humans. Neurological deficits observed in symptomatic mice with biotinidase deficiency are similar to those seen in symptomatic children with the disorder. Using a battery of functional neurological assessment tests, the symptomatic mice performed poorly compared to wild-type mice. Demyelination, axonal degeneration, ventriculomegaly, and corpus callosum compression were found in the brains of untreated, symptomatic enzyme-deficient mice. With biotin treatment, the symptomatic mice improved neurologically and the white matter abnormalities resolved. These functional and anatomical findings and their reversal with biotin therapy are similar to those observed in untreated, symptomatic and treated individuals with biotinidase deficiency. The mouse with biotinidase deficiency appears to be an appropriate animal model in which to study the neurological abnormalities and the effects of treatment of the disorder.
Keywords: Biotin, Biotin-responsive metabolic disorders, Biotinidase deficiency, Multiple carboxylase, Deficiency, Motor neuron impairment, Somatosensory impairment, Axonal damage, Transgenic knockout model
Introduction
Biotin is the coenzyme for all four human biotin dependent carboxylases: propionyl-CoA carboxylase (PCC), β-methylcrotonyl-CoA carboxylase (MCC), pyruvate carboxylase (PC), and acetyl-CoA carboxylase (ACC) (Moss and Lane, 1971). PCC is involved in the catabolism of several branched-chain amino-acids and odd-carbon chain fatty acids by converting propionyl-CoA tomethylmalonyl-CoA, which then enters the tricarboxylic acid cycle. MCC is involved in leucine catabolism by the conversion of β-methylcrotonyl-CoA to β-methylglutaconyl-CoA. PC catalyzes the conversion of pyruvate to oxaloacetate, an intermediate in the biosynthesis of phosphoenolpyruvate and, ultimately, to glucose. ACC catalyzes the formation of malonyl-CoA from acetyl-CoA, the first committed step in the biosynthesis of fatty acid. Deficiencies of the carboxylases and the consequential accumulation of abnormal metabolic intermediates have profound effect on other pathways resulting in severe clinical consequences (Wolf and Feldman, 1982). Holocarboxylase synthetase (HCS) catalyzes the covalent attachment of biotin to ε-amino groups of the conserved lysyl-residues of apocarboxylases; thereby transforming apocarboxylases to holocarboxylases (Sweetman, 1981). Biocytin (biotinyl-ε-lysine) and small biotinyl-peptides are released during the proteolytic turnover of the holocarboxylases. Biotinidase hydrolyzes biocytin and small biotinyl-peptides, thus recycling free biotin (Craft et al., 1985; Pispa, 1965). Thus, children with genetic defect in either HCS or biotinidase results in multiple carboxylase deficiency (MCD).
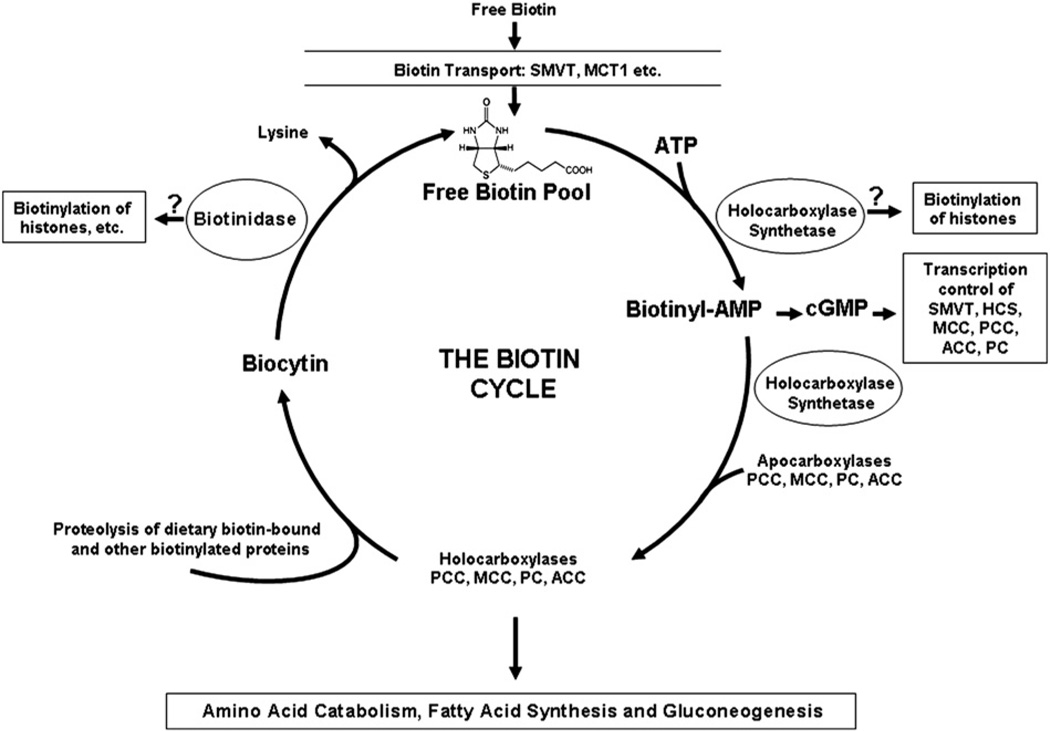
Biotin deficiency in humans is rare because biotin is continuously being recycled by the action of biotinidase. Biotin is also obtained exogenously from the diet, either directly as free biotin from sources, such as fruits and vegetables, or in a protein-conjugated form which must be made bioavailable by biotinidase. The quantity of free biotin that comes directly from the diet is small. Thus, biotinidase plays a crucial role in maintaining the free biotin pool in the body and deficient biotinidase activity results in late-onset MCD in children. The known and putative functions of biotinidase are shown in the biotin cycle in Fig. 1.
Fig. 1.
The biotin cycle and the known and putative roles. Free, unbound, biotin is the coenzyme for four carboxylases in humans and mice: propionyl-CoA carboxylase (PC), β-methylcrotonyl-CoA carboxylase (MCC), pyruvate carboxylase (PC), and acetyl-CoA carboxylase (ACC). Holocarboxylase synthetase (HCS) catalyzes the covalent attachment of free biotin to apocarboxylases forming holocarboxylases. The holocarboxylases participate in amino acid catabolism, fatty acid synthesis, and gluconeogenesis. Biotinidase releases biotin from biocytin and/or biotinylated peptides generated from the proteolytic turnover of holocarboxylases, thereby recycling biotin and resupplying the free biotin pool. Biotinidase also mediates the release of biotin from biocytin and/or biotinylated peptides generated by the proteolysis of dietary proteins. Biotinidase may posttranslationally modify histones by biotinylating them in the presence of biocytin and removing the biotinyl moiety from histones. HCS can also biotinylate histones and produces biotinyl-AMP, which can then participate in the cGMP signaling pathway regulating gene expression. Free biotin is absorbed in intestine, recaptured in the kidney and transported in the cytoplasm by transporter proteins, sodium-dependent multivitamin transporter (SMVT) and monocarboxylate transporter 1 (MCT1).
