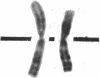Abstract
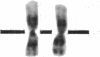
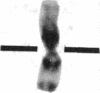
Normal human fibroblasts were fused to carcinogen-transformed baby hamster kidney (BHK) cells and found to be able to suppress the anchorage-independent transformed phenotype of the hamster cells. This suppression was not due to interspecies incompatibility, for transformation could be effectively expressed in hybrids if either the human or the BHK parent had initially been transformed by a dominantly acting viral genome. Upon growth of suppressed hybrids, loss of human chromosomes was accompanied by the re-expression of transformation. Karyotype analysis indicated that only human chromosome 1 was retained in all hybrids that were suppressed and was lost in all hybrids in which transformation was re-expressed. Cytological evidence for the presence or absence of chromosome 1 was confirmed by electrophoretic identification of the human isozyme for phosphoglucomutase 1. Clones re-expressing transformation were isolated from two suppressed hybrids and in both cases loss of suppression was accompanied by the loss of human chromosome 1. Thus, the maintenance of suppression in these cross-species hybrids appears to require the continued presence of normal human chromosome 1. These findings raise the possibility that the frequent involvement of human chromosome 1 in potentially inactivating aberrations in human tumors may reflect a suppressor role for this chromosome in human malignancy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avilès D., Jami J., Rousset J. P., Ritz E. Tumor x host cell hybrids in the mouse: chromosomes from the normal cell parent maintained in malignant hybrid tumors. J Natl Cancer Inst. 1977 May;58(5):1391–1399. doi: 10.1093/jnci/58.5.1391. [DOI] [PubMed] [Google Scholar]
- Barrett J. C., Ts'o P. O. Evidence for the progressive nature of neoplastic transformation in vitro. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3761–3765. doi: 10.1073/pnas.75.8.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict W. F., Banerjee A., Mark C., Murphree A. L. Nonrandom chromosomal changes in untreated retinoblastomas. Cancer Genet Cytogenet. 1983 Dec;10(4):311–333. doi: 10.1016/0165-4608(83)90090-0. [DOI] [PubMed] [Google Scholar]
- Benedict W. F., Weissman B. E., Mark C., Stanbridge E. J. Tumorigenicity of human HT1080 fibrosarcoma X normal fibroblast hybrids: chromosome dosage dependency. Cancer Res. 1984 Aug;44(8):3471–3479. [PubMed] [Google Scholar]
- Bouck N., Di Mayorca G. Evaluation of chemical carcinogenicity by in vitro neoplastic transformation. Methods Enzymol. 1979;58:296–302. doi: 10.1016/s0076-6879(79)58145-2. [DOI] [PubMed] [Google Scholar]
- Bouck N., di Mayorca G. Chemical carcinogens transform BHK cells by inducing a recessive mutation. Mol Cell Biol. 1982 Feb;2(2):97–105. doi: 10.1128/mcb.2.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck N., di Mayorca G. Somatic mutation as the basis for malignant transformation of BHK cells by chemical carcinogens. Nature. 1976 Dec 23;264(5588):722–727. doi: 10.1038/264722a0. [DOI] [PubMed] [Google Scholar]
- Brito-Babapulle V., Atkin N. B. Break points in chromosome #1 abnormalities of 218 human neoplasms. Cancer Genet Cytogenet. 1981 Nov;4(3):215–225. doi: 10.1016/0165-4608(81)90015-7. [DOI] [PubMed] [Google Scholar]
- Brodeur G. M., Green A. A., Hayes F. A., Williams K. J., Williams D. L., Tsiatis A. A. Cytogenetic features of human neuroblastomas and cell lines. Cancer Res. 1981 Nov;41(11 Pt 1):4678–4686. [PubMed] [Google Scholar]
- Carney D. N., Edgell C. J., Gazdar A. F., Minna J. D. Suppression of malignancy in human lung cancer (A549/8) times mouse fibroblast (3T3-4E) somatic cell hybrids. J Natl Cancer Inst. 1979 Feb;62(2):411–415. [PubMed] [Google Scholar]
- Comings D. E. A general theory of carcinogenesis. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3324–3328. doi: 10.1073/pnas.70.12.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mayorca G., Greenblatt M., Trauthen T., Soller A., Giordano R. Malignant transformation of BHK21 clone 13 cells in vitro by nitrosamines--a conditional state. Proc Natl Acad Sci U S A. 1973 Jan;70(1):46–49. doi: 10.1073/pnas.70.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. P., Burtenshaw M. D., Brown B. B., Hennion R., Harris H. The analysis of malignancy by cell fusion. IX. Re-examination and clarification of the cytogenetic problem. J Cell Sci. 1982 Aug;56:113–130. doi: 10.1242/jcs.56.1.113. [DOI] [PubMed] [Google Scholar]
- Francke U., Oliver N. Quantitative analysis of high-resolution trypsin-giemsa bands on human prometaphase chromosomes. Hum Genet. 1978 Dec 18;45(2):137–165. doi: 10.1007/BF00286957. [DOI] [PubMed] [Google Scholar]
- Geurts van Kessel A. H., den Boer W. C., van Agthoven A. J., Hagemeijer A. Decreased tumorigenicity of rodent cells after fusion with leukocytes from normal and leukemic donors. Somatic Cell Genet. 1981 Nov;7(6):645–656. doi: 10.1007/BF01538754. [DOI] [PubMed] [Google Scholar]
- Goyette M., Petropoulos C. J., Shank P. R., Fausto N. Expression of a cellular oncogene during liver regeneration. Science. 1983 Feb 4;219(4584):510–512. doi: 10.1126/science.6297003. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Hall A., Marshall C. J., Spurr N. K., Weiss R. A. Identification of transforming gene in two human sarcoma cell lines as a new member of the ras gene family located on chromosome 1. Nature. 1983 Jun 2;303(5916):396–400. doi: 10.1038/303396a0. [DOI] [PubMed] [Google Scholar]
- Honey N. K., Shows T. B. The tumor phenotype and the human gene map. Cancer Genet Cytogenet. 1983 Nov;10(3):287–310. doi: 10.1016/0165-4608(83)90058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett O., Macpherson I. The basis of the tumorigenicity of BHK 21 cells. Int J Cancer. 1968 Sep 15;3(5):654–662. doi: 10.1002/ijc.2910030514. [DOI] [PubMed] [Google Scholar]
- Jonasson J., Harris H. The analysis of malignancy by cell fusion. VIII. Evidence for the intervention of an extra-chromosomal element. J Cell Sci. 1977 Apr;24:255–263. doi: 10.1242/jcs.24.1.255. [DOI] [PubMed] [Google Scholar]
- Kitchin R. M., Gadi I. K., Smith B. L., Sager R. Genetic analysis of tumorigenesis: X. Chromosome studies of transformed mutants and tumor-derived CHEF/18 cells. Somatic Cell Genet. 1982 Sep;8(5):677–689. doi: 10.1007/BF01542860. [DOI] [PubMed] [Google Scholar]
- Klinger H. P., Shows T. B. Suppression of tumorigenicity in somatic cell hybrids. II. Human chromosomes implicated as suppressors of tumorigenicity in hybrids with Chinese hamster ovary cells. J Natl Cancer Inst. 1983 Sep;71(3):559–569. [PubMed] [Google Scholar]
- Klinger H. P. Suppression of tumorigenicity in somatic cell hybrids. I. Suppression and reexpression of tumorigenicity in diploid human X D98AH2 hybrids and independent segregation of tumorigenicity from other cell phenotypes. Cytogenet Cell Genet. 1980;27(4):254–266. doi: 10.1159/000131494. [DOI] [PubMed] [Google Scholar]
- Klinger H. P. Suppression of tumorigenicity. Cytogenet Cell Genet. 1982;32(1-4):68–84. doi: 10.1159/000131688. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Genetics and etiology of human cancer. Adv Hum Genet. 1977;8:1–66. doi: 10.1007/978-1-4615-8267-0_1. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr, Hethcote H. W., Brown B. W. Mutation and childhood cancer: a probabilistic model for the incidence of retinoblastoma. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5116–5120. doi: 10.1073/pnas.72.12.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucherlapati R., Shin S. I. Genetic control of tumorigenicity in interspecific mammalian cell hybrids. Cell. 1979 Mar;16(3):639–648. doi: 10.1016/0092-8674(79)90037-0. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Cellular oncogenes and multistep carcinogenesis. Science. 1983 Nov 18;222(4625):771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- Marshall C. J., Dave H. Suppression of the transformed phenotype in somatic cell hybrids. J Cell Sci. 1978 Oct;33:171–190. doi: 10.1242/jcs.33.1.171. [DOI] [PubMed] [Google Scholar]
- Mitelman F. Catalogue of chromosome aberrations in cancer. Cytogenet Cell Genet. 1983;36(1-2):1–515. doi: 10.1159/000131930. [DOI] [PubMed] [Google Scholar]
- Mitelman F. Restricted number of chromosomal regions implicated in aetiology of human cancer and leukaemia. 1984 Jul 26-Aug 1Nature. 310(5975):325–327. doi: 10.1038/310325a0. [DOI] [PubMed] [Google Scholar]
- Morse H., Hays T., Rose B., Robinson A. Chromosome 1 abnormalities in relapse and terminal stages in childhood leukemia. Med Pediatr Oncol. 1979;7(1):9–16. doi: 10.1002/mpo.2950070103. [DOI] [PubMed] [Google Scholar]
- Murphree A. L., Benedict W. F. Retinoblastoma: clues to human oncogenesis. Science. 1984 Mar 9;223(4640):1028–1033. doi: 10.1126/science.6320372. [DOI] [PubMed] [Google Scholar]
- Müller R., Tremblay J. M., Adamson E. D., Verma I. M. Tissue and cell type-specific expression of two human c-onc genes. Nature. 1983 Aug 4;304(5925):454–456. doi: 10.1038/304454a0. [DOI] [PubMed] [Google Scholar]
- Nishikura K., ar-Rushdi A., Erikson J., Watt R., Rovera G., Croce C. M. Differential expression of the normal and of the translocated human c-myc oncogenes in B cells. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4822–4826. doi: 10.1073/pnas.80.15.4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak S., Strong L. C., Ferrell R. E., Trindade A. Familial renal cell carcinoma with a 3;11 chromosome translocation limited to tumor cells. Science. 1982 Sep 3;217(4563):939–941. doi: 10.1126/science.7112106. [DOI] [PubMed] [Google Scholar]
- Perucho M., Goldfarb M., Shimizu K., Lama C., Fogh J., Wigler M. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981 Dec;27(3 Pt 2):467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Biological implications of consistent chromosome rearrangements in leukemia and lymphoma. Cancer Res. 1984 Aug;44(8):3159–3168. [PubMed] [Google Scholar]
- Rowley J. D. Mapping of human chromosomal regions related to neoplasia: evidence from chromosomes 1 and 17. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5729–5733. doi: 10.1073/pnas.74.12.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOKER M., MACPHERSON I. SYRIAN HAMSTER FIBROBLAST CELL LINE BHK21 AND ITS DERIVATIVES. Nature. 1964 Sep 26;203:1355–1357. doi: 10.1038/2031355a0. [DOI] [PubMed] [Google Scholar]
- Sager R., Kovac P. E. Genetic analysis of tumorigenesis: I. Expression of tumor-forming ability in hamster hybrid cell lines. Somatic Cell Genet. 1978 May;4(3):375–392. doi: 10.1007/BF01542849. [DOI] [PubMed] [Google Scholar]
- Santos E., Martin-Zanca D., Reddy E. P., Pierotti M. A., Della Porta G., Barbacid M. Malignant activation of a K-ras oncogene in lung carcinoma but not in normal tissue of the same patient. Science. 1984 Feb 17;223(4637):661–664. doi: 10.1126/science.6695174. [DOI] [PubMed] [Google Scholar]
- Shows T. B., Sakaguchi A. Y., Naylor S. L. Mapping the human genome, cloned genes, DNA polymorphisms, and inherited disease. Adv Hum Genet. 1982;12:341–452. doi: 10.1007/978-1-4615-8315-8_5. [DOI] [PubMed] [Google Scholar]
- Smith B. L., Sager R. Multistep origin of tumor-forming ability in Chinese hamster embryo fibroblast cells. Cancer Res. 1982 Feb;42(2):389–396. [PubMed] [Google Scholar]
- Solomon E. Recessive mutation in aetiology of Wilms' tumour. Nature. 1984 May 10;309(5964):111–112. doi: 10.1038/309111a0. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. J., Der C. J., Doersen C. J., Nishimi R. Y., Peehl D. M., Weissman B. E., Wilkinson J. E. Human cell hybrids: analysis of transformation and tumorigenicity. Science. 1982 Jan 15;215(4530):252–259. doi: 10.1126/science.7053574. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. J., Flandermeyer R. R., Daniels D. W., