Abstract
Purpose
Type I collagen accumulates during liver fibrosis primarily because α-complex protein-2 (αCP2), encoded by the poly(rC) binding protein 2 (PCBP2) gene, binds to the 3′ end of the collagen mRNA and increases its half-life. This study aimed to reverse the pro-fibrogenic effect of alcohol on hepatic stellate cells (HSCs) by silencing the PCBP2 gene with siRNA.
Methods
The silencing effects of a series of predesigned PCBP2 siRNAs were evaluated in the rat hepatic stellate cell line, HSC-T6. The pro-fibrogenic effects of alcohol on the expression levels of PCBP2 and type-I collagen were examined by several methods. The effect of PCBP2 siRNA on the stability of type I collagen α1(I) mRNA was investigated by an in vitro mRNA decay assay.
Results
We identified one potent PCBP2 siRNA that reversed the alcohol-induced expression of PCBP2 in HSCs. The decay rate of the collagen α1(I) mRNA increased significantly in HSCs treated with the PCBP2 siRNA.
Conclusion
This study provides the first evidence that alcohol up-regulates the expression of PCBP2, which subsequently increases the half-life of collagen α1(I) mRNA. Silencing of PCBP2 using siRNA may provide a promising strategy to reverse the alcohol-induced pro-fibrogenic effects in HSCs.
Keywords: alcoholic liver fibrosis, mRNA stability, PCBP2, siRNA, type I collagen
INTRODUCTION
Alcoholic liver fibrosis is a wound healing process that results from chronic liver injuries caused by alcohol abuse (1), and it is the most common type of liver fibrosis/cirrhosis in western developed countries, accounting for more than 50% of cirrhosis cases (1,2). Excessive consumption of alcohol causes several stages of alcoholic liver diseases, such as steatosis and steatohepatitis, before alcoholic liver fibrosis becomes evident. Alcoholic liver fibrosis is characterized by the excessive accumulation of extracellular matrix (ECM) in pericentral and perisinusoidal areas of the liver (3,4). Liver fibrogenesis is a dynamic process that involves the transformation of quiescent hepatic stellate cells (HSCs) into myofibroblast-like cells, which migrate to the site of liver injury and account for the approximately ~50-fold increase of ECM in fibrotic liver relative to normal liver (3,5,6). Of the 12 different types of collagen in the liver, type I collagen is the most abundant component of the accumulated ECM in fibrotic liver.
Type I collagen is a heterotrimer of two α1(I) and one α2 (I) polypeptide chains encoded by the COL1A1 and COL1A2 genes, respectively (7,8). The accumulation of type I collagen in fibrotic liver is primarily due to an increase in the half-life of its mRNA from 1.5 h in quiescent HSCs to greater than 24 h in activated primary HSCs (9,10). The transcription rate of the collagen α1 (I) gene is only two times higher in activated HSCs than in quiescent HSC. This observation suggests that post-transcriptional regulation plays a key role in the accumulation of type I collagen (11). The enhanced stability of the collagen α1(I) mRNA is mediated by the 3′ untranslated region (3′-UTR) of the transcript (2). Brenner et al. have identified an RNA-binding protein encoded by the PCBP2 gene, αCP2, that binds to the C-rich region of the 3′-UTR of collagen α1(I) mRNA with a high affinity (Kd=2nM), leading to stabilization of the mRNA in activated HSCs, but not in quiescent HSCs (12). Although the mechanism has not been fully elucidated, post-transcriptional modification of αCP2 in activated HSCs may account for this difference between activated and quiescent HSCs (6). Recombinant αCP2 specifically binds to the 30 nt C-rich region of the collagen α1(I) mRNA 3′-UTR, and even a single nucleotide change in the binding site could abolish the binding affinity of αCP2 (6). Furthermore, a mutant collagen α1(I) mRNA lacking the binding region is not stabilized by αCP2 in activated primary HSCs (12,13). Additionally, competition of αCP2 in activated HSCs results in a decreased steady-state level of the collagen α1(I) mRNA (12).
αCP belongs to the K-homology (KH) domain super-family of RNA binding proteins. Of the four αCP family members (αCP1, αCP2, αCP3 and αCP4), αCP1 and αCP2 are the most abundantly expressed isoforms that play important roles in mRNA stabilization (14,15). Both αCP1 and αCP2 have been reported to stabilize the mRNAs of tyrosine hydroxylase (16), erythropoietin (17), α-globin (18), and β-globin (19). In particular, αCP2 has been identified as the αCP isoform that binds to the 3′-UTR region of the collagen α1(I) mRNA (6,12,13,20). Therefore, blocking PCBP2 expression may decrease the stability of collagen α1(I) mRNA and thereby reverse the accumulation of type I collagen in fibrotic liver.
The aim of this study was to reverse the accumulation of type I collagen in fibrotic liver by blocking PCBP2 with synthetic siRNAs. We demonstrated that alcohol increases the expression of PCBP2 as well as that of type I collagen in HSCs and showed that inhibition of PCBP2 using siRNA reverses the alcohol-induced up-regulation of type I collagen.
MATERIALS AND METHODS
Materials
PCBP2 siRNAs were purchased from Ambion Inc. (Austin, TX) and Invitrogen Corp. (Carlsbad, CA), and the scrambled siRNA (negative control siRNA) was obtained from Ambion Inc. (Austin, TX). Lipofectamine-2000 and TRIzol reagent were obtained from Invitrogen Corp. (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals Inc. (Lawrenceville, GA). Other cell culture reagents including Dulbecco’s modified Eagle’s medium (DMEM), Dulbecco’s phosphate-buffered saline (DPBS), penicillin and streptomycin were obtained from Mediatech, Inc. (Manassas, VA). Bovine serum albumin (BSA) was purchased from Sigma-Aldrich Corporation (St. Louis, MO). Light cycler 480 SYBER-1 master mix was purchased from Roche (Indianapolis, IN), and the reverse transcription reagents were purchased from Applied Biosystems, Inc. (Foster City, CA).
Cell Culture
Rat hepatic stellate cells (HSC-T6, kindly provided by Dr. Scott L. Friedman, Mount Sinai School of Medicine, New York, NY) were cultured in DMEM medium supplemented with 10% FBS, penicillin (100 units/mL) and streptomycin (100 µg/mL). Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2. The culture medium was changed every other day, and the cells were passaged when they reached 80–90% confluence. Incubation of HSC-T6 cells with ethanol was conducted according to previous reports, with minor modifications (21–23). For alcohol treatment, HSC-T6 cells were grown in 24-well plates at a density of 2 × 104 cells/well in antibiotic-free DMEM medium supplemented with 10% FBS. After 12 h, the medium was replaced with fresh medium containing 10% FBS and alcohol (50 mM or 100 mM) for the alcohol exposure studies. Cells that have been treated with alcohol were kept in an airtight box to avoid evaporation. The cellular toxicity of these reagents was determined by the MTT assay.
Design and Synthesis of siRNA
Nine siRNAs (Table I) targeting PCBP2 (Accession # NM_001013223.1) were designed using siRNA Target Finder (Ambion, Austin, TX), BLOCK-iT RNAi Designer (Invitrogen Corp., Carlsbad, CA.), siRNA Target Finder (GeneScript), and siRNA target Designer (Promega). The siRNAs are 19 nt in length with a 3′ overhang of two thymidine. To avoid cross-silencing of non-target genes, all of the designed siRNA sequences were blasted against the rat genome database. A scrambled siRNA (Ambion, Inc. Austin, TX) that does not target any gene was used as the negative control (NC).
Table 1.
Predesigned PCBP2 siRNA
| Start site | Name | Sense sequence of the siRNA |
|---|---|---|
| 296 | siR-1 | 5′-GUUGGCAGUAUCAUCGGAA-3′ |
| 326 | siR-2 | 5′-UCUGUUAAGAAGAUGCGCG-3′ |
| 378 | siR-3 | 5′-GGAAUUGUCCUGAGAGAAU-3′ |
| 543 | siR-4 | 5′-GUCAGUGUGGCUCUCUUAU-3′ |
| 545 | siR-5 | 5′-CAGUGUGGCUCUCUUAUUG-3′ |
| 572 | siR-6 | 5′-GGUUGCAAGAUCAAGGAAA-3′ |
| 684 | siR-7 | 5′-CCAUCAUUGAGUGUGUCAA-3′ |
| 905 | siR-8 | 5′-GAGGCCUAUACCAUUCAAG-3′ |
| 1487 | siR-9 | 5′-GUCAGUGUGGCUCUCUUAU-3′ |
siRNA Transfection
Cells were transfected with siRNA using Lipofectamine-2000 as previously reported (24,25). Briefly, cells were seeded in 24-well plates at a density of 1 × 104 cells/well in antibiotic-free medium 12 h before transfection. The transfection mixture was prepared by mixing a 1.25-µl aliquot of 20 µM synthetic siRNA and a 1-µl aliquot of Lipofectamine-2000 with two separate aliquots of 50 µl of serum-free OPTI MEM®-I (Invitrogen) and then combining the two solutions at room temperature for 25 min to form a complex. The cells were washed with DPBS, and 100 µl of the transfection mixture was added to each well along with 400 µl of DMEM medium containing 10% FBS to produce a final concentration of 50 nM siRNA per well. The transfected cells were incubated for 24 h before being harvested for RNA and protein isolation.
