Abstract
Central nervous system (CNS) damage associated with psychostimulant dependence may be an ongoing, degenerative process with adverse effects on neuropsychiatric function. However, the molecular mechanisms regarding how altered energy regulation affects immune response in the context of substance use disorders are not fully understood. This review summarizes the current evidence regarding the effects of psychostimulant [particularly 3,4-methylenedioxy-N-methylamphetamine (MDMA) and methamphetamine] exposure on brain energy regulation, immune response, and neuropsychiatric function. Importantly, the neuropsychiatric impairments (e.g., cognitive deficits, depression, and anxiety) that persist following abstinence are associated with poorer treatment outcomes – increased relapse rates, lower treatment retention rates, and reduced daily functioning. Qualifying the molecular changes within the CNS according to the exposure and use patterns of specifically abused substances should inform the development of new therapeutic approaches for addiction treatment.
Keywords: Methamphetamine; 3,4-Methylenedioxy-N-methylamphetamine (MDMA); Neurodegenerative diseases; Inflammation; Oxidative stress; Bioenergetics
1. Introduction
Increasingly, in addition to neurotoxic effects on neurotransmitter systems (e.g., dopamine, serotonin, and glutamate), changes in cellular bioenergetics, redox (reduction–oxidation) balance, and immune function are thought to contribute to the development and persistence of central nervous system (CNS) impairments associated with exposure to psychostimulants, including amphetamine derivatives [e.g., 3,4-methylenedioxy-N-methylamphetamine (MDMA), methamphetamine] and other drugs of abuse (e.g., cocaine). Methamphetamine and MDMA abuse, in particular, are associated with long-term damage to regions of the brain that control cognitive and psychiatric function, which can persist for months or years following abstinence. The neuropsychiatric impairments that last following abstinence are associated with poorer treatment outcomes, including increased relapse rates, lower treatment retention rates, and reduced daily functioning.
2. Brain energy regulation and psychostimulant exposure
Acute and, in particular, chronic exposure to abused substances can lead to changes in brain metabolism and energy requirements, which are complex and often brain-region specific. For example, MDMA increases extracellular glucose in multiple brain regions (Pachmerhiwala et al., 2010), especially within striatum (Darvesh et al., 2002; Gramsbergen and Cumming, 2007). MDMA also reduces the brain concentration of glycogen, one of the main energy reserves in brain (Darvesh and Gudelsky, 2004; Darvesh et al., 2002). Consistent with these preclinical observations, human studies using positron emission tomography (PET) with 2-[18F]-fluoro-2-deoxy-d-glucose (FDG) show that FDG uptake rates are globally reduced in “ecstasy” (MDMA) users (most notably in the striatum) and tend to be negatively correlated with cumulative “ecstasy” doses (Buchert et al., 2001; Obrocki et al., 2002; Volkow et al., 2003). In an earlier report the analog of “ecstasy,” 3,4-methylenedioxyethamphetamine (MDE) and methamphetamine were shown to induce cortical hypometabolism and cerebellar hypermetabolism (Gouzoulis-Mayfrank et al., 1999). More recently, Sailasuta and colleagues measured glial metabolic flux rate in frontal brain structures and observed a 50% reduction in the oxidative rate in recovering brain (at least seven days abstinent) following methamphetamine abuse (Sailasuta et al., 2010). Methamphetamine exposure also significantly inhibits glucose uptake by neurons and astrocytes, and neurons appear to be more sensitive to methamphetamine's effects on glucose uptake than astrocytes. Evidence suggests that the ability of astrocytes to use fatty acid oxidation as an alternative source of energy may contribute to this differential sensitivity (Abdul Muneer et al., 2011a).
Psychostimulant-induced alterations in energy regulation are accompanied by oxidative and nitrosative stress as well as by reductions in antioxidant defense mechanisms (Capela et al., 2009; Quinton and Yamamoto, 2006; Song et al., 2010). Methamphetamine increases reactive oxygen species production and oxidative stress, mainly in brain regions containing dopamine neurons (Cubells et al., 1994) – putatively due to the release of dopamine following methamphetamine exposure (Graham, 1978). Reactive oxygen species are produced following methamphetamine exposure in a dose-dependent manner in rat striatal synaptosomes (Pubill et al., 2005) for example, and MDMA and other psychostimulants (e. g., cocaine) also increase free radical generation (e.g., superoxide), lipid peroxidation (a measure of damaged cellular membranes from reactive oxygen species), and nitric oxide-dependent protein nitration [a common effect associated with oxidative stress, particularly at sites of inflammation (Galinanes and Matata, 2002)]. Human in vitro studies and animal models show increase in the expression of neuronal and inducible nitric oxide synthase, increased nitric oxide synthase activity, and increased nitric oxide production following exposure to psychostimulants (Friend et al., 2013; Loftis and Janowsky, 2000; Permpoonputtana and Govitrapong, 2013). It was recently shown that the combination of MDMA and cocaine augments oxidative/nitrosative stress in mouse striatum, leading to an increase in protein nitration and lipid peroxidation but does not potentiate dopaminergic neurotoxicity (Peraile et al., 2013). Interestingly, although it was originally hypothesized that exposure to MDMA and cocaine (e.g., polysubstance use) would produce further redox imbalance and potentiate brain damage, exposure to cocaine increased glutathione peroxidase activity, a cellular antioxidant defense mechanism, and may have provided some neuroprotection for dopaminergic neurons (Peraile et al., 2013). Thus, it is apparent that multiple mechanisms underlie the cellular adaptations – neurotoxic as well as potentially neuroprotective – associated with psychostimulants and other substances of abuse.