Biotinidase deficiency, if untreated, can cause serious neurological and cutaneous consequences (Wolf, 2001; Wolf et al., 1983a). Children with profound biotinidase deficiency (less than 10% of mean normal serum activity) can exhibit neurological symptoms, including hypotonia, seizures, ataxia, optic atrophy, sensorineural hearing loss, spastic paraparesis and developmental delay (Wolf, 1992). Metabolic acidosis, hyperammonemia and/or organic acidemia/uria are common biochemical features of symptomatic children. Although many symptomatic children show no central nervous system (CNS) abnormalities during radiological investigation, the most commonly reported abnormal findings are CNS atrophy, decreased white matter mass and ventricular enlargement. Affected children respond markedly to biotin treatment; however, a delay in diagnosis and failure to rapidly initiate therapy can result in life-long neurological deficits, such as hearing loss, impaired visual acuity, and developmental delay (Wolf, 2001). To prevent irreversible consequences of the disorder, newborn screening for biotinidase deficiency is incorporated into essentially all newborn screening programs in the United States and in many countries throughout the world (Wolf, 1991;Wolf and Heard, 1990).
With the advent of newborn screening for biotinidase deficiency, most children with the disorder are identified presymptomatically, biotin therapy is initiated, and the adverse effects of the disorder are prevented. Newborn screening has also closed the window-of-opportunity to study the natural history of the disorder. Therefore, in order to study the neuropathophysiology of biotinidase deficiency we have developed a transgenic knock-out mouse with profound biotinidase deficiency (Pindolia et al., 2011). Mice with biotinidase deficiency exhibit many of the clinical and biochemical characteristics of untreated children with biotinidase deficiency and, when symptomatic, similarly respond to biotin treatment. In the current study, we have characterized the clinical neurological deficits and the alterations in the brain of symptomatic mice and have demonstrated their reversibility with biotin treatment.
Material and methods
Mouse with biotinidase deficiency
The development and characterization of the biotinidase-deficient (BD) mouse used in this study has been described previously by Pindolia et al. (2011). Briefiy, the chimera with the gene knock-out was generated followed by initial backcross with C57BL/6 background mouse for 10 generations to obtain the biotinidase gene knock-out mouse with C57BL/6 genetic background. Approval was obtained from the Institutional Animal Care and Use Committee (IACUC) for maintaining the breeding colonies. Standard breeder diet (Harlan Teklad, Madison, WI) was fed to the mice used for breeding until the litters were weaned and used for specific experiments described below. Both the BD and wildtype (WT) mice were obtained from the breeding colonies.
Animal care, housing and dietary regiment
IACUC approval was obtained for all procedures performed in this project. All mice were maintained under standard housing conditions in a 12-hour light–dark cycle with free access to food and water. All mice, regardless of their biotinidase activity status, remain asymptomatic when fed a standard rodent diet that contains the daily requirement of 0.2 mg/kg of biotin. Only the BD mice develop the disease specific symptoms after they are fed a custom-formulated, biotin-deficient diet for two weeks. The biotin-deficient diet contains essentially no biotin (far less than the detection limit of 0.007 mg/kg) which is prepared with alcohol-extracted casein as the sole source of protein. These symptomatic BD mice are designated as BDs mice. WT mice remained asymptomatic when fed the biotin-deficient diet for the same period of time. A group of BDs mice were treated with 100 µg biotin/kg body weights in 100 µl of saline by injection each day (designated BDt).
Weight measurements
Three-week-old WT and BD mice were placed on the biotin-deficient diet immediately after being weaned. All mice were weighed twice per week and monitored for their overall health and growth.
Flexion assessments
Flexion assessment is another form of measurement used to assess functional neurological status (Marklund et al., 2009). The mouse is held by the tail while freely suspended. The score is based on movement of the following three flexions: forelimb, hindlimb, and head. Scores are 0 = normal with no flexion deficit; 1 = one flexion is deficient; 2 = two flexions are deficient; and 3 = all three flexions are deficient.
Motor-neuronal assessments
We adopted a general staging method to assess motor-neuron deficits based on gross observation of motor neuron function as previously described (Zhang et al., 2008). Mice were assigned to one of six stages of physical impairment: stage 0 = normal; stage 1 = loss of tail tone; stage 2 = hindlimb weakness; stage 3 = hindlimb paralysis; stage 4 = hind and forelimb paralysis; and stage 5 = moribund.
Foot-fault functional test
Mice were placed on an elevated (6.4 cm high) grid floor (48 cm × 4.5 cm with 1.0 cm2 openings) until 100 steps were taken. While walking on the grid, the mice typically place their paws on the wire frame for foot holds. With each weight-bearing step, the paw may fall or slip between the wires. This is referred to as “foot-fault.” The total steps taken on the grid using forelimb were counted and the total number of foot faults for each forelimb was recorded (Liu et al., 2011; Stroemer et al., 1998).
Pinna and corneal reflex
The pinna reflex was measured by observing a head shake or movement when the auditory meatus was touched. The corneal reflex was gauged by an eye blink when the cornea was lightly touched with fine cotton fibers. Reflex scoring has been described earlier (Paylor et al., 1999). Mice were scored as follows: 0 = No deficient response for both pinna and corneal reflex; 1 = one reflex, either corneal or pinna, is deficient; 2 = both corneal and pinna reflexes are deficient (Paylor et al., 1999).
Tissue preparation
For histological studies, mice were anesthetized on the 25th day after initiating the biotin-deficient diet. The brains of anesthetized mice were fixed by transcardial perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde before being removed and embedded in paraffin. The tissue was then cut into a series of six µm-thick sections at bregma (−1mm to +1mm). Every tenth coronal section for a total five sections was used for staining.