The alcohol treatment experiments began with the same siRNA transfection procedure. After the initial 24-h incubation period, the medium was replaced with fresh medium containing 100 mM alcohol, and the cells were incubated for an additional 24, 48 or 72 h.
Detection of αCP2 and Type I Collagen by Western Blotting
The αCP2 protein was prepared as follows. The HSC-T6 cells were lysed with ice-cold RIPA buffer (150 mM NaCl, 50 mM Tris base pH 8.0, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, 1 mM PMSF and 1 mM Na3VO4) containing freshly added protease inhibitor cocktail (Roche, Indianapolis, IN). After a 5-min incubation on ice, the cells were scraped, and the lysate was collected by centrifugation at 12,000 g for 10 min. The total protein content was determined using a BCA protein assay kit (Pierce, Rockford, IL). Equal amounts of protein samples (15 µg/well) were resolved on a 10% SDS-PAGE gel.
The cell lysate was prepared for the type I collagen western blot by processing with 10 mg/ml pepsin solution (10:1, v/v, protein/pepsin) for 2 h as previously described (26,27). The proteins were precipitated in 4.5 M NaCl overnight at 4°C, redissolved in a buffer containing 0.5 M urea and then resolved on a 4–12% gradient NuPAGE gel (Invitrogen, Carlsbad, CA).
The separated proteins were electrophoretically transferred to a nitrocellulose membrane, blocked with a 5% nonfat dry milk solution, and subsequently incubated with the anti-PCBP2 antibody (Geneway Biotech Inc., San Diego, CA) or anti-type I collagen antibody (Rockland Immunochemical Inc., Gilbertsville, PA) for 2 h at room temperature. The protein was then visualized using horseradish peroxidase-conjugated secondary antibodies and a chemoluminescence detection kit. The membrane was also re-probed with anti-β-actin antibodies (Rockland Immunochemical Inc., Gilbertsville, PA) as an internal control.
Real-Time RT-PCR
Total RNA was isolated using TRIzol reagent, and reverse transcribed into cDNA using random hexamer primer and MultiScribe reverse transcriptase reagent. Two hundred nanograms of the cDNA were amplified by Real-Time RT-PCR using Light Cycler 480 SYBR Green-1 Master mix on a Light Cycler 480 System (Roche, Indianapolis, IN). The comparative threshold (Ct) method was used to calculate the amount of mRNA in treated samples relative to the control group (28). Each treatment was performed in triplicate, and the results are given as the mean value. The primers used for the study were as follows: PCBP2: 5′-ACCAATAGCACAGC TGCCAGTAGA-3′ (forward primer) and 5′-AGTCTC CAACATGACCACGCAGAT-3′ (reverse primer); type I collagen mRNA: 5′-TGGTCCCAAAGGTTCTCCTGGT- 3′ (forward primer) and 5′-TTAGGTCCAGGGAATCC CATCACA-3′ (reverse primer); type I collagen pre-mRNA 5′-CCAGCCGCAAAGAGTCTACATGTC-3′ (forward primer) and 5′-TCACCTTCTCATCCCTC CTAA-3′ (reverse primer). We used 18s ribosomal RNA as an internal control, with the following primers: 5 ′ -GTCTGTGATGCCCTTAGATG-3′ (forward) and 5′-AGCTTATGACCC GCACTTAC -3′ (reverse).
Immunostaining of Type-I Collagen and αCP2
HSC-T6 cells (2 × 104) were plated on a Lab-Tek chamber slide (Nunc, Inc., Naperville, IL) and incubated for 12 h, the medium was then replaced with fresh medium containing 100 mM alcohol, and the cells were incubated for another 48 h. The cells were then fixed with 10% paraformaldehyde at room temperature for 20 min. Non-specific binding sites were blocked with 1% BSA in PBS for 1 hr. The cells were subsequently incubated with anti-collagen type I or anti-αCP2 antibodies for 2 h in a humidified chamber. The cells were then washed with 1% BSA containing PBS buffer and incubated with FITC-conjugated goat anti-rabbit antibodies for 1 hr. Fluorescence images were obtained with a Leica DMI 3000B fluorescence microscope (Leica Microsystems Inc., Wetzlar, Germany). DAPI (Vector laboratories, Inc., Burlingame, CA) was used for nuclear counterstaining according to the manufacturer’s instruction.
Cell Viability Assay
The MTT assay was employed to assess cell viability. Briefly, HSC-T6 cells were plated in 96-well plates at a density of 4 × 103 cells/well in DMEM medium supplemented with 10% FBS for 24 h. The medium was then replaced with fresh medium containing alcohol (50 mM or 100 mM). After 48 h of incubation, MTT was added (0.5 mg/well) and incubated at 37°C for 2 hr. The supernatant was removed, and 200 µl of DMSO was added to solubilize the formazan products. After gentle agitation the optical density was measured at 570 nm. All experiments were performed in triplicate.
In Vitro mRNA Decay Assay
The in vitro mRNA decay assay was performed as described previously with modifications (13,29,30). HSC-T6 cells were incubated with alcohol or siRNA for 24 h, followed by incubation with actinomycin D (Act-D, 1 µM) for different time periods at 37°C to inhibit transcription. At each designated time-point, the cells were harvested for RNA isolation. The collagen α1(I) mRNA levels were quantified with real-time RT-PCR, and the results are presented as percentage of mRNA remaining versus time using a semi-logarithmic scale on the y-axis. The mRNA half-life was calculated using Graph Pad prism software (Graph Pad Software, Inc., La Jolla, CA) by fitting the data with a one-phase exponential decay model using non-linear regression.
Statistical Analysis
Data are expressed as the mean±standard deviation (SD). The difference between any two groups was determined by one-way analysis of variance (ANOVA). P<0.05 was considered to be statistically significant.
RESULTS
Effects of Alcohol on HSC-T6 Cells
It has been shown by several groups that alcohol and acetaldehyde (the metabolite of alcohol) induce the expression of type I collagen in primary hepatic stellate cells (27,31–33). In this study, we used HSC-T6 as an in vitro model to examine the pro-fibrotic effects of alcohol and the possible anti-fibrotic effects of PCBP2 siRNA. HSC-T6 is an immortalized rat liver stellate cell line that retains the major features of cytokine signaling, retinoid metabolism, neuronal gene expression, and fibrogenesis (34). Because of the important role of HSCs in liver fibrogenesis, HSC-T6 cells have been widely used in liver fibrosis research (35–38).