3. Brain energetics, immune response, and neuropsychiatric symptoms accompanying psychostimulant exposure
Drug induced alterations in energy utilization, oxidative/nitrosative stress and immune response are associated with significant cognitive impairments and mood disturbances (Loftis and Huckans, 2013; Parrott, 2006). PET studies report altered brain glucose metabolism that correlates with severity of psychiatric symptoms in the limbic and orbitofrontal regions of adults who used methamphetamine (Chang et al., 2007). Kim and colleagues found that methamphetamine dependent adults have dose-dependent frontal hypometabolism that is associated with frontal executive dysfunction (Kim et al., 2009). Methamphetamine users have also been observed to display white matter hypertrophy and reduced hippocampal volume in comparison to human controls (Thompson et al., 2004). Verbal learning and recall deficits of recreational MDMA users are similarly correlated with glucose hypometabolism in prefrontal and parietal cortices (Bosch et al., 2013). Mild to marked loss of serotonin transporter binding in cerebral cortex/hippocampus has also been observed in chronic MDMA users (Kish et al., 2010), with these reductions in serotonin transporter binding being previously associated with the memory deficits common in humans with a history of recreational MDMA use (McCann et al., 2008). Comparable findings are reported using animal models of substance abuse.
A recent preclinical study found that mice treated with MDMA displayed higher oxidative damage than ethanol-treated mice 72 h after drug exposure, and this increase in oxidative injury was accompanied by impaired long-term memory, as evidenced on both object recognition and radial arm maze tests (Ros-Simo et al., 2013). Correspondingly, developmental studies show that maternal methamphetamine and MDMA exposure can induce cognitive impairments in offspring that are associated with damage to the hippocampus and other brain regions (Siegel et al., 2011; Zuloaga et al., 2013; Skelton et al., 2008; Schaefer et al., 2013). Consistent with and bridging these findings are studies showing that psychostimulants induce alterations in neurotrophic factors which may also play a role in cognitive dysfunction (Hemmerle et al., 2012; Chen et al., 2012). For example, MDMA significantly suppresses neurite outgrowth of PC12 cells (a catecholaminergic cell line often used in neurotoxicological studies) induced by nerve growth factor (Kaizaki et al., 2010). Thus, in combination with altered brain energetics and immune function, changes in neurotrophic factor signaling may additionally contribute to the adverse CNS and neuropsychiatric effects associated with abuse of MDMA and/or methamphetamine.
Protracted abstinence can reverse some drug induced alterations in brain neurochemical systems (Volkow et al., 2001b), but other mechanisms, such as impaired bioenergetics and immune response may contribute to and perpetuate the mood and cognitive impairments that persist following stimulant exposure. Indeed, cognitive deficits ostensibly caused by ‘heavy’ usage or the dependence on or abuse of psychostimulant drugs are not reversed by six months of abstinence (Potter et al., 2013). Studies show that adults with a history of methamphetamine abuse have significantly higher metabolism than control subjects in the parietal cortex and significantly lower metabolism in the thalamus, caudate, and putamen (Berman et al., 2008; Volkow et al., 2001a). These metabolic differences are present even in former methamphetamine abusers that have been abstinent for at least 11 months (Volkow et al., 2001a). Further, methamphetamine-induced alterations in parietal cortex metabolism correlate with performance on the Grooved Pegboard task, indicating that hyperactivity of the parietal cortex may have functional significance. Volkow and associates hypothesize that higher parietal activity in adults with a history of methamphetamine abuse could reflect reactive gliosis (Berman et al., 2008; Volkow et al., 2001a). In line with these observations, a global pattern of microglial activation and microgliosis persists in the brains of methamphetamine addicted adults for at least two years into abstinence (Sekine et al., 2008).
There is a critical balance between the metabolic systems regulating energy and the protective mechanisms regulating immunity. Exposure to drugs of abuse, such as MDMA and methamphetamine can adversely affect this balance, and this imbalance has been implicated in potentiating brain damage in various neurological conditions (e.g., Matarese et al., 2008). Among the questions yet to be answered is how altered energy regulation affects immune response in the context of substance use disorders. Based on the evidence available to date, there are at least three mechanisms by which impaired bioenergetics may affect immune function and inflammatory processes and contribute to persistent neuropsychiatric impairments.
3.1. Redox imbalance
The redox environment can have significant impact on CNS function, as it modulates cell proliferation, apoptosis, cell adhesion molecules [e.g., intercellular adhesion molecule 1 (ICAM-1) and monocyte chemotactic protein-1 (MCP-1)], and pro-inflammatory cytokines. In the brain, methamphetamine and MDMA cause neurodegeneration, in part, through nitric oxide and the generation of peroxynitrite (superoxide can react with nitric oxide, giving rise to peroxynitrite) and peroxynitrite is released predominantly by inflammatory cells (Halliwell, 2006; Matata and Galinanes, 2002). Further, reactive oxygen species are generated in response to cytokine signaling as well as during mitochondrial oxidative metabolism, and methamphetamine exposure results in mitochondrial oxidative damage and T-cell dysfunction (Potula et al., 2010). We have previously reported increased ICAM-1 [also known as Cluster of Differentiation 54 (CD54)] and MCP-1 [also known as chemokine (C–C motif) ligand 2 (CCL2)] in plasma of both humans and mice following methamphetamine exposure (Loftis et al., 2011). In human participants, methamphetamine-induced changes in cytokine and chemokine expression were accompanied by increased cognitive impairments across multiple domains, including attention, memory, and learning – suggesting that chronic immune dysregulation may be related to neuropsychiatric deficits in methamphetamine users (Loftis et al., 2011).
3.2. Protein glycation
Protein glycation (i.e., glucose chemically attaching to proteins and nucleic acids without the aid of enzymes) can lead to increased immunogenicity of endogenous proteins (Brownlee et al., 1984). Consequently, proteins glycated by methamphetamine (or other drugs of abuse) may evoke an abnormal immune response via the generation of advanced glycation endproducts (AGEs) (Dickerson et al., 2004) – potentially leading to autoimmunity, an abundance of non-specifically activated T-cells, or systemic inflammation. In a currently underway trial, we have observed a significant correlation between increased T-cell proliferation and poorer working memory within patients in remission from methamphetamine dependence (Huckans et al., 2012).
3.3. Altered energy utilization at the blood brain barrier (BBB)
Many drugs of abuse, including psychostimulants, are proinflammatory which leads to BBB disruption. In both preclinical and clinical studies, methamphetamine, MDMA, cocaine, and nicotine, produce BBB dysfunction through alterations in tight junction protein expression and conformation, increased glial activation, increased enzyme activation related to BBB cytoskeleton remodeling, and induction of neuroinflammatory pathways (Carvalho et al., 2012). Indeed, methamphetamine-induced interference of glucose uptake and transport at the endothelium can disrupt the energy requirement of the BBB function and integrity, and impairment of glucose transporter protein-1 at the brain endothelium by methamphetamine may contribute to energy-associated disruption of BBB integrity (Abdul Muneer et al., 2011b). In other studies, BBB permeability has been assessed using immunoglobulin G (IgG) immunostaining, as IgG leakage from serum into the brain is a marker of vasculature damage (Torres et al., 2011; Urrutia et al., 2013). Torres and colleagues report increased IgG immunostaining in rat hypothalamus following MDMA exposure (Torres et al., 2011). Similarly, mice treated with methamphetamine show increased IgG striatal immunoreactivity, which colocalizes with areas of greater activity of matrix metalloproteinase-9, an enzyme that can degrade junction proteins and change the permeability of the BBB (Urrutia et al., 2013). Oxidative stress and hyperthermia can also increase BBB dysfunction which in turn, can exacerbate neuroinflammation after methamphetamine or MDMA exposure (Yamamoto and Bankson, 2005; Yamamoto and Raudensky, 2008). Thus, alterations in BBB integrity resulting from altered energy utilization and inflammation produces a cascade resulting in further BBB disruption and increased penetration of immune cells into the CNS (Kousik et al., 2012) – putatively contributing to neuropsychiatric symptoms during and following substance abuse.
4. Future implications
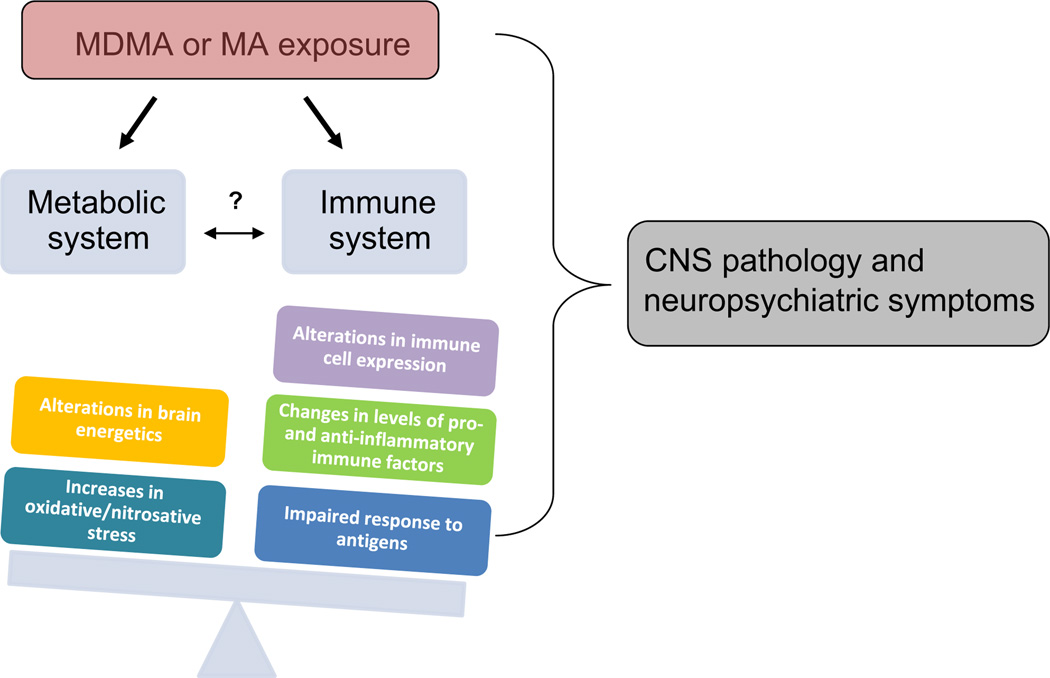
It is apparent that multiple mechanisms underlie the cellular adaptations – neurotoxic as well as potentially neuroprotective – associated with psychostimulants and other substances of abuse; however, the extent to which mono- or polysubstance abuse impacts regulation of brain energy utilization remains poorly understood. Furthermore, there is limited information regarding how the effects of exercise, diet, chronic viral infection(s) (e.g., hepatitis C, human immunodeficiency virus), other co-morbid clinical conditions, or environmental factors impact brain energy utilization and immune response in the context of chronic exposure to, or withdrawal from, abused substances. It is likely that such factors may synergize with the effects of substances of abuse to impact brain energy utilization and inflammatory processes, and these reviewed factors are presented graphically in Fig. 1 to illustrate how MDMA or methamphetamine exposure can adversely affect the balance between metabolic and immune systems and produce CNS damage and precipitate neuropsychiatric impairments. Given the probable synergistic or additive effects of poly substance abuse on CNS functioning and psychiatric sequelae, more research is needed to evaluate “the interplay between molecular regulation of brain energy utilization and brain and/or behavioral changes resulting from chronic exposure to abused substances” (National Institutes of Health, 2013).
Fig. 1.
MDMA or methamphetamine (MA) exposure can adversely affect the balance between metabolic and immune systems and contribute to CNS damage and neuropsychiatric impairments (e.g., cognitive deficits, depression, and anxiety) (e.g., Parrott, 2006). The question mark in the figure indicates that there are questions yet to be answered regarding how drug-induced changes in energy regulation affect immune function (and vice versa).
5. Summary
Taken together, studies suggest that CNS damage associated with psychostimulant dependence may be an ongoing, degenerative process with adverse effects on neuropsychiatric function. Peraile and colleagues aptly note that oxidative and nitrosative stress play a key role in the pathogenesis of neurodegenerative diseases such as Alzheimer's disease, amyotrophic lateral sclerosis, Huntington's disease, Parkinson's disease, and brain ischemia/ reperfusion injury – as well as substance use disorders (Peraile et al., 2013). In addition, a growing literature demonstrates that exposure to psychostimulants and other drugs of abuse alters peripheral and central immune functions (In et al., 2004, 2005; Liang et al., 2008; Martinez et al., 2009; Ye et al., 2008) and that immune factors such as cytokines, chemokines, and adhesion molecules likely play a role in the development of substance-induced neuronal injury and neuropsychiatric impairment (Loftis et al., 2011). Importantly, the neuropsychiatric impairments that persist following abstinence are associated with poorer treatment outcomes, including increased relapse rates, lower treatment retention rates, and reduced daily functioning (Aharonovich et al., 2003; Bowden et al., 2001; Sadek et al., 2007). Qualifying the molecular changes within the CNS according to the exposure and use patterns of specifically abused substances should inform the development of new therapeutic approaches for addiction treatment.
Acknowledgments
The research discussed in this review was in part supported by National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH) Grants (DA028537 and DA018165). This material was also supported with resources and the use of facilities at the Portland Veterans Affairs (VA) Medical Center and Oregon Health & Sciences University. Dr. Jennifer Loftis is a Research Scientist at the Portland VA Medical Center. Dr. Luke Downey is supported by a National Health and Medical Research Council (NH&MRC) biomedical fellowship (APP1054279).
Footnotes
Chemical compounds studied in this article:
Cocaine (PubChem CID: 5760)
2-Fluoro-2-deoxy-d-glucose (PubChem CID: 315411)
Methamphetamine (PubChem CID: 10836)
3,4-Methylenedioxyethamphetamine (MDE) (PubChem CID: 105039)
3,4-Methylenedioxy-N-methylamphetamine (MDMA) (PubChem CID: 1615)
References
- Abdul Muneer PM, Alikunju S, Szlachetka AM, Haorah J. Methamphetamine inhibits the glucose uptake by human neurons and astrocytes: stabilization by acetyl-L-carnitine. PLoS One. 2011a;6:e19258. doi: 10.1371/journal.pone.0019258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul Muneer PM, Alikunju S, Szlachetka AM, Murrin LC, Haorah J. Impairment of brain endothelial glucose transporter by methamphetamine causes blood-brain barrier dysfunction. Mol. Neurodegener. 2011b;6:23. doi: 10.1186/1750-1326-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Depend. 2003;71:207–211. doi: 10.1016/s0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SM, Voytek B, Mandelkern MA, Hassid BD, Isaacson A, Monterosso J, Miotto K, Ling W, London ED. Changes in cerebral glucose metabolism during early abstinence from chronic methamphetamine abuse. Mol. Psychiatry. 2008;13:897–908. doi: 10.1038/sj.mp.4002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OG, Wagner M, Jessen F, Kuhn KU, Joe A, Seifritz E, Maier W, Biersack HJ, Quednow BB. Verbal memory deficits are correlated with prefrontal hypometabolism in (18)FDG PET of recreational MDMA users. PLoS One. 2013;8:e61234. doi: 10.1371/journal.pone.0061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden SC, Crews FT, Bates ME, Fals-Stewart W, Ambrose ML. Neurotoxicity and neurocognitive impairments with alcohol and drug-use disorders: potential roles in addiction and recovery. Alcohol Clin. Exp. Res. 2001;25:317–321. [PubMed] [Google Scholar]
- Brownlee M, Vlassara H, Cerami A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann. Intern. Med. 1984;101:527–537. doi: 10.7326/0003-4819-101-4-527. [DOI] [PubMed] [Google Scholar]
- Buchert R, Obrocki J, Thomasius R, Vaterlein O, Petersen K, Jenicke L, Bohuslavizki KH, Clausen M. Long-term effects of ‘ecstasy’ abuse on the human brain studied by FDG PET. Nucl. Med. Commun. 2001;22:889–897. doi: 10.1097/00006231-200108000-00007. [DOI] [PubMed] [Google Scholar]
- Capela JP, Carmo H, Remiao F, Bastos ML, Meisel A, Carvalho F. Molecular and cellular mechanisms of ecstasy-induced neurotoxicity: an overview. Mol. Neurobiol. 2009;39:210–271. doi: 10.1007/s12035-009-8064-1. [DOI] [PubMed] [Google Scholar]
- Carvalho M, Carmo H, Costa VM, Capela JP, Pontes H, Remiao F, Carvalho F, Bastos Mde L. Toxicity of amphetamines: an update. Arch. Toxicol. 2012;86:1167–1231. doi: 10.1007/s00204-012-0815-5. [DOI] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl. 1):S16–S32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Chen PH, Huang MC, Lai YC, Chen PY, Liu HC. Serum brain-derived neurotrophic factor levels were reduced during methamphetamine early withdrawal. Addict. Biol. 2012 doi: 10.1111/j.1369-1600.2012.00444.x. [Epub ahead of print] PubMed PMID: 22458544. [DOI] [PubMed] [Google Scholar]
- Cubells JF, Rayport S, Rajendran G, Sulzer D. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J. Neurosci. 1994;14:2260–2271. doi: 10.1523/JNEUROSCI.14-04-02260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvesh AS, Gudelsky GA. The relationship between hyperthermia and glycogenolysis in 3,4-methylenedioxymethamphetamine-induced serotonin depletion in rats. Neurotoxicol. Teratol. 2004;26:571–577. doi: 10.1016/j.ntt.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Darvesh AS, Shankaran M, Gudelsky GA. 3,4-Methylenedioxymethamphetamine produces glycogenolysis and increases the extracellular concentration of glucose in the rat brain. J. Pharmacol. Exp. Ther. 2002;301:138–144. doi: 10.1124/jpet.301.1.138. [DOI] [PubMed] [Google Scholar]
- Dickerson TJ, Yamamoto N, Ruiz DI, Janda KD. Immunological consequences of methamphetamine protein glycation. J. Am. Chem. Soc. 2004;126:11446–11447. doi: 10.1021/ja047690h. [DOI] [PubMed] [Google Scholar]
- Friend DM, Son JH, Keefe KA, Fricks-Gleason AN. Expression and activity of nitric oxide synthase isoforms in methamphetamine-induced striatal dopamine toxicity. J. Pharmacol. Exp. Ther. 2013;344:511–521. doi: 10.1124/jpet.112.199745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinanes M, Matata BM. Protein nitration is predominantly mediated by a peroxynitrite-dependent pathway in cultured human leucocytes. Biochem. J. 2002;367:467–473. doi: 10.1042/BJ20020825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Schreckenberger M, Sabri O, Arning C, Thelen B, Spitzer M, Kovar KA, Hermle L, Bull U, Sass H. Neurometabolic effects of psilocybin, 3,4-methylenedioxyethylamphetamine (MDE) and d-methamphetamine in healthy volunteers. A double-blind, placebo-controlled PET study with [18F]FDG. Neuropsychopharmacology. 1999;20:565–581. doi: 10.1016/S0893-133X(98)00089-X. [DOI] [PubMed] [Google Scholar]
- Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol. Pharmacol. 1978;14:633–643. [PubMed] [Google Scholar]
- Gramsbergen JB, Cumming P. Serotonin mediates rapid changes of striatal glucose and lactate metabolism after systemic 3,4-methylenedioxymethamphetamine (MDMA, "Ecstasy") administration in awake rats. Neurochem. Int. 2007;51:8–15. doi: 10.1016/j.neuint.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and neurodegeneration: where are we now? J. Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- Hemmerle AM, Dickerson JW, Herring NR, Schaefer TL, Vorhees CV, Williams MT, Seroogy B. (±)3,4 methylenedioxymethamphetamine (“ecstasy”) treatment modulates expression of neurotrophins and their receptors in multiple regions of adult rat brain. J. Comp. Neurol. 2012;520:2459–2474. doi: 10.1002/cne.23048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckans M, Buenafe A, Huan J, Vandenbark AA, Loftis JM. Methamphetamine addiction is associated with increased T-cell immunoreactivity and neuropsychiatric impairment. Brain, Behavior, and Immunity. 2012;26(Supp 1):S25–S26. [Google Scholar]
- In SW, Son EW, Rhee DK, Pyo S. Modulation of murine macrophage function by methamphetamine. J Toxicol Environ Health A. 2004;67:1923–1937. doi: 10.1080/15287390490514589. [DOI] [PubMed] [Google Scholar]
- In SW, Son EW, Rhee DK, Pyo S. Methamphetamine administration produces immunomodulation in mice. J Toxicol Environ Health A. 2005;68:2133–2145. doi: 10.1080/15287390500177156. [DOI] [PubMed] [Google Scholar]
- Kaizaki A, Tanaka S, Tsujikawa K, Numazawa S, Yoshida T. Recreational drugs, 3,4-Methylenedioxymethamphetamine (MDMA), 3,4-methylenedioxyamphetamine (MDA) and diphenylprolinol, inhibit neurite outgrowth in PC12 cells. J. Toxicol. Sci. 2010;35:375–381. doi: 10.2131/jts.35.375. [DOI] [PubMed] [Google Scholar]
- Kim YT, Lee SW, Kwon DH, Seo JH, Ahn BC, Lee J. Dose-dependent frontal hypometabolism on FDG-PET in methamphetamine abusers. J. Psychiatr. Res. 2009;43:1166–1170. doi: 10.1016/j.jpsychires.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Lerch J, Furukawa Y, Tong J, McCluskey T, Wilkins D, Houle S, Meyer J, Mundo E, Wilson AA. Decreased cerebral cortical serotonin transporter binding in ecstasy users: a positron emission tomography/[11C] DASB and structural brain imaging study. Brain. 2010;133:1779–1797. doi: 10.1093/brain/awq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousik SM, Napier TC, Carvey PM. The effects of psychostimulant drugs on blood brain barrier function and neuroinflammation. Front. Pharmacol. 2012;3:121. doi: 10.3389/fphar.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Wang X, Chen H, Song L, Ye L, Wang SH, et al. Methamphetamine enhances HIV infection of macrophages. Am J Pathol. 2008;172:1617–1624. doi: 10.2353/ajpath.2008.070971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Choi D, Hoffman W, Huckans MS. Methamphetamine causes persistent immune dysregulation: a cross-species, translational report. Neurotox. Res. 2011;20:59–68. doi: 10.1007/s12640-010-9223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Huckans M. Substance use disorders: Psychoneuroimmunological mechanisms and new targets for therapy. Pharmacol. Ther. 2013;139:289–300. doi: 10.1016/j.pharmthera.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. Regulation of NMDA receptor subunits and nitric oxide synthase expression during cocaine withdrawal. J. Neurochem. 2000;75:2040–2050. doi: 10.1046/j.1471-4159.2000.0752040.x. [DOI] [PubMed] [Google Scholar]