Ventriculomegaly assessment
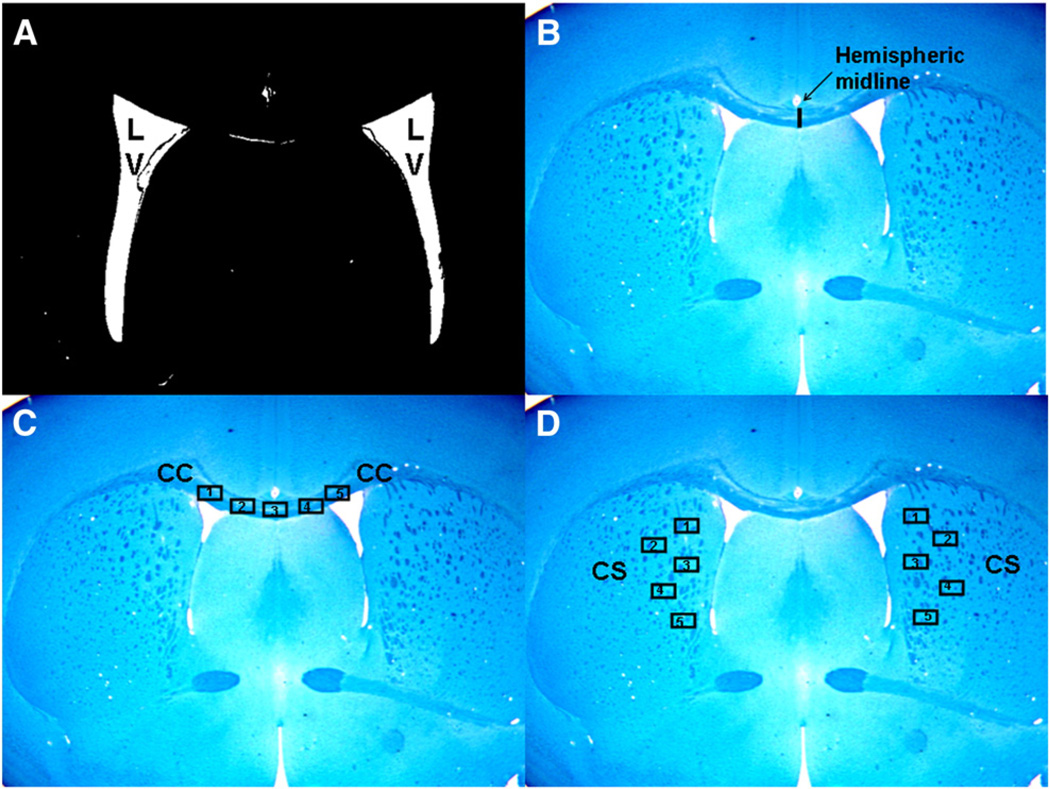
The brain sections were stained with Bielschowsky silver. Slides from WT and BDs mice were analyzed. Using the digitized images, lateral ventricle area from both sides was quantitatively analyzed using Image J software (downloaded from the NIH website) using a previously described procedure (Davis et al., 2007). In brief, the absolute scale was initially set using a hematocytometer and then the lateral ventricular areas were highlighted and measured (shown in Fig. 3A).
Fig. 3.
Ventriculomegaly and white matter assessments. Shown is the procedural guide for assessing CNS abnormality. Digitized images at low magnification (2×) depicting methodology for making ventriculomegaly assessments are shown in Figs. 3A and B. Assessments of myelination and axonal changes are depicted also at low magnification (2×) in Figs. 3C and D. Five distinct fields in CC region and five distinct fields in CS region were evaluated. The mean stained area of these fields were calculated for each mouse in the group and the final data were then plotted in the graph as shown in Fig. 6.
Using a previously described procedure, corpus callosum (CC) thickness at hemispheric midline was measured (Davis et al., 2007). Image J software was used to analyze WT mice and BDs mice. In brief, the absolute scale was first set using a hemocytometer followed by drawing a line along the hemispheric midline of the CC and measuring the length of the line. This line represented the CC thickness (shown in Fig. 3B).
Myelin and axonal staining
Axonal abundance was assessed by Bielschowsky silver staining and myelin abundance was assessed by Luxol fast-blue staining in the region of white matter bundles of the corpus striatum (CS) and CC (Irving et al., 2001). Briefly, for Bielschowsky silver staining, slides were placed in 20% silver nitrate in the dark and followed by sequential treatment with sodium hydroxide and sodium thiosulfate. For Luxol Fast Blue staining, slides were stained in Luxol fast blue solution, washed in 95% alcohol, and then placed in lithium carbonate.
Quantitative assessment of myelin and axons
Bielschowsky silver and Luxol fast blue stained slides were photographed and digitized under a 20 × objective (Olympus BX40) using a 3-CCD color camera (Sony DXC-970MD) interfaced with an MCID image analysis system (Imaging Research, St. Catherines, Canada). The contrasts for the digitized images were set at a point that would allow calculating the quantity of stained material in each axonal bundle. The axons and myelin present in each axonal bundle was assessed by measuring stained area for Bielschowsky silver and Luxol fast blue, respectively. Data were analyzed in a blinded manner according to a published protocol (Calza et al., 2002; Chen et al., 2003). Five separate fields were analyzed and the mean value calculated for each slide and then for each animal. The final data are presented as the mean percent of positive area (±SEM) for all the animals in a particular experimental group.
Statistical analysis
For comparison of Ventriculomegaly and CC thickness in WT and BDs groups, Student's T-test was employed. One-way ANOVA analysis was used to compare the results of foot fault in WT, BDs, and BDt mice at day 25. Additionally, One-way ANOVA analysis was performed to establish the statistical significance among the mean percent of stained positive area (for myelin and axon in the CS and CC) of WT, BDs, and treated, biotinidase-deficient (BDt) mice. If an overall p<0.05 was found, then a Tukey–Kramer's post hoc test was used.
Results
BD mice become symptomatic when fed biotin-deficient diet
Three-week-old mice were placed on the biotin-deficient diet for 25 days. As described in the Material and methods section, regardless of their biotinidase activity status, all mice remained asymptomatic when fed a standard rodent diet. To produce symptoms in BD mice, it is necessary to feed them the biotin-deficient diet. All experimental mice were monitored for symptoms in addition to observing their general appearance, skin tone, fur loss and/or discoloration, degree of hydration, physical activity and weight measurements.