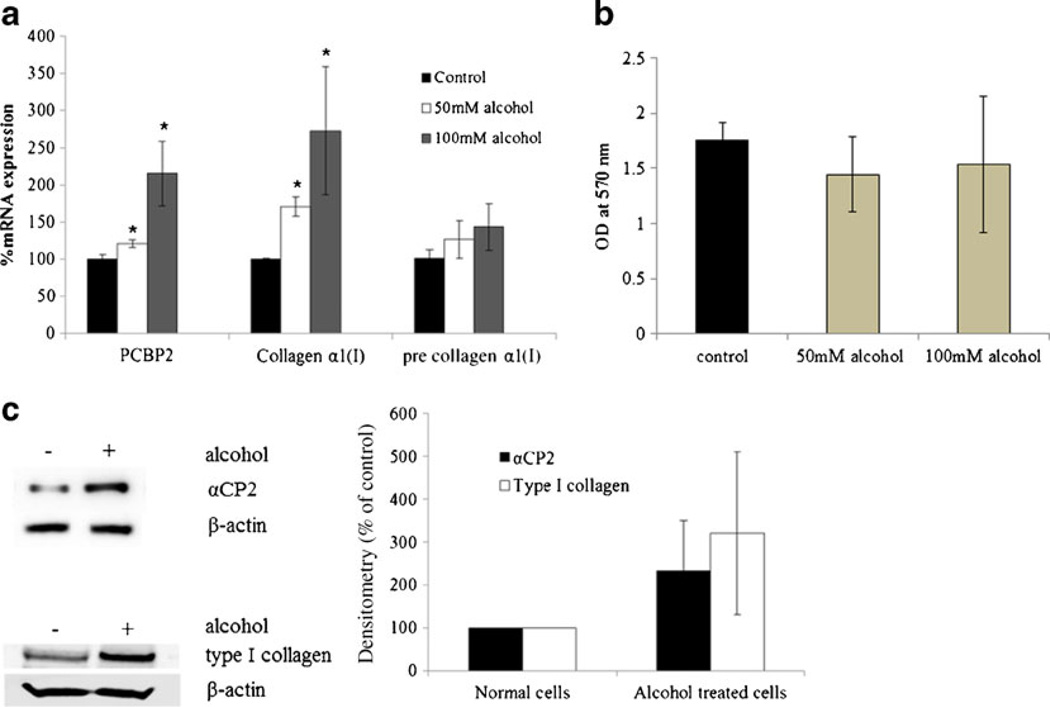
We first incubated HSC-T6 cells with alcohol (50 mM and 100 mM) for 48 h to promote pro-fibrotic activation. These concentrations of alcohol have been found in the blood of patients with alcoholic liver diseases (39,40) and are sufficient to generate hepatic vasoconstriction in rats (41,42). The levels of PCBP2 mRNA, collagen α1(I) mature mRNA, and collagen α1(I) pre-mRNA were determined using real-time RT-PCR (Fig. 1a). Exposure to 100 mM alcohol increased the level of collagen α1(I) mature mRNA by 2.7-fold, which is consistent with previous reports using primary hepatic stellate cells (31,33). Exposure to alcohol also increased PCBP2 expression by 2.15-fold. The up-regulation of PCBP2 and collagen α1(I) by alcohol was concentration dependent. Interestingly, the level of collagen α1(I) mRNA in cells treated with 100 mM alcohol for 48 h was similar to that of cells treated with 50 mM alcohol for 72 h (data not shown).
Fig. 1.
The effect of alcohol on the expressions of PCBP2 and type I collagen in HSC-T6 cells. HSC-T6 cells were incubated with alcohol (50 mM and 100 mM) for 48 h, and then the protein and RNA were extracted and quantified. The alcohol-containing medium was changed approximately every 6 h to maintain the alcohol concentration. (a) The levels of PCBP2 mRNA, collagen α1(I) mature mRNA, and collagen α1(I) pre-mRNA were quantified using real-time RT-PCR. Results are presented as the mean±SD (n=3). (*P<0.05; **P<0.01). (b) The effect of alcohol on cell viability. After incubation with alcohol for 48 h, the cell viability was determined by MTT assay. Results are represented as the mean±SD (n=3). (c) The effect of alcohol (100 mM) on the expression of αCP2 protein and type I collagen. All values were normalized against β actin with the NC set to 100 percent. Results are presented as the mean±SD (n=3).
Although incubation with alcohol substantially increased the level of collagen α1(I) mature mRNA, the effect on the collagen α1(I) pre-mRNA was negligible (p=0.211 and 0.345 for 50 mM and 100 mM alcohol, respectively). This result indicates that alcohol modulates the collagen α1(I) gene at the post-transcriptional level rather than the transcriptional level. Because alcohol also induces the PCBP2 gene, which produces a protein that stabilizes the collagen α1(I) mRNA at the post-transcriptional level (10), we hypothesize that alcohol induces pro-fibrogenic effects in HSCs by up-regulating PCBP2. Incubation with alcohol had a negligible effect on cell viability (p=0.112 and 0.924 for 50 mM and 100 mM, Fig. 1b), indicating that HSCs readily tolerate the alcohol doses used in this study. Because 100 mM alcohol produced a more significant increase in the levels of PCBP2 and collagen α1(I), we used this concentration for all further experiments. Incubation with alcohol also increased the expression of αCP2 (Fig. 1c) and type I collagen (Fig. 1d) at the protein level. These results are consistent with the stimulation effect at the mRNA level.
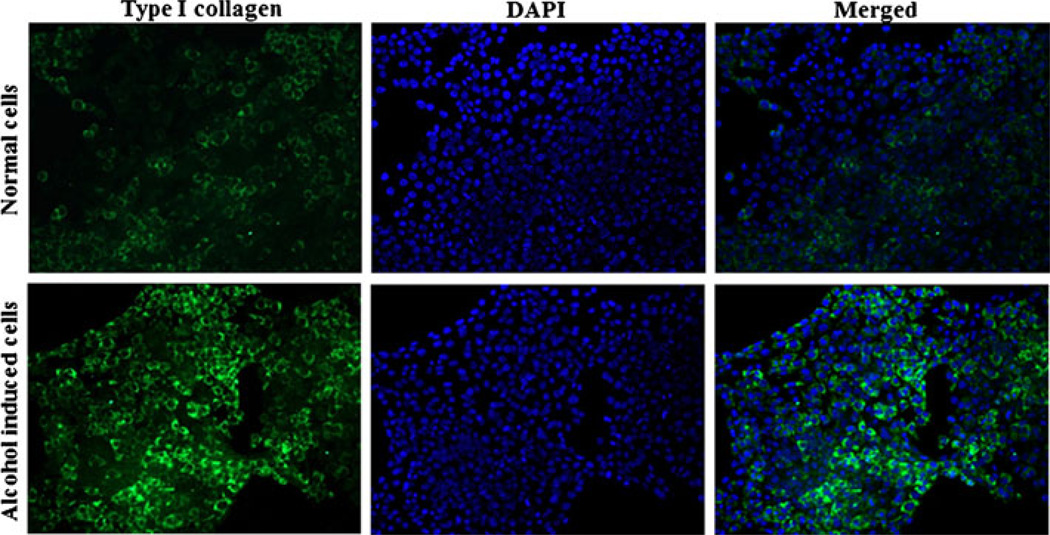
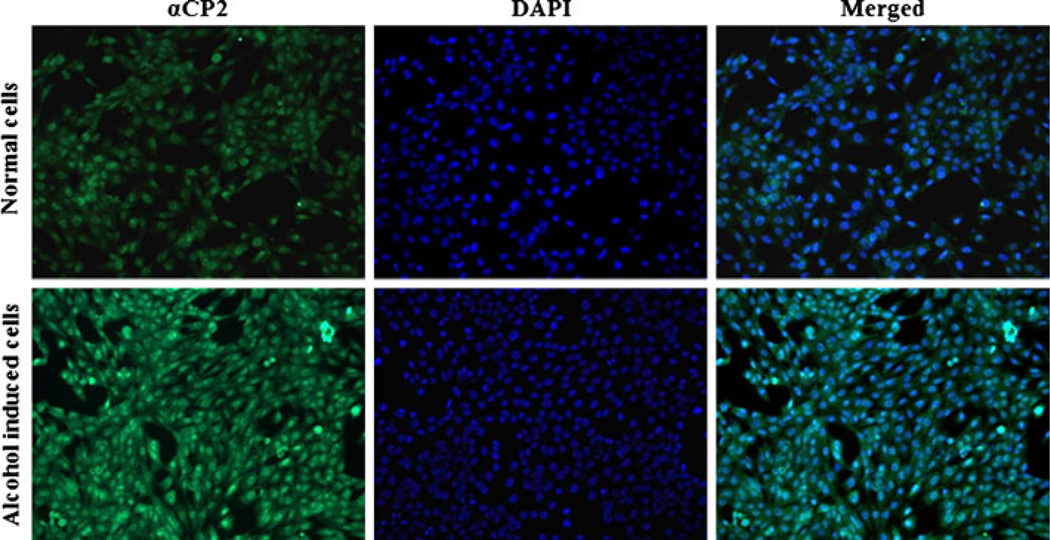
We also examined the effect of alcohol on the expression of αCP2 and type I collagen in HSC-T6 cells with immunostaining. HSC-T6 cells that had been incubated with 100 mM alcohol for 48 h exhibited increased expression of type I collagen (Fig. 2) and αCP2 (Fig. 3) relative to non-treated cells. These results further confirmed the observations presented in Fig. 1.
Fig. 2.
Alcohol induces the expression of type I collagen in HSC-T6 cells. HSCs were grown on a chamber slide and incubated with 100 mM alcohol for 48 h. The cells were then fixed and stained first with the anti-type I collagen antibody and then with the FITC-labeled secondary antibody.
Fig. 3.
Alcohol induces the expression of αCP2 in HSC-T6 cells. HSCs were grown on a chamber slide and incubated with 100 mM alcohol for 48 h. The cells were then fixed and stained first with the anti-αCP2 antibody and then with the FITC-labeled secondary antibody.