- Martinez LR, Mihu MR, Gacser A, Santambrogio L, Nosanchuk JD. Methamphetamine enhances histoplasmosis by immunosuppression of the host. J Infect Dis. 2009;200:131–141. doi: 10.1086/599328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matata BM, Galinanes M. Peroxynitrite is an essential component of cytokines production mechanism in human monocytes through modulation of nuclear factor-kappa B DNA binding activity. J. Biol. Chem. 2002;277:2330–2335. doi: 10.1074/jbc.M106393200. [DOI] [PubMed] [Google Scholar]
- Matarese G, Procaccini C, De Rosa V. The intricate interface between immune and metabolic regulation: a role for leptin in the pathogenesis of multiple sclerosis? J. Leukoc. Biol. 2008;84:893–899. doi: 10.1189/jlb.0108022. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Vranesic M, Palermo M, Mathews WB, Ravert HT, Dannals RF, Ricaurte GA. Positron emission tomographic studies of brain dopamine and serotonin transporters in abstinent (±) 3, 4- methylenedioxymethamphetamine (“ecstasy”) users: relationship to cognitive performance. Psychopharmacology. 2008;200:439–450. doi: 10.1007/s00213-008-1218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health, National Institute on Drug Abuse. Substance Use Disorders and Molecular Regulation of Brain Energy Utilization. 2013 http://grants.nih.gov/grants/guide/rfa-files/RFA-DA-14-005.html.
- Obrocki J, Schmoldt A, Buchert R, Andresen B, Petersen K, Thomasius R. Specific neurotoxicity of chronic use of ecstasy. Toxicol. Lett. 2002;127:285–297. doi: 10.1016/s0378-4274(01)00511-2. [DOI] [PubMed] [Google Scholar]
- Pachmerhiwala R, Bhide N, Straiko M, Gudelsky GA. Role of serotonin and/or norepinephrine in the MDMA-induced increase in extracellular glucose and glycogenolysis in the rat brain. Eur. J. Pharmacol. 2010;644:67–72. doi: 10.1016/j.ejphar.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC. MDMA in humans: factors which affect the neuropsychobiological profiles of recreational ecstasy users, the integrative role of bioenergetic stress. J. Psychopharmacol. 2006;20:147–163. doi: 10.1177/0269881106063268. [DOI] [PubMed] [Google Scholar]
- Peraile I, Granado N, Torres E, Gutierrez-Lopez MD, Moratalla R, Colado MI, O'Shea E. Cocaine potentiates MDMA-induced oxidative stress but not dopaminergic neurotoxicity in mice: implications for the pathogenesis of free radical-induced neurodegenerative disorders. Psychopharmacol. (Berl.) 2013 doi: 10.1007/s00213-013-3142-5. [DOI] [PubMed] [Google Scholar]
- Permpoonputtana K, Govitrapong P. The anti-inflammatory effect of melatonin on methamphetamine-induced proinflammatory mediators in human neuroblastoma dopamine SH-SY5Y cell lines. Neurotox. Res. 2013;23:189–199. doi: 10.1007/s12640-012-9350-7. [DOI] [PubMed] [Google Scholar]
- Potter A, Downey L, Stough C. Cognitive function in ecstasy naive abstinent drug dependants and MDMA users. Curr. Drug Abuse Rev. 2013;6:71–76. doi: 10.2174/1874473711306010008. [DOI] [PubMed] [Google Scholar]
- Potula R, Hawkins BJ, Cenna JM, Fan S, Dykstra H, Ramirez SH, Morsey B, Brodie MR, Persidsky Y. Methamphetamine causes mitrochondrial oxidative damage in human T lymphocytes leading to functional impairment. J. Immunol. 2010;185:2867–2876. doi: 10.4049/jimmunol.0903691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pubill D, Chipana C, Camins A, Pallàs M, Camarasa J, Escubedo E. Free radical production induced by methamphetamine in rat striatal synaptosomes. Toxicol. Appl. Pharmacol. 2005;204:57–68. doi: 10.1016/j.taap.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Quinton MS, Yamamoto BK. Causes and consequences of methamphetamine and MDMA toxicity. AAPS J. 2006;8:E337–E347. doi: 10.1007/BF02854904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros-Simo C, Moscoso-Castro M, Ruiz-Medina J, Ros J, Valverde O. Memory impairment and hippocampus specific protein oxidation induced by ethanol intake and 3, 4-Methylenedioxymethamphetamine (MDMA) in mice. J. Neurochem. 2013;125:736–746. doi: 10.1111/jnc.12247. [DOI] [PubMed] [Google Scholar]