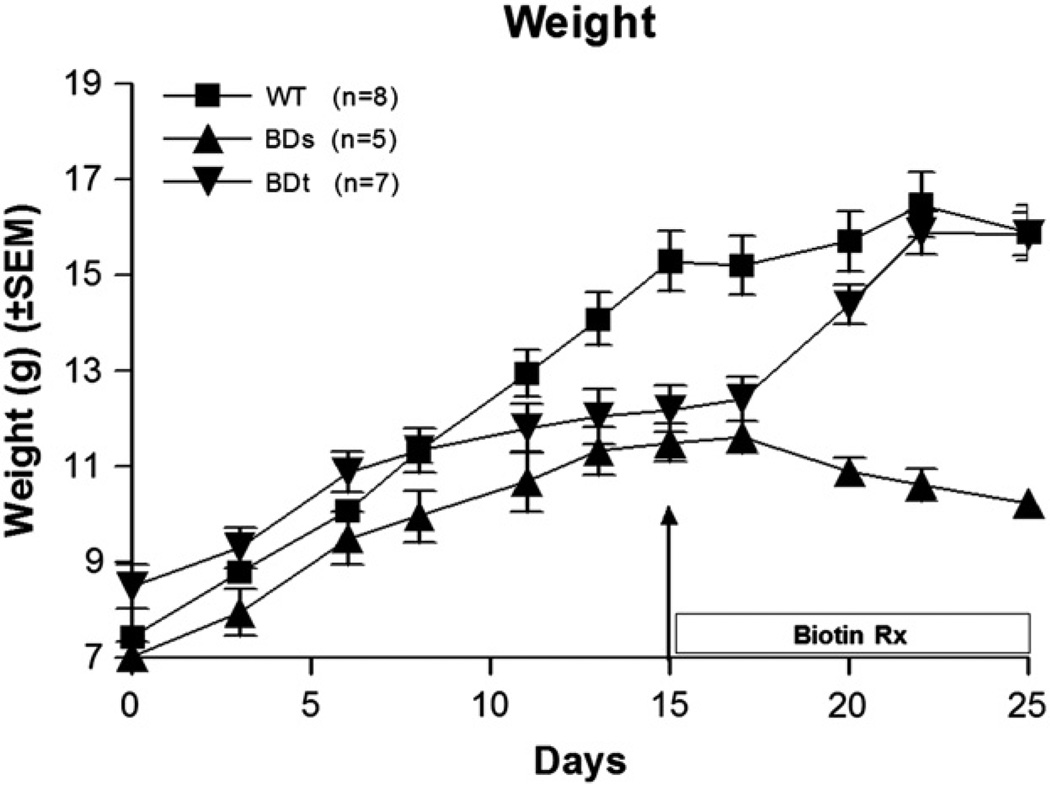
Weight measurements recorded with time on the biotin-deficient diet are shown in Fig. 2. All BD mice (n = 12) initially showed weight gain, but this tapered off considerably, as they become symptomatic, compared to the continuous weight gain of WT mice (n = 8). At day 15, seven BDs mice were treated with intra-peritoneal injections of biotin (100 µg/kg/day in 100 µl of normal saline), whereas five other BDs mice received intra-peritoneal injections of 100 µl of normal saline without biotin as a control for the BDt mice (n = 7). These mice began to regain the weight within a week of initiating treatment. By day 25, the weight of the BDt mice was comparable to that of WT mice whereas the weight of the BDs mice (n = 5) continued to deteriorate. At the end of the experiment, the mean weight of the BDs mice was 40% lighter than the WT and BDt mice.
Fig. 2.
Monitoring of weight changes as BD mice become symptomatic. Weight changes were monitored once the three-week-old weaned mice were fed biotin-deficient diet. At the start of the experiment the mice weight ranged from 7 to 8.5 g and BD mice continue to gain weight comparable to that of the WT mice. Weight gains for BD mice noticeably slowed down at about seven days and continued to do so until about day 15 as they became symptomatic. Once the biotin treatment was initiated, the BD symptomatic mice, labeled as BDt began to regain weight. On day 25, when the experiment was terminated, the weight of WT and BDt mice were both in the range of 15–16 g.
In contrast to the BDt mice that received biotin injections, BDs mice that were administered normal saline injections exhibited progressive graying of their fur and eventually, in some animals, developed alopecia. Graying of the fur began around the eyes and subsequently became more evident over the rest of the body. Hair loss in most cases began on the abdomen and spread to the remainder of the body. Biotin treatment resulted in marked improvement in skin tone and degree of hydration. In addition, many of the mice that developed alopecia began to grow back their body fur.
Ventriculomegaly and corpus callosum compression
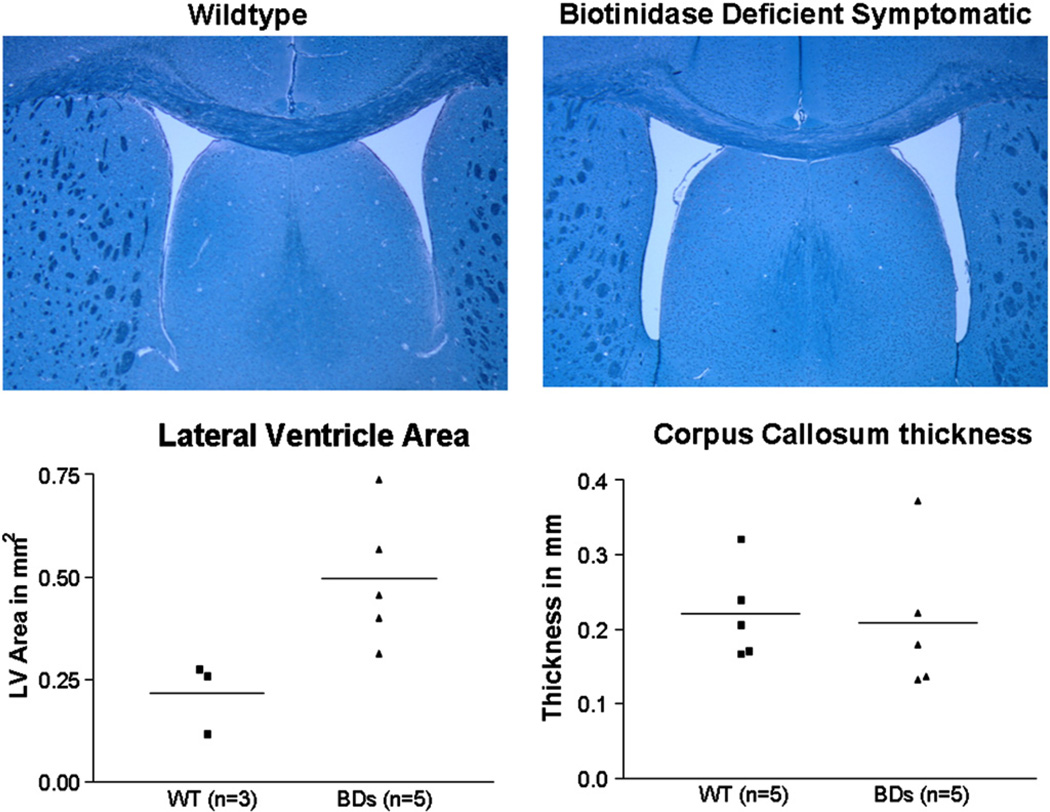
Changes in lateral ventricular area and compression of CC were noted in the histological brain samples. Representative images of the brain sections from WT and BDs mice are shown in Fig. 4. The mean area of the lateral ventricles in the brain slides of WT mice was 0.22 ± 0.05 mm2 (n = 3). The mean area of the BDs mice was larger at 0.50 ± 0.07 mm2 (n = 5). The difference in the areas of lateral ventricles between these two groups was statistically significant (p<0.05).
Fig. 4.
Ventriculomegaly and CC compression in neurologically compromised symptomatic mice (BDs mice). In the top panel, Luxol Fast blue staining of WT and BDs mice at 4× magnification are shown. Mean lateral ventricle volumes were greater in the brains of BDs mice compared to those of WT mice (p<0.05). Consequently, the CC thicknesses measured parasagittally, close to hemispheric midline, were also compressed in the BDs mice; however the statistical significance could not be established.
The mean thickness of CC in the brains of WT mice was 0.22 ± 0.03 mm (n = 5), whereas the mean thickness of CC of BDs mice was 0.21 ± 0.04 (n = 5). CC thickness varies considerably even within the WT mice. Although, there was a mean decrease in CC thickness in the BDs mice, the degree of compression was still within the low normal range. For the mean compression of CC it was not possible to obtain statistical significance.
BD mice develop reversible neurological deficits
WT mice remained asymptomatic throughout the 25 days that they were on biotin-deficient diet, whereas BD mice began to develop symptoms at around 10 to 12 days. The symptoms progressively worsened with time in five mice out of the 12 BD mice that were not treated with biotin. The non-treated symptomatic mice appeared lethargic, displayed varying degrees of tremors and hypotonia, graying of their fur and, in the most cases, eventual fur loss.
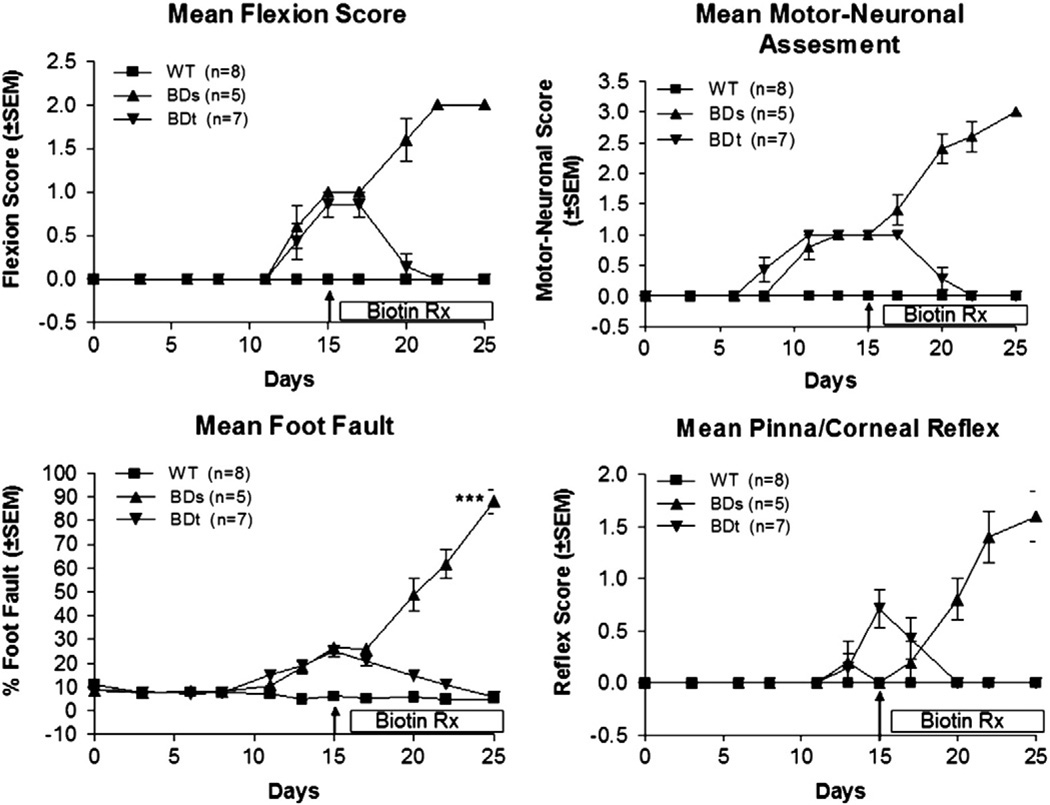
Functional neuronal deficits were assessed by foot-fault, flexion, pinna/corneal reflex, and motor-neuron score throughout the experimental period. Neurological deficits, based on the four functional neurological assessment tests, were observed in all BD mice beginning between the 8th and 15th day as shown in Fig. 5. In general, loss of pinna/corneal reflex and loss of flexion occurred relatively late, at about the 10th to 15th days, compared to the development of foot fault and the motor-neuronal deficits. Worsening of pinna/corneal reflex and loss of flexion became more pronounced after about the 15th day on the biotin-deficient diet. All neurological deficits worsened through day 25 when the experiment was eventually terminated.
Fig. 5.
Neuronal deficit assessment. Functional neuronal deficits were assessed by a battery of functional neurological assessment tests (flexion, motor-neuron scoring, foot fault and pinna/corneal reflex). The mean values (±SEM) for the four different assessments are plotted versus the days the mice were fed a biotin-deficient diet. The differences in mean neurological deficit, when compared between WT and BDs mice, became apparent in about two weeks on the diet and they were pronounced at day 25. These neurological deficits improved after biotin treatment, as seen by comparison of BDs and BDt group of mice at day 25.
The remaining seven symptomatic BD mice were treated with biotin. As shown in Fig. 5, BDt mice showed improvement of all of their neurological deficits within a week of initiating biotin treatment with continuous improvements in overall behavior and general appearance. At the end of the experiment, after 10 days of biotin treatment, BDt mice performed essentially the same as the WT mice. Conversely, the BDs mice, that received intra-peritoneal injections of normal saline without biotin, continued to perform progressively worse on their neurological assessments scores. These results clearly demonstrated the efficacy of biotin treatment in reversing the neurological deficits.
Neurological deficit is consistent with myelopathy
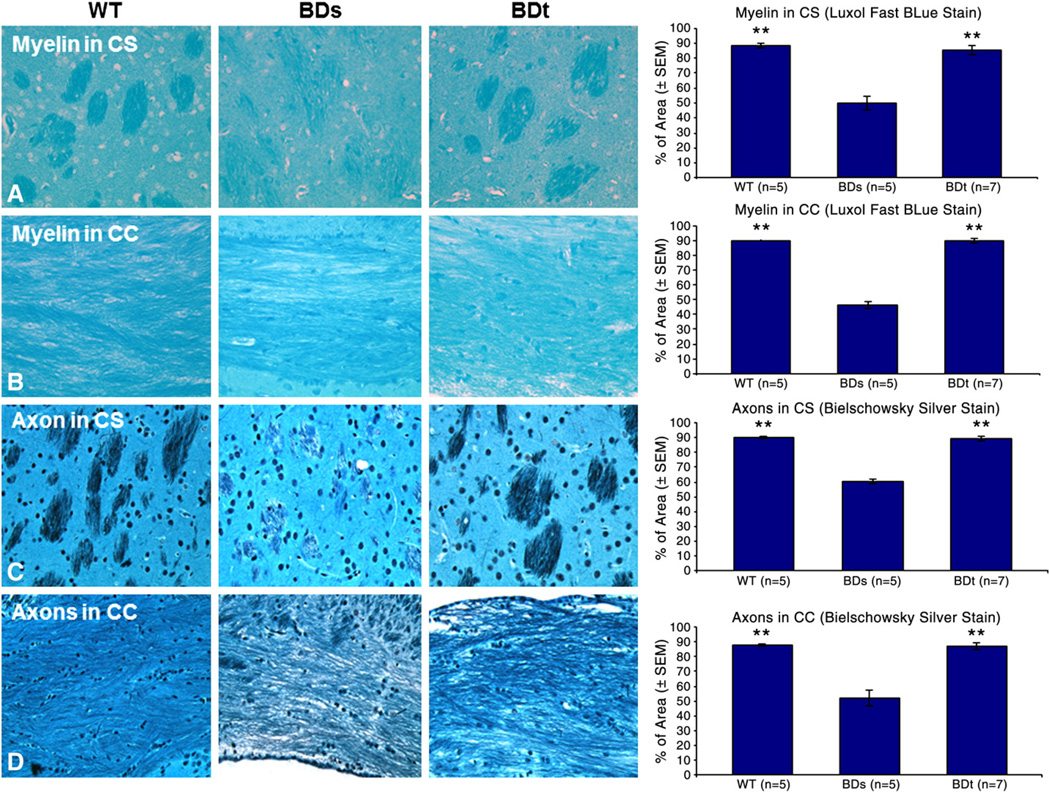
Luxol fast-blue was used to assess the degree of myelination and the Bielschowsky silver stain was used to assess axonal changes. For better representation of the white matter pathology, two slides were analyzed from each mouse. Fig. 3 shows the lower magnification schematics for myelin and axonal analysis in the region of CC (Fig. 3C) and CS (Fig. 3D). As shown in Figs. 3C and D, white matter deficits (demyelination/remyelination and axonal degeneration/regeneration) were evaluated in five distinct fields from each hemisphere in the regions of CS and in five distinct fields in the region of CC. The quantity of axons and myelin present in each axonal bundle was assessed by measuring the percent of stained area at 20× magnifications as presented in Fig. 6. Myelopathy in BDs mice and the cerebral remyelination and axonal regeneration in BDt mice were evaluated and compared with the findings in the brains of WT mice as shown in Fig. 6. The data are presented as the mean percent stained-positive area (±SEM) for each group in Fig. 6.
Fig. 6.
CNS demyelination and subsequent axonal degeneration in neurologically compromised BDs mice. Axon bundles and myelin abundance in two different regions of white matter, CS and CC, were assessed bilaterally. BDs mice showed symmetrical decreases of myelin and are stained for axon compared to those of WT asymptomatic mice. These white matter defects were reversed following biotin treatment. The double star (**) on the bars indicate difference being statistical significant (p<0.01) when either WT or BDt groups were compared with BDs group.
Mean percent of stained area for myelin in CS of WT mice (n = 5) was 88.2 ± 1.6. In BDs mice, the mean percent of stained area for myelin in CS was 48.5 ± 4.0 (n = 5). The reduction in staining for myelin in BDs mice compared to that of WT mice was statistically significant (p<0.01). Recovery after 10 days of biotin treatment was remarkable as demonstrated by the regeneration of myelin. Increase in the mean percent of stained area for myelin in CS of BDt mice compared to that of BDs mice was significant (p<0.01). BDt mice (n = 7) regenerated their myelin as indicated by the increase in stained area for myelin to a mean of 85.4 ± 2.8, which was comparable to that of the mean percent of myelin staining of WT mice (p>0.05).
Similarly, the mean percent of stained area for myelin in the CC of BDs mice was reduced. As shown in the graph, the mean percent of stained area for WT mice (n = 5) was 89.9 ± 0.3. In BDs mice (n = 5), the mean percent of stained area for myelin in CC was 46.4 ± 2.6. The reduction in staining for myelin in BDs mice compared to that of WT mice was statistically significant (p<0.01). Similar to the myelin regeneration in the region of CC, the myelin recovery after 10 days of biotin treatment was remarkable; increase in mean percent of stained area for myelin in CC of BDt mice compared to that of BDs mice (n = 5) was significant (p<0.01). BDt mice regenerated their myelin as demonstrated by the increase of stained area for myelin to 89.7 ± 1.38 which was comparable to the mean percent of myelin staining for WT mice.
Since the loss of axonal myelination can directly affect axon integrity, we also evaluated axon degeneration in BDs mice and axon regeneration in BDt mice by Bielschowsky silver staining. For more accurate evaluation, we assessed axonal status in both CS and CC. As shown in Fig. 6, the mean percent of stained area for axons in the CS of WT mice (n = 5) was 90.0 ± 2.0. In BDs mice (n = 5), the mean percent of stained area for axons in CS was 60.3 ± 0.6. The difference in the amount of axon present in BDs mice compared to that of the WT mice was statistically significant (p<0.01). After 10 days of biotin treatment, the axonal recovery was remarkable. BDt mice (n = 7) regenerated their axons as indicated by the increase in stained area to a mean percent of 89.1 ± 1.4. Increase in stained area for axons in CS of BDt mice compared to that of BDs mice was significant (p<0.01). At the end of the experiment, post-recovery of the axonal staining had returned to normal and was comparable to the degree of axonal staining in WT mice.
Similar to changes in the amount of myelin in both regions of CS and CC, we observed decreases in the staining for axons in CC and CS in symptomatic BDs mice and it returned towards normal when they were treated with biotin. As shown in Fig. 6, the mean percent of axonic staining in CC of WT mice (n = 5) was 87.8 ± 0.6, whereas in BDs mice (n = 5), the mean percent of staining for axons in CC was 52.0 ± 5.2. The difference in staining of axons in BDs mice compared to that in WT mice was statistically significant (p<0.01). After biotin treatment for 10 days, the BDt mice (n = 7) regenerated their axons as indicated by the increase of axonal staining to a mean percent of 87.1 ± 2.5. Increase in the mean percent of axonal staining in the CC of BDt mice compared to that in BDs mice was significant (p<0.01) and the axonal mass was comparable to that of WT mice.
Discussion
Our early investigation has established that biotinidase deficiency was the major cause for the late-onset MCD (Wolf et al., 1983a). We characterized the variability in the clinical expression of the disorder (Wolf et al., 1983b) and developed and performed the initial newborn screening for biotinidase deficiency (Heard et al., 1984; Wolf and Heard, 1990; Wolf et al., 1985). Subsequently, we isolated and cloned the human biotinidase gene (Cole et al., 1994), characterized its genomic organization (Knight et al., 1998), analyzed over one hundred known mutation that cause biotinidase deficiency (Pindolia et al., 2010), and predicted the enzyme's three-dimensional structure (Pindolia et al., 2007). In the past many investigators, including us, have used rats made biotin-deficient by feeding them diet containing egg white in attempt as a putative animal model for biotinidase deficiency. However, this model system was inadequate because biotinidase activity was still intact and the endogenous biotin continued to be recycled in this animal. Therefore, we have developed the biotinidase deficient transgenic mouse that is better suited model system. This mouse when fed simply the biotin-deficient diet, it in fact develops biotin deficiency that is clinically relevant for inherited biotinidase deficiency disorder. This mouse exhibits most of the clinical and biochemical features of the disorder in humans. This is the first study to evaluate neurological deficits in the BD mice and relates them with the neurological deficit observed in humans with the deficiency.
Most individuals with untreated profound biotinidase deficiency develop cutaneous and neurological symptoms during early childhood. If they remain untreated, they can develop progressive encephalopathy during episodes of acute metabolic deterioration (Wolf, 2001). The most common neurological symptoms include hypotonia, seizures, ataxia, spasticity, developmental delay, sensorineural hearing loss and optic atrophy.
Neuroimaging studies of the CNS of symptomatic children with biotinidase deficiency often exhibit white matter abnormalities, ventricular dilation, and cerebral atrophy (Wolf, 1992). However, many symptomatic children who had imaging studies performed exhibited normal brain findings. Because these children are usually diagnosed, treated with biotin and improve, there have been only a few histopathological studies of children with confirmed biotinidase deficiency who have died prior to definitive diagnosis and treatment (Wolf, 1992).
To understand better the neurological abnormalities associated with biotinidase deficiency, we used our recently developed knockout mouse with biotinidase deficiency. For symptom development, BD mice are required to be on a biotin-deficient diet for about two weeks. It is important to emphasis that only the BD mice become symptomatic and develop neurological symptoms when fed the biotin-deficient diet, whereas the WT mice remain asymptomatic during the same period. Observation that the WT mice remain asymptomatic when fed the biotin-deficient diet confirms the critical role biotinidase plays in recycling biotin.
We observed that the clinical neurological deficits observed in symptomatic children with profound biotinidase deficiency are similar to those seen in the symptomatic BDs mice. BDs mice appear lethargic and jittery and exhibit generalized growth retardation. In addition, ventriculomegaly and CC compression was also noted in the BDs mice similar to that observed in symptomatic children with the disorder.
The foot fault assessment protocol has been widely used in experimental mice because of its simplicity and objective nature. In our current study it proved to be a reliable method of assessing neurological deficits. It complemented the motor neuronal (i.e., gross observation and flexion assessment) and sensory neuronal assessments (i.e., pinna/corneal reflux). Based on these functional neurological assessments, the BDs mice performed poorly compared to that of WT mice.
Histological evaluation of the brains of BDs mice show demyelination and axonal degeneration compared to the degree of myelination and axonal status of WT mice. The degree of demyelination and axonal degeneration in BDs mice correlates with the neurological deficits observed in the BDs mice. Similarly, the remyelination and axonal regeneration after the biotin treatment correlated with the recovery in neurological deficits seen in BDt mice. The improvement in symptoms of BD mice treated with biotin correlates with the improvement in central nervous system abnormalities observed in treated symptomatic individuals with biotinidase deficiency.
Biotin treatment did not result in as pronounced restoration of the structure of corpus callosum and corpus striatum. Biotin supplementation also did not dramatically improve the ventricular edema and corpus callosum compression. It is possible that a longer duration of biotin treatment is necessary to reverse the alterations in brain morphology. It is also possible, that these changes, once they occur, are not entirely reversible.
The rapid rate of recovery of the BDs mice after biotin treatment is remarkable. Acute biotin deficiency may affect oligodendrocytes and axonal functions rather than their viability. Decreased activities of the biotin-dependent carboxylases, particularly those in the mitochondria, caused by biotin deficiency may result in decreased production and secretion of myelin basic protein, thereby altering myelination. Rapid restoration of carboxylase activities with biotin supplementation likely causes the restoration of oligodendrocytes and axonal function.
Biotinidase catalyzes the release of biotin from prototypically degraded holocarboxylases and biotinylated dietary proteins. This reaction is important for biotin reutilization and replenishing the free biotin pool (Hymes and Wolf, 1996). Free biotin is required for converting each of the apocarboxylases into holocarboxylases. Biotin is also important for regulating gene expression of certain enzymes of energy metabolism pathways (Perez-Monjaras et al., 2008; Zemplini et al., 2009). Brain pathology observed in this study possibly is caused by metabolic encephalopathy and its resultant impact on lipid synthesis. This subsequently affects myelination and axon integrity.
The etiology of the neurological changes observed in symptomatic individuals with biotinidase deficiency is not known. However, it has been speculated that it is due to the accumulation of abnormal metabolites in the brain, including lactic acid, ammonia, and other organic acids (Wolf, 2001). However, not all symptomatic individuals show abnormal findings on neuroimaging. We reason that biotin treatment response and the reversal of the CNS changes would correlate with a better prognosis than for those who continue to exhibit residual deficits.
The mouse with biotinidase deficiency appears to be an appropriate model in which to study the neurological abnormalities and the effects of treatment of the disorder. In addition, these mice will afford us an opportunity to correlate the neurological alterations seen in symptomatic animals with the biochemical changes and better explain their etiologies. Moreover, the degree of reversal of abnormal findings may correlate with prognosis in symptomatic individuals or in those who are poorly compliant with treatment.
Acknowledgments
This work was funded by the Safra Research Foundation, National Institute of Neurological Diseases and Stroke RO1 NS047682 and PO1 NS23393, and American Heart Association grant 0750048Z. We thank Megan Jordan for technical assistance.