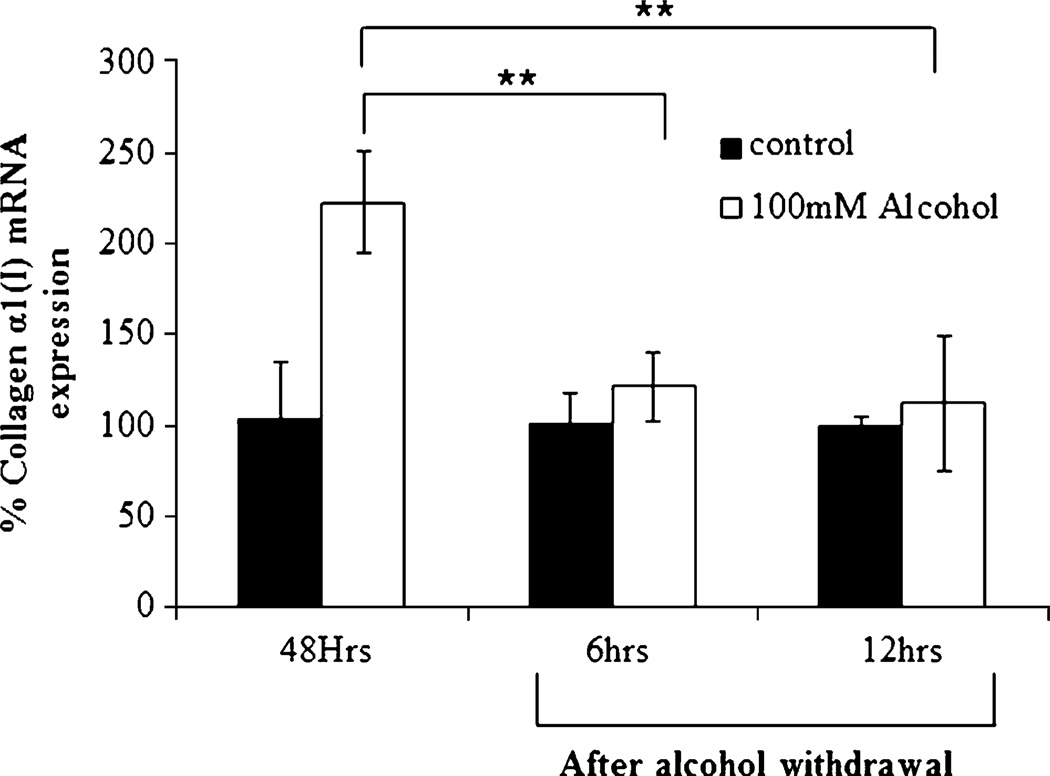
To examine whether exposure to alcohol permanently induces collagen expression, HSC-T6 cells were incubated with 100 mM alcohol for 48 h, and then the medium was replaced with alcohol-free medium. After incubation for up to 12 h, real-time RT-PCR was used to evaluate the ablation effect of alcohol. As illustrated in Fig. 4, the alcohol-induced expression of collagen α1(I) mRNA dropped significantly following the withdrawal of the alcohol. These data suggest that chronic consumption of alcohol is necessary to induce liver fibrosis and that discontinuing the use of alcohol will definitely improve the efficacy of alcoholic liver fibrosis treatment.
Fig. 4.
Effect of alcohol withdrawal on the expression of collagen α1(I) mRNA. After incubation of HSC-T6 cells with 100 mM alcohol for 48 h, the medium was replaced with alcohol-free medium. Real-time RT-PCR was performed after 6 and 12 h. Results are presented as the mean±SD (n=3) (*P<0.05; **P<0.01).
Silencing of PCBP2 with Synthetic siRNAs
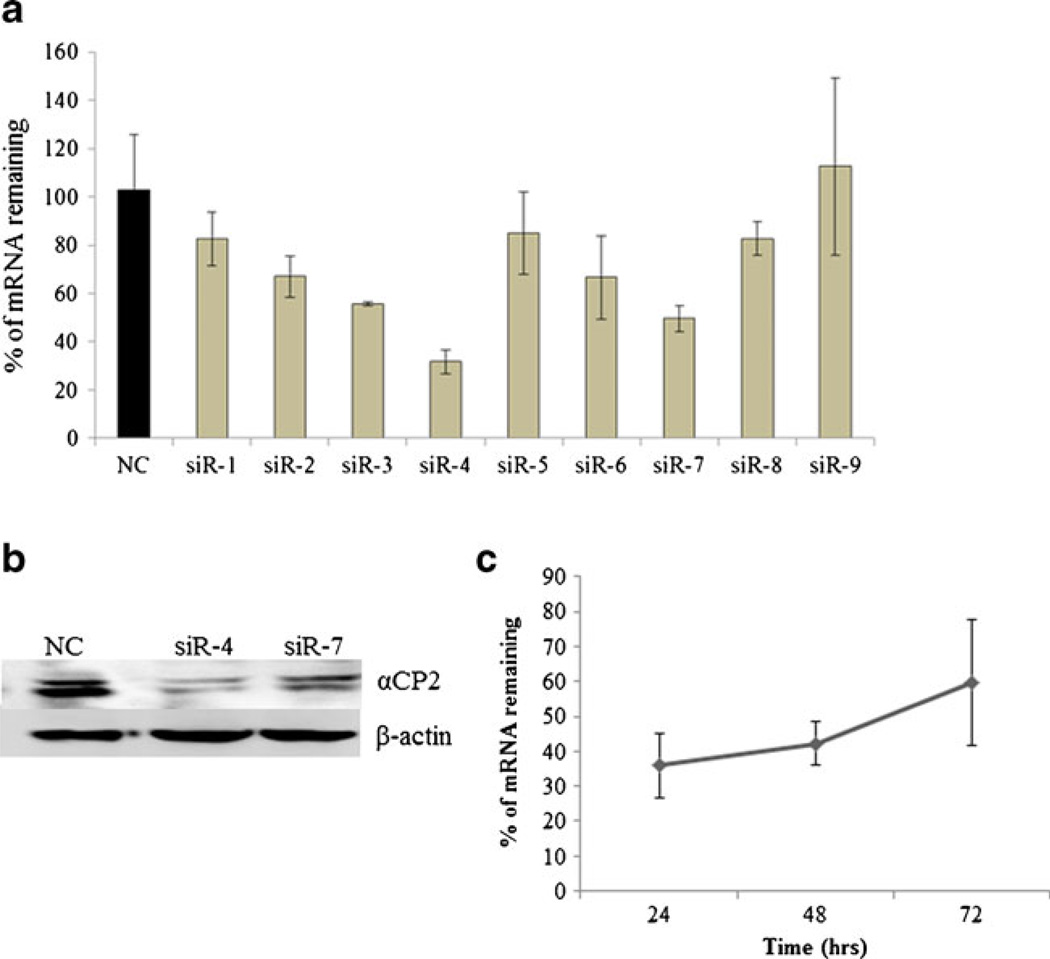
Having shown that alcohol up-regulates the expression of PCBP2, which subsequently increases the half-life of collagen α1(I) mRNA, we next designed nine synthetic siRNAs that targeted different regions of the rat PCBP2 mRNA (Table I). These siRNA duplexes were transfected into HSC-T6 cells at a final concentration of 50 nM following complexation with Lipofectamine-2000. Seven of the nine siRNAs exhibited significant silencing effects on the PCBP2 mRNA in comparison to the scrambled siRNA (NC) (Fig. 5a). Among them, siR-4 and siR-7 exhibited the highest silencing effects, up to ~70% and 50%, respectively. The silencing effect was further confirmed at the protein level using western blotting (Fig. 5b). Both siR-4 and siR-7 inhibited the expression of PCBP2 at the protein level, and siR-4 produced the greatest inhibition. We investigated the silencing duration of siR-4 and found that the effect peaked at 24 h post-transfection (Fig. 5c).
Fig. 5.
Silencing effect of PCBP2 siRNAs in HSC-T6 cells. HSC-T6 cells were transfected with PCBP2 siRNAs (in complex with Lipofectamine-2000) at a dose of 50 nM. (a) Silencing effects at the mRNA level. Results are presented as the mean±SD (n=3). (b) Silencing effects of siR-4 and siR-7 at the protein level. (c) Time course of the silencing effect of siR-4 on the expression of PCBP2. The gene silencing effect was determined by real-time RT-PCR at 24, 48 and 72 h post-transfection. Results are presented as the mean±SD (n=3).
Effect of PCBP2 siRNA on the Level of Collagen α1(I) mRNA
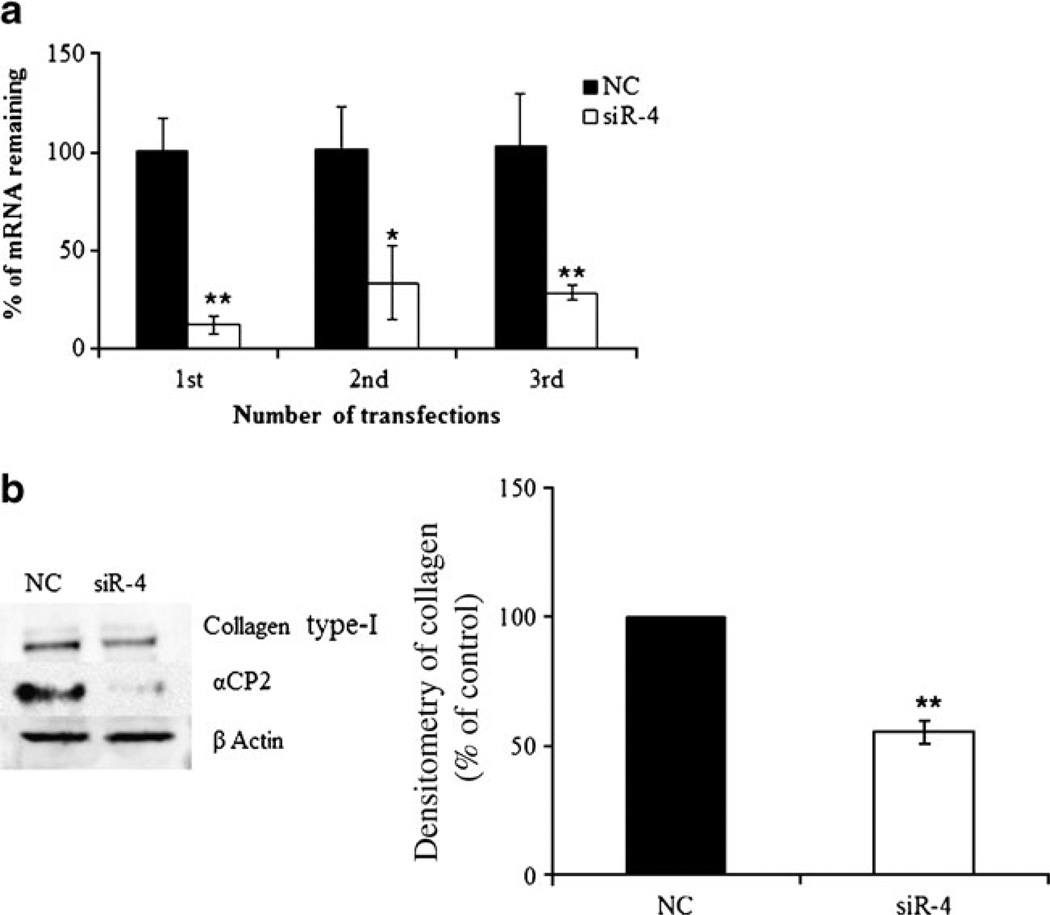
Because the PCBP2 protein product stabilizes collagen α1 (I) mRNA in activated HSCs, we next investigated whether silencing the PCBP2 gene could reverse the alcohol-induced accumulation of collagen α1(I) mRNA. Although siR-4 substantially silenced PCBP2, the effect on the level of collagen α1(I) mRNA was negligible (data not shown). This result suggests that the transient silencing of PCBP2 mRNA by a single transfection with siR-4 may not reduce the level of αCP2 protein for long enough to affect the stability of the collagen α1(I) mRNA. We therefore performed multiple transfections of siR-4 to sustain the silencing effect in HSCT6 cells over a longer period of time. After having been transfected with siR-4 for 36 h, the HSC-T6 cells were trypsinized and seeded in a new 24-well plate and incubated for 12 h in fresh medium before the next round of transfection. The HSC-T6 cells were transfected three times with siR-4, continuously silencing the PCBP2 gene for approximately 6 days. The silencing effect on PCBP2 was maintained throughout the multiple transfection experiments (Fig. 6). Western blot analysis for type I collagen was performed to confirm these results (Fig. 6b). We observed a significant decrease in type I collagen at the protein level after three transfections, suggesting that continuous silencing of PCBP2 could reduce the stability of the collagen α1(I) mRNA and thereby decrease the level of protein expression. These results suggested that the αCP2-mediated stabilization of the collagen α1(I) mRNA is not affected by the transient silencing of the PCBP2 gene but that it can be altered by sustained silencing.
Fig. 6.
Effect of PCBP2 siRNA on PCBP2 and type I collagen after multiple siRNA transfections. HSC-T6 cells were transfected with the PCBP2 siRNA (siR-4) at a dose of 50 nM for 36 h. The cells were then trypsinized and cultured in 24-well plates for another 12 h before the next round of transfection. The transfection process was repeated twice, and the expressions of PCBP2 (a) at the mRNA level were determined by real-time RT-PCR. Results are presented as the mean±SD (n= 3). (b) Western blot demonstrating the silencing of type-I collagen after the third transfection with siR-4. The bar graph illustrates the densitometric density of the western blot bands. All of the values were normalized against β actin with the NC set to 100 percent. Results are presented as the mean±SD (n=3) (*P<0.05; **P<0.01).
Effect of PCBP2 siRNA on HSCs in the Presence of Alcohol
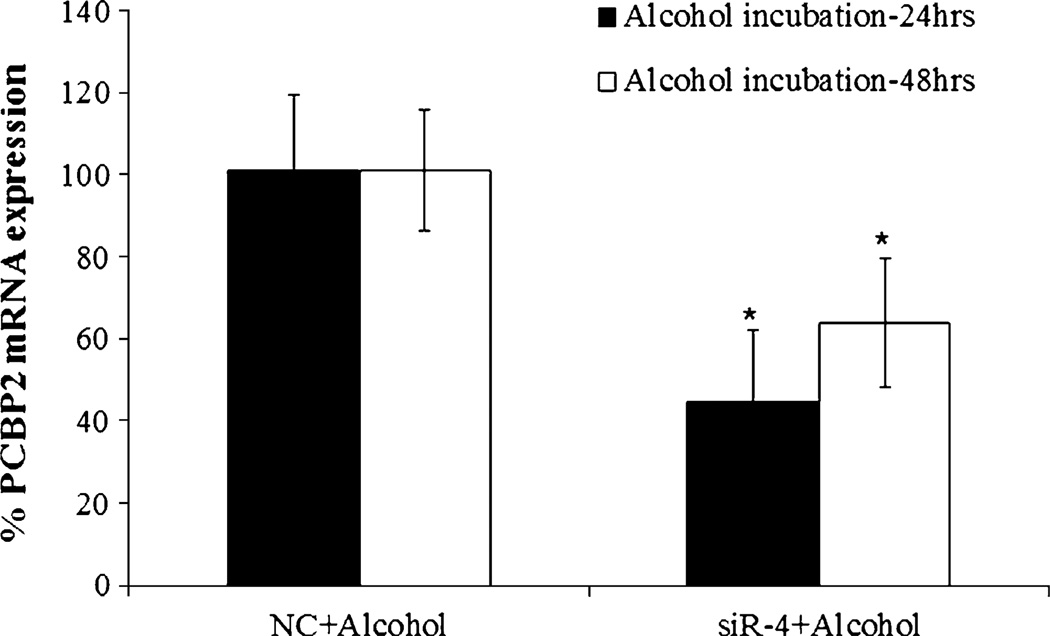
To determine whether the PCBP2 siRNA inhibits alcohol-induced expression of PCBP2 in HSCs, we transfected HSC-T6 cells with siR-4 for 24 h and then incubated the cells with alcohol for another 24 or 48 h. As shown in Fig. 7, siR-4 significantly silenced the expression of PCBP2 even in the presence of alcohol, suggesting that treatment with siR-4 may reverse the up-regulation of PCBP2 expression in alcoholic liver fibrosis. The silencing effect of siR-4 was smaller after 48 h of exposure to alcohol than it was that after 24 h of exposure, which is consistent with the previous experiments (see Fig. 5c).
Fig. 7.
Effect of the PCBP2 siRNA on the expression of PCBP2 in HSC-T6 cells following exposure to alcohol. HSC-T6 cells were grown in 24-well plates at a density of 5 × 103 cells per well for 12 h and then transfected with PCBP2 siRNA at a dose of 50 nM. After 24 h of transfection with siRNA, the cells were incubated with medium containing100 mM alcohol for another 24 or 48 h. The relative expression levels of mRNA were determined by real-time RT-PCR, and the results are presented as the mean±SD (n=3) (*P<0.05).