- Sadek JR, Vigil O, Grant I, Heaton RK. The impact of neuropsychological functioning and depressed mood on functional complaints in HIV-1 infection and methamphetamine dependence. J. Clin. Exp. Neuropsychol. 2007;29:266–276. doi: 10.1080/13803390600659384. [DOI] [PubMed] [Google Scholar]
- Sailasuta N, Abulseoud O, Harris KC, Ross BD. Glial dysfunction in abstinent methamphetamine abusers. J. Cereb. Blood Flow Metab. 2010;30:950–960. doi: 10.1038/jcbfm.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TL, Grace CE, Braun AA, Amos-Kroohs RM, Graham DL, Skelton MR, Williams MT, Vorhees CV. Cognitive impairments from developmental exposure to serotonergic drugs: citalopram and MDMA. Int. J. Neuropsychopharmacol. 2013;16:1383–1394. doi: 10.1017/S1461145712001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL. Methamphetamine causes microglial activation in the brains of human abusers. J. Neurosci. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JA, Park BS, Raber J. Methamphetamine exposure during brain development alters the brain acetylcholine system in adolescent mice. J. Neurochem. 2011;119:89–99. doi: 10.1111/j.1471-4159.2011.07418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton MR, Williams MT, Vorhees CV. Developmental effects of 3,4 methylenedioxymethamphetamine: a review. Behav. Pharmacol. 2008;19:91–111. doi: 10.1097/FBP.0b013e3282f62c76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BJ, Moon KH, Upreti VV, Eddington ND, Lee IJ. Mechanisms of MDMA (ecstasy)-induced oxidative stress, mitochondrial dysfunction, and organ damage. Curr. Pharm. Biotechnol. 2010;11:434–443. doi: 10.2174/138920110791591436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J. Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres E, Gutierrez-Lopez MD, Mayado A, Rubio A, O'Shea E, Colado MI. Changes in interleukin-1 signal modulators induced by 3,4-methylenedioxymethamphetamine (MDMA): regulation by CB2 receptors and implications for neurotoxicity. J. Neuroinflamm. 2011;8:53. doi: 10.1186/1742-2094-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrutia A, Rubio-Araiz A, Gutierrez-Lopez MD, ElAli A, Hermann DM, O'Shea E, Colado MI. A study on the effect of JNK inhibitor, SP600125, on the disruption of blood-brain barrier induced by methamphetamine. Neurobiol. Dis. 2013;50:49–58. doi: 10.1016/j.nbd.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Wong C, Logan J. Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. Am. J. Psychiatry. 2001a;158:383–389. doi: 10.1176/appi.ajp.158.3.383. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan, J.,Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am. J. Psychiatry. 2001b;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J. Clin. Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Bankson MG. Amphetamine neurotoxicity: cause and consequence of oxidative stress. Crit. Rev. Neurobiol. 2005;17:87–117. doi: 10.1615/critrevneurobiol.v17.i2.30. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Raudensky J. The role of oxidative stress, metabolic compromise, and inflammation in neuronal injury produced by amphetamine-related drugs of abuse. J. Neuroimmune Pharmacol. 2008;3:203–217. doi: 10.1007/s11481-008-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Peng JS, Wang X, Wang YJ, Luo GX, Ho WZ. Methamphetamine enhances Hepatitis C virus replication in human hepatocytes. J Viral Hepat. 2008;15:261–270. doi: 10.1111/j.1365-2893.2007.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Siegel JA, Acevedo SF, Agam M, Raber J. Developmental methamphetamine exposure results in short- and long-term alterations in hypothalamic-pituitary-adrenal-axis-associated proteins. Dev. Neurosci. 2013;35:338–346. doi: 10.1159/000351278. [DOI] [PMC free article] [PubMed] [Google Scholar]