References
- Calza L, Fernandez M, Giuliani A, Aloe L, Giardino L. Thyroid hormone activates oligodendrocyte precursors and increases a myelin-forming protein and NGF content in the spinal cord during experimental allergic encephalomyelitis. Proc. Natl. Acad. Sci. U. S. A. 2002;99:3258–3263. doi: 10.1073/pnas.052704499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann. Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- Cole H, Reynolds TR, Buck GB, Lockyer JM, Denson T, Spence JE, Hymes J, Wolf B. Human serum biotinidase: cDNA cloning, sequence and characterization. J. Biol. Chem. 1994;269:6566–6570. [PubMed] [Google Scholar]
- Craft DV, Goss NH, Chandramouli N, Wood HG. Purification of biotinidase from human plasma and its activity on biotinyl peptides. Biochemistry. 1985;24:2471–2476. doi: 10.1021/bi00331a012. [DOI] [PubMed] [Google Scholar]
- Davis RE, Swiderski RE, Rahmouni K, Nishimura DY, Mullins RF, Aggassandian K, Philp AR, Seary CC, Andrews MP, Thompson S, Berry CJ, Thedens DR, Yang B, Weiss RM, Cassell MD, Stone EM, Sheffield VC. A knockin mouse model of the Bardet-Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proc. Natl. Acad. Sci. U. S. A. 2007;104:19422–19427. doi: 10.1073/pnas.0708571104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard GS, Secor McVoy JR, Wolf B. A screening method for biotinidase deficiency in newborns. Clin. Chem. 1984;30:125–127. [PubMed] [Google Scholar]
- Hymes J, Wolf B. Biotinidase and its role in biotin metabolism. Clin. Chim. Acta. 1996;255:1–11. doi: 10.1016/0009-8981(96)06396-6. [DOI] [PubMed] [Google Scholar]
- Irving EA, Bentley DL, Parsons AA. Assessment of white matter injury following prolonged focal cerebral ischemia in the rat. Acta Neuropathol. 2001;102:627–635. doi: 10.1007/s004010100416. [DOI] [PubMed] [Google Scholar]
- Knight HC, Reynolds TR, Meyers GA, Pomponio RJ, Buck GA, Wolf B. Structure of the human biotinidase gene. Mamm. Genome. 1998;9:327–330. doi: 10.1007/s003359900760. [DOI] [PubMed] [Google Scholar]
- Liu Z, Li Y, Zhang RL, Cui Y, Chopp M. Bone marrow stromal cells promote skilled motor recovery and enhance contralesional axonal connections after ischemic stroke in adult mice. Stroke. 2011;42:740–744. doi: 10.1161/STROKEAHA.110.607226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund N, Morales D, Clausen F, Hanell A, Kiwanuka O, Pitkanen A, Gimbel DA, Philipson O, Lannfelt L, Hillered L, Strittmatter SM, McIntosh TK. Functional outcome is impaired following traumatic brain injury in aging Nogo-A/B-deficient mice. Neuroscience. 2009;163:540–551. doi: 10.1016/j.neuroscience.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J, Lane MD. The biotin-dependent enzymes. Adv. Enzymol. 1971;35:321–442. doi: 10.1002/9780470122808.ch7. [DOI] [PubMed] [Google Scholar]
- Paylor R, Hirotsune S, Gambello MJ, Yuva-Paylor L, Crawlely JN, Wyndhaw-Boris A. Impaired learning and motor behavior in heterozygous Pafah1b1 (Lis1) mutant mice. Learn. Mem. 1999;6:521–537. doi: 10.1101/lm.6.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Monjaras A, Cervantes-Roldan R, Menses-Morales I, Gravel RA, Reyes-Carmona S, Solorzano-Vargus S, Gonzalez-Noriega A, Leon-Del-Rio A. Impaired biotinidase activity disrupts holocarboxylase synthetase expression in late onset multiple carboxylase deficiency. J. Biol. Chem. 2008;283:34150–34158. doi: 10.1074/jbc.M806985200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pindolia K, Jensen K, Wolf B. Three dimensional structure of human biotinidase: computer modeling and functional correlations. Mol. Genet. Metab. 2007;92:13–22. doi: 10.1016/j.ymgme.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Pindolia K, Jordan M, Wolf B. Analysis of mutations causing biotinidase deficiency. Hum. Mutat. 2010;31:983–991. doi: 10.1002/humu.21303. [DOI] [PubMed] [Google Scholar]
- Pindolia K, Jordan M, Guo C, Matthews N, Mock D, Strovel E, Blitzer M, Wolf B. Development and characterization of a mouse with profound biotinidase deficiency: a biotin-responsive neurocutaneous disorder. Mol. Genet. Metab. 2011;102:161–169. doi: 10.1016/j.ymgme.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pispa J. Animal biotinidase. Ann. Med. Exp. Biol. Fenn. 1965;43(Suppl. 5):1–39. [PubMed] [Google Scholar]
- Stroemer RP, Kent TA, Hulsebosch CE. Enhanced neocortical neural sprouting, synaptogenesis, and behavioral recovery with D-amphetamine therapy after neocortical infarction in rats. Stroke. 1998;29:2381–2393. doi: 10.1161/01.str.29.11.2381. [DOI] [PubMed] [Google Scholar]
- Sweetman L. Two forms of biotin-responsive multiple carboxylase deficiency. J. Inherit. Metab. Dis. 1981;4:53–54. doi: 10.1007/BF02263587. [DOI] [PubMed] [Google Scholar]
- Wolf B. Worldwide survey of neonatal screening for biotinidase deficiency. J. Inherit. Metab. Dis. 1991;14:923–927. doi: 10.1007/BF01800475. [DOI] [PubMed] [Google Scholar]
- Wolf B. Disorders of biotin metabolism: treatable neurological syndromes. In: Rosenberg R, Prusiner SB, DiMauro S, Barchi RL, Kunkel LM, editors. The Molecular and Genetic Basis of Neurological Disease. Stoneham, Mass: Butterworth Publishers; 1992. pp. 569–581. [Google Scholar]
- Wolf B. Disorders of biotin metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001. pp. 3935–3962. [Google Scholar]
- Wolf B, Feldman GL. The biotin-dependent carboxylase deficiencies. Am. J. Hum. Genet. 1982;34:699–716. [PMC free article] [PubMed] [Google Scholar]
- Wolf B, Heard GS. Screening for biotinidase deficiency in newborns: worldwide experience. Pediatrics. 1990;85:512–517. [PubMed] [Google Scholar]
- Wolf B, Grier RE, Allen RJ, Goodman SI, Kien CL. Biotinidase deficiency: the enzymatic defect in late-onset multiple carboxylase deficiency. Clin. Chim. Acta. 1983a;131:273–281. doi: 10.1016/0009-8981(83)90096-7. [DOI] [PubMed] [Google Scholar]
- Wolf B, Grier RE, Allen RJ, Goodman SI, Kien CL, Parker WD, Howell DM, Hurst DL. Phenotypic variation in biotinidase deficiency. J. Pediatr. 1983b;103:233–237. doi: 10.1016/s0022-3476(83)80351-5. [DOI] [PubMed] [Google Scholar]
- Wolf B, Heard GS, Jefferson LG, Proud VK, Nance WE, Weissbecker KA. Clinical findings in four children with biotinidase deficiency detected through a statewide neonatal screening program. N. Engl. J. Med. 1985;313:16–19. doi: 10.1056/NEJM198507043130104. [DOI] [PubMed] [Google Scholar]
- Zemplini J, Wijeratne SS, Hassan YI. Biotin. Biofactors. 2009;35:36–46. doi: 10.1002/biof.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Zhang C, Zhang L, Roberts C, Lu M, Kapke A, Cui Y, Ninomiya M, Nagafuji T, Albala B, Zhang ZG, Chopp M. Synergistic effect of an endothelin type A receptor antagonist. Stroke. 2008;39:2830–2836. doi: 10.1161/STROKEAHA.108.515684. [DOI] [PMC free article] [PubMed] [Google Scholar]