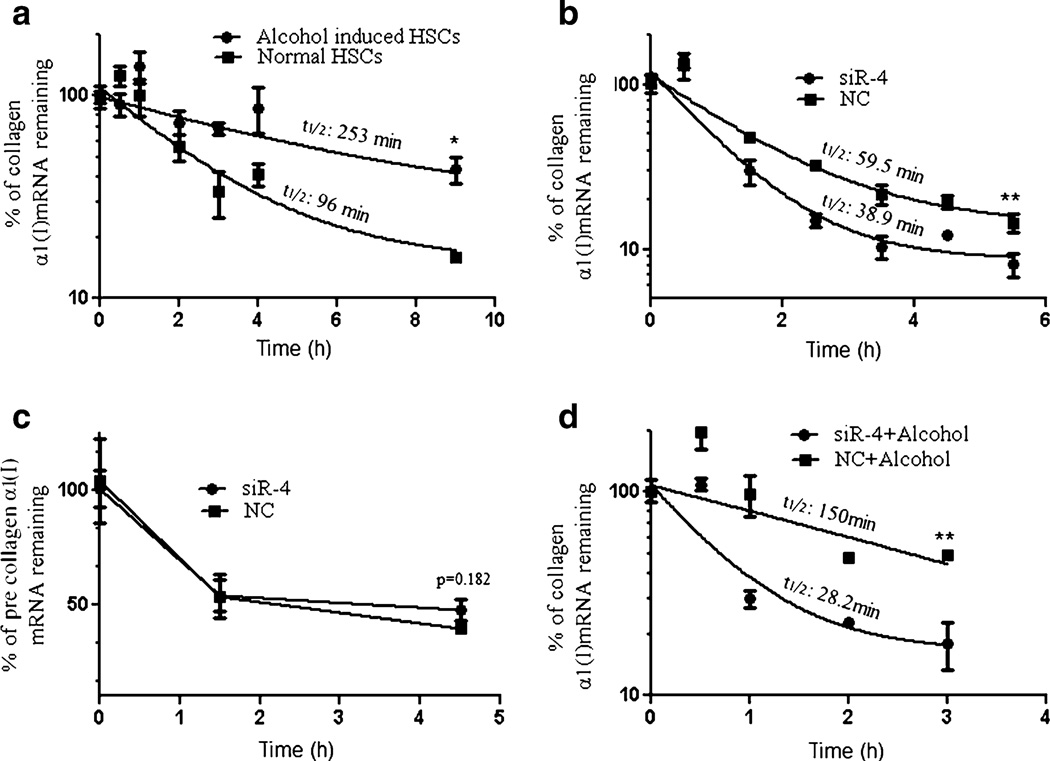
Because PCBP2 enhances the stability of collagen α1(I) mRNA, we next sought to determine whether silencing PCBP2 increases the decay rate of collagen α1(I) mRNA. We first evaluated the effect of alcohol on the decay of collagen α1(I) mRNA. Exposure to alcohol increased the half-life of collagen α1(I) mRNA from 96 min to 253 min in HSC-T6 cells (Fig. 8a). In the absence of alcohol, siR-4 reduced the half-life of collagen α1(I) mRNA from 59.5 min to 38.9 min (Fig. 8b). In contrast, the stability of the collagen α1(I) pre-mRNA was not affected (Fig. 8c). These results are consistent with previous reports that PCBP2 regulates the post-transcriptional activity of the collagen α1 (I) mRNA (12,13).
Fig. 8.
Effects of alcohol and the PCBP2 siRNA on the stability of collagen α1(I) mRNA. (a) Effect of exposure to alcohol on the stability of collagen α1(I) mRNA. (b) Effect of PCBP2 siRNA on the stability of mature collagen α1(I) mRNA. (c) Effect of PCBP2 siRNA on the stability of collagen α1(I) pre-mRNA. (d) Effect of PCBP2 siRNA on the stability of collagen α1(I) mRNA following exposure to alcohol. After incubation with siRNA and/or alcohol, the cells were incubated with actinomycin D (1 µM) to inhibit transcription. At each designated time-point, the mRNA levels were determined by real-time RT-PCR and are presented as the percentage of mRNA remaining versus time. Results are presented as the mean±SD (n=3) (*P<0.05; **P<0.01)
In the presence of both alcohol and siR-4, the half-life of the collagen α1(I) mRNA was dramatically reduced from 150 min to 28.2 min, a value similar to the half-life of collagen α1(I) mRNA in HSC-T6 cells treated with scrambled siRNA in the absence of alcohol (Fig. 8d). These results suggest a promising potential of PCBP2 siRNA for the reversal of the accumulated type I collagen in alcoholic liver fibrosis.
DISCUSSION
Alcohol abuse is one of the most common causes of liver fibrosis/cirrhosis in western developed countries. Similar to fibrosis caused by other chronic liver injuries, alcoholic liver fibrosis is characterized by excessive accumulation of the extracellular matrix, particularly type I collagen, in the liver (2,11,36). Despite extensive efforts, there is still no standard treatment for alcoholic liver fibrosis. Reversing the accumulation of type I collagen is an unavoidable challenge for all therapeutic approaches. Because type I collagen is a heterotrimer of two α1(I) and one α2(I) polypeptide chains, collagen α1(I) mRNA is considered an attractive target for anti-fibrotic therapy (10,12,13,36,37). Collagen α1(I) mRNA generally exists in two forms, a 4.7 kb mRNA and a 5.7 kb mRNA, both of which can form a complex with the RNA binding protein αCP2 at the 3′-UTR site in activated HSCs (13,43,44). An α-complex RNA binding protein encoded by the PCBP2 gene, αCP2, is found in excess in cytoplasmic extracts of activated HSCs. This protein binds to the C-rich region in the 3′-UTR site of the collagen α1(I) mRNA, leading to stabilization of the mRNA and subsequent accumulation of type I collagen in the liver (45,46). Of the four αCP family members, αCP1 and αCP2 are most abundantly expressed and play important roles in mRNA stabilization (14,15). Both αCP1 and αCP2 have been reported to stabilize the mRNAs of tyrosine hydroxylase (16), erythropoietin (17), α-globin (18) and β-globin (19). In particular, αCP2 has been identified as the only isoform that binds to the 3′-UTR region of the collagen α1 (I) mRNA (6,12,13,20). Thus, inhibition of αCP2 is expected to have a more significant effect on the stability of collagen α1(I) mRNA than on other mRNAs that can be stabilized by different αCP isoforms. For example, Chkheidze et al. have shown that αCP1, αCP2, and αCP2- KL are capable of forming an α-complex with the 3′-UTR of α-globin, and each of these three αCP isoforms alone is sufficient to form the α-complex and stabilize the α-globin mRNA (47). Therefore, the silencing of αCP2 alone is not likely to significantly change the expression of the α-globin mRNA.
In this study, we investigated two hypotheses: (i) HSC-T6 cells over-express αCP2 and type-I collagen following exposure to alcohol, and (ii) PCBP2 siRNA can reverse the alcohol-induced accumulation of collagen α1(I) mRNA in HSC-T6 cells. Although it has been demonstrated that alcohol stimulates the expression of type I collagen in primary hepatic stellate cells, the mechanism is not fully understood. In addition, technical difficulties associated with the isolation of primary hepatic stellate cells limit their application as an in vitro model to evaluate anti-fibrotic agents. We therefore chose HSC-T6, an immortalized rat hepatic stellate cell line (34), to evaluate the effects of alcohol and PCBP2 siRNAs.
Consistent with observations using primary hepatic stellate cells (31,32), we found that alcohol induces the expression of collagen α1(I) mRNA in HSC-T6 cells (Fig. 1). However, there was no significant change in the level of collagen α1(I) pre-mRNA, suggesting the important role of alcohol on post-transcriptional, but not transcriptional regulation of the PCBP2 gene. More importantly, we demonstrated for the first time that alcohol exposure induces the expression of PCBP2 in HSC-T6 cells. Immunostaining experiments further confirmed the induction effects of alcohol on both type I collagen and αCP2 (Figs. 2 and 3). Collectively, these data suggested that alcohol stimulates the production of type I collagen by inducing the PCBP2 gene, whose products stabilize the collagen α1(I) mRNA. These results also provide a sound rationale for reducing the accumulation of type I collagen in alcoholic liver fibrosis by silencing the PCBP2 gene.
Subsequently, we identified a potent PCBP2 siRNA (siR-4) and examined whether the silencing of PCBP2 results in a reduction of the level of collagen α1(I) mRNA in HSCs. To our surprise, we did not observe a significant reduction of type I collagen following the silencing of PCBP2 by siR-4 (data not shown). We postulated that pre-existing αCP2 proteins in HSC-T6 cells maintain the stabilization of the collagen α1(I) mRNA after the transitory silencing of PCBP2. Therefore, we performed multiple siRNA transfections to extend the silencing effects for up to six days. As shown in Fig. 6, continuous silencing of PCBP2 was able to reverse the increased expression of type I collagen at the protein levels. This result suggests that multiple administrations of the PCBP2 siRNA will be required for future in vivo studies.
After demonstrating that siR-4 can silence the PCBP2 gene even in the presence of alcohol (Fig. 7), we next determined if siR-4 could reverse the alcohol-induced accumulation of type I collagen in HSCs. It has been generally assumed that the accumulation of type I collagen in liver fibrosis is mainly due to the increased stability of the collagen α1(I) mRNA (10,12,13). We performed mRNA decay assays to evaluate the effects of alcohol induction and siRNA treatment on the half-life of the collagen α1(I) mRNA. Exposure to alcohol increased the half-life of the collagen α1(I) mRNA by 2.6-fold relative to control HSCT6 cells (Fig. 8a). To our knowledge, this is the first evidence of the induction effect of alcohol on the half-life of collagen α1(I) mRNA in HSC-T6 cells. After blocking transcription for 24 h, only 4% of the collagen α1(I) mRNA remained in the control HSC-T6 cells, whereas more than 34% of the collagen α1(I) mRNA remained in the HSC-T6 cells exposed to alcohol (data not shown).
We next compared the effect of PCBP2 siRNA on the stability of mature collagen α1(I) mRNA and collagen α1(I) pre-mRNA in HSC-T6 cells. While siR-4 significantly reduced the half-life of the mature collagen α1(I) mRNA, the effect on the collagen α1(I) pre-mRNA was negligible (Fig. 8b and c). In the presence of alcohol, the induction effect of siR-4 on the collagen α1(I) mRNA decay rate was more remarkable. The half-life was reduced by more than five-fold (Fig. 8d). Together, these results confirmed the hypothesis that PCBP2 siRNA reduces the stability of collagen α1(I) mRNA by silencing the PCBP2 gene, which produces a protein that stabilizes the collagen α1(I) mRNA.
We observed up to a 30% decrease in the level of collagen α1(I) mRNA because of its destabilization after multiple transfections that silenced the PCBP2 gene for up to six days (data not shown). However, this reduction effect on collagen α1(I) mRNA was not statistically significant, possibly due to the variation of real-time RT-PCR. Therefore, we confirmed the down-regulation effect at the protein level by western blotting (Fig. 6b). Although these effects were moderate, the reduction of the half-life of the collagen α1(I) mRNA by PCBP2 siRNA was more significant (Fig. 8). Two factors may account for this difference. First, the destabilization of collagen by PCBP2 siRNA is a slow indirect process because it directly silences the PCBP2 gene rather than the collagen α1(I) mRNA. Second, because of the large quantity of collagen in HSCs, the levels of mRNA and protein are not as sensitive to silencing as the mRNA half-life in a short period of time. We are planning to construct shRNA to silence the PCBP2 gene in HSCs over an extended period of time to observe the effects of prolonged inhibition on the levels of type I collagen mRNA and protein.
In conclusion, the observations in this study provide the first evidence that exposure to alcohol induces the expression of αCP2, which plays a key role in the accumulation of type I collagen in hepatic stellate cells. The siRNA-mediated knockdown of the PCBP2 gene may provide a way to reverse alcoholic liver fibrosis by increasing the decay rate of the accumulated type I collagen mRNA.
ACKNOWLEDGMENTS
This project has been supported by a grant (1R21AA017960- 01A1) from the National Institute of Alcohol Abuse and Alcoholism (NIAAA) at NIH and CA53596 to Wan. We also would like to express thanks for the financial support from a start-up package at the University of Missouri—Kansas City.
Contributor Information
Ravi S. Shukla, Division of Pharmaceutical Sciences, School of Pharmacy, University of Missouri-Kansas City, 2464 Charlotte Street, Kansas City, MO 64108, USA
Bin Qin, Division of Pharmaceutical Sciences, School of Pharmacy, University of Missouri-Kansas City, 2464 Charlotte Street, Kansas City, MO 64108, USA.
Yu-Jui Yvonne Wan, Department of Pharmacology, Toxicology, and Therapeutics, University of Kansas Medical Center, 3901 Rainbow Boulevard, Kansas City, KS 66212, USA.
Kun Cheng, Email: chengkun@umkc.edu, Division of Pharmaceutical Sciences, School of Pharmacy, University of Missouri-Kansas City, 2464 Charlotte Street, Kansas City, MO 64108, USA.
REFERENCES
- 1.Cheng K, Mahato RI. Gene modulation for treating liver fibrosis. Crit Rev Ther Drug Carrier Syst. 2007;24:93–146. doi: 10.1615/critrevtherdrugcarriersyst.v24.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegmund SV, Dooley S, Brenner DA. Molecular mechanisms of alcohol-induced hepatic fibrosis. Dig Dis. 2005;23:264–274. doi: 10.1159/000090174. [DOI] [PubMed] [Google Scholar]
- 3.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George J, Chandrakasan G. Molecular characteristics of dimethylnitrosamine induced fibrotic liver collagen. Biochim Biophys Acta. 1996;1292:215–222. doi: 10.1016/0167-4838(95)00202-2. [DOI] [PubMed] [Google Scholar]
- 5.LaBrecque DR, Gitnick GL, Moody FG. Diseases of the liver and biliary tract. St Louis: Mosby-Year Book; 1992. [Google Scholar]
- 6.Lindquist JN, Marzluff WF, Stefanovic B. Fibrogenesis. III. Posttranscriptional regulation of type I collagen. Am J Physiol Gastrointest Liver Physiol. 2000;279:G471–G476. doi: 10.1152/ajpgi.2000.279.3.G471. [DOI] [PubMed] [Google Scholar]
- 7.Vuorio E, de Crombrugghe B. The family of collagen genes. Annu Rev Biochem. 1990;59:837–872. doi: 10.1146/annurev.bi.59.070190.004201. [DOI] [PubMed] [Google Scholar]
- 8.Joseph J, Kandala JC, Veerapanane D, Weber KT, Guntaka RV. Antiparallel polypurine phosphorothioate oligonucleotides form stable triplexes with the rat alpha1(I) collagen gene promoter and inhibit transcription in cultured rat fibroblasts. Nucleic Acids Res. 1997;25:2182–2188. doi: 10.1093/nar/25.11.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato M, Suzuki S, Senoo H. Hepatic stellate cells: unique characteristics in cell biology and phenotype. Cell Struct Funct. 2003;28:105–112. doi: 10.1247/csf.28.105. [DOI] [PubMed] [Google Scholar]
- 10.Lindquist JN, Stefanovic B, Brenner DA. Regulation of collagen alpha1(I) expression in hepatic stellate cells. J Gastroenterol. 2000;35(Suppl 12):80–83. [PubMed] [Google Scholar]
- 11.Siegmundand SV, Brenner DA. Molecular pathogenesis of alcohol-induced hepatic fibrosis. Alcohol Clin Exp Res. 2005;29:102S–109S. doi: 10.1097/01.alc.0000189275.97419.58. [DOI] [PubMed] [Google Scholar]
- 12.Lindquist JN, Parsons CJ, Stefanovic B, Brenner DA. Regulation of alpha1(I) collagen messenger RNA decay by interactions with alphaCP at the 3′-untranslated region. J Biol Chem. 2004;279:23822–23829. doi: 10.1074/jbc.M314060200. [DOI] [PubMed] [Google Scholar]
- 13.Stefanovic B, Hellerbrand C, Holcik M, Briendl M, Aliebhaber S, Brenner DA. Posttranscriptional regulation of collagen alpha1(I) mRNA in hepatic stellate cells. Mol Cell Biol. 1997;17:5201–5209. doi: 10.1128/mcb.17.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makeyev AV, Liebhaber SA. The poly(C)-binding proteins: a multiplicity of functions and a search for mechanisms. RNA. 2002;8:265–278. doi: 10.1017/s1355838202024627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makeyev AV, Eastmond DL, Liebhaber SA. Targeting a KH-domain protein with RNA decoys. RNA. 2002;8:1160–1173. doi: 10.1017/s135583820202808x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paulding WR, Czyzyk-Krzeska MF. Regulation of tyrosine hydroxylase mRNA stability by protein-binding, pyrimidine-rich sequence in the 3′-untranslated region. J Biol Chem. 1999;274:2532–2538. doi: 10.1074/jbc.274.4.2532. [DOI] [PubMed] [Google Scholar]
- 17.Czyzyk-Krzeska MF, Bendixen AC. Identification of the poly(C) binding protein in the complex associated with the 3′ untranslated region of erythropoietin messenger RNA. Blood. 1999;93:2111–2120. [PubMed] [Google Scholar]
- 18.Ji X, Kong J, Liebhaber SA. In vivo association of the stability control protein alphaCP with actively translating mRNAs. Mol Cell Biol. 2003;23:899–907. doi: 10.1128/MCB.23.3.899-907.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J, Russell JE. Structural and functional analysis of an mRNP complex that mediates the high stability of human beta-globin mRNA. Mol Cell Biol. 2001;21:5879–5888. doi: 10.1128/MCB.21.17.5879-5888.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindquist JN, Kauschke SG, Stefanovic B, Burchardt ER, Brenner DA. Characterization of the interaction between alphaCP(2) and the 3′-untranslated region of collagen alpha1(I) mRNA. Nucleic Acids Res. 2000;28:4306–4316. doi: 10.1093/nar/28.21.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato J, Sato Y, Inui N, Nakano Y, Takimoto R, Takada K, et al. Ethanol induces transforming growth factor-alpha expression in hepatocytes, leading to stimulation of collagen synthesis by hepatic stellate cells. Alcohol Clin Exp Res. 2003;27:58S–63S. doi: 10.1097/01.ALC.0000078614.44983.97. [DOI] [PubMed] [Google Scholar]
- 22.Nieto N, Greenwel P, Friedman SL, Zhang F, Dannenberg AJ, Cederbaum AI. Ethanol and arachidonic acid increase alpha 2(I) collagen expression in rat hepatic stellate cells overexpressing cytochrome P450 2E1. Role of H2O2 and cyclooxygenase-2. J Biol Chem. 2000;275:20136–20145. doi: 10.1074/jbc.M001422200. [DOI] [PubMed] [Google Scholar]
- 23.Anania FA, Womack L, Jiang M, Saxena NK. Aldehydes potentiate alpha(2)(I) collagen gene activity by JNK in hepatic stellate cells. Free Radic Biol Med. 2001;30:846–857. doi: 10.1016/s0891-5849(01)00470-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin B, Cheng K. Silencing of the IKKepsilon gene by siRNA inhibits invasiveness and growth of breast cancer cells. Breast Cancer Res. 12:R74. doi: 10.1186/bcr2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tai W, Qin B, Cheng K. Inhibition of breast cancer cell growth and invasiveness by dual silencing of HER-2 and VEGF. Mol Pharm. 7:543–556. doi: 10.1021/mp9002514. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed N, Dreier R, Gopferich A, Grifka J, Grassel S. Soluble signalling factors derived from differentiated cartilage tissue affect chondrogenic differentiation of rat adult marrow stromal cells. Cell Physiol Biochem. 2007;20:665–678. doi: 10.1159/000107728. [DOI] [PubMed] [Google Scholar]
- 27.Grassel S, Rickert M, Opolka A, Bosserhoff A, Angele P, Grifka J, et al. Coculture between periosteal explants and articular chondrocytes induces expression of TGF-beta1 and collagen I. Rheumatology (Oxford) 2010;49:218–230. doi: 10.1093/rheumatology/kep326. [DOI] [PubMed] [Google Scholar]
- 28.Mahato R, Qin B, Cheng K. Blocking IKKalpha expression inhibits prostate cancer invasiveness. Pharm Res. 2010 doi: 10.1007/s11095-010-0351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brewer G. Regulation of c-myc mRNA decay in vitro by a phorbol ester-inducible, ribosome-associated component in differentiating megakaryoblasts. J Biol Chem. 2000;275:33336–33345. doi: 10.1074/jbc.M006145200. [DOI] [PubMed] [Google Scholar]
- 30.Opyrchal M, Anderson JR, Sokoloski KJ, Wilusz CJ, Wilusz J. A cell-free mRNA stability assay reveals conservation of the enzymes and mechanisms of mRNA decay between mosquito and mammalian cell lines. Insect Biochem Mol Biol. 2005;35:1321–1334. doi: 10.1016/j.ibmb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Fontana L, Jerez D, Rojas-Valencia L, Solis-Herruzo JA, Greenwel P, Rojkind M. Ethanol induces the expression of alpha 1(I) procollagen mRNA in a co-culture system containing a liver stellate cell-line and freshly isolated hepatocytes. Biochim Biophys Acta. 1997;1362:135–144. doi: 10.1016/s0925-4439(97)00056-2. [DOI] [PubMed] [Google Scholar]
- 32.Tsutsumi M, Takada A, Takase S, Shimanaka K, Enyama K. Effects of ethanol and its metabolites on collagen synthesis by cultured rat liver cells. J Gastroenterol Hepatol. 1989;4:229–240. doi: 10.1111/j.1440-1746.1989.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 33.Maher JJ, Zia S, Tzagarakis C. Acetaldehyde-induced stimulation of collagen synthesis and gene expression is dependent on conditions of cell culture: studies with rat lipocytes and fibroblasts. Alcohol Clin Exp Res. 1994;18:403–409. doi: 10.1111/j.1530-0277.1994.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 34.Vogel S, Piantedosi R, Frank J, Lalazar A, Rockey DC, Friedman SL, et al. An immortalized rat liver stellate cell line (HSC-T6): a new cell model for the study of retinoid metabolism in vitro. J Lipid Res. 2000;41:882–893. [PubMed] [Google Scholar]
- 35.Cheng K, Yang N, Mahato RI. TGF-beta1 gene silencing for treating liver fibrosis. Mol Pharm. 2009;6:772–779. doi: 10.1021/mp9000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng K, Ye Z, Guntaka RV, Mahato RI. Biodistribution and hepatic uptake of triplex-forming oligonucleotides against type alpha1(I) collagen gene promoter in normal and fibrotic rats. Mol Pharm. 2005;2:206–217. doi: 10.1021/mp050012x. [DOI] [PubMed] [Google Scholar]
- 37.Cheng K, Ye Z, Guntaka RV, Mahato RI. Enhanced hepatic uptake and bioactivity of type alpha1(I) collagen gene promoter-specific triplex-forming oligonucleotides after conjugation with cholesterol. J Pharmacol Exp Ther. 2006;317:797–805. doi: 10.1124/jpet.105.100347. [DOI] [PubMed] [Google Scholar]
- 38.Ye Z, Cheng K, Guntaka RV, Mahato RI. Targeted delivery of a triplex-forming oligonucleotide to hepatic stellate cells. Biochemistry. 2005;44:4466–4476. doi: 10.1021/bi047529j. [DOI] [PubMed] [Google Scholar]
- 39.Hamlyn AN, Brown AJ, Sherlock S, Baron DN. Casual blood-ethanol estimations in patients with chronic liver disease. Lancet. 1975;2:345–347. doi: 10.1016/s0140-6736(75)92781-6. [DOI] [PubMed] [Google Scholar]
- 40.Israel Y, Britton RS, Orrego H. Liver cell enlargement induced by chronic alcohol consumption: studies on its causes and consequences. Clin Biochem. 1982;15:189–192. doi: 10.1016/s0009-9120(82)90048-0. [DOI] [PubMed] [Google Scholar]
- 41.Hijioka T, Sato N, Matsumura T, Yoshihara H, Takei Y, Fukui H, et al. Ethanol-induced disturbance of hepatic microcirculation and hepatic hypoxia. Biochem Pharmacol. 1991;41:1551–1557. doi: 10.1016/0006-2952(91)90153-v. [DOI] [PubMed] [Google Scholar]
- 42.Oshita M, Sato N, Yoshihara H, Takei Y, Hijioka T, Fukui H, et al. Ethanol-induced vasoconstriction causes focal hepatocellular injury in the isolated perfused rat liver. Hepatology. 1992;16:1007–1013. doi: 10.1002/hep.1840160425. [DOI] [PubMed] [Google Scholar]
- 43.Maatta A, Penttinen RP. A fibroblast protein binds the 3′-untranslated region of pro-alpha 1(I) collagen mRNA. Biochem J. 1993;295(Pt 3):691–698. doi: 10.1042/bj2950691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maatta A, Penttinen RP. Nuclear and cytoplasmic alpha 1 (I) collagen mRNA-binding proteins. FEBS Lett. 1994;340:71–77. doi: 10.1016/0014-5793(94)80175-4. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Kiledjian M, Weiss IM, Liebhaber SA. Detection and characterization of a 3′ untranslated region ribonucleoprotein complex associated with human alpha-globin mRNA stability. Mol Cell Biol. 1995;15:1769–1777. doi: 10.1128/mcb.15.3.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss IM, Liebhaber SA. Erythroid cell-specific determinants of alpha-globin mRNA stability. Mol Cell Biol. 1994;14:8123–8132. doi: 10.1128/mcb.14.12.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chkheidze AN, Lyakhov DL, Makeyev AV, Morales J, Kong J, Liebhaber SA. Assembly of the alpha-globin mRNA stability complex reflects binary interaction between the pyrimidine-rich 3′ untranslated region determinant and poly(C) binding protein alphaCP. Mol Cell Biol. 1999;19:4572–4581. doi: 10.1128/mcb.19.7.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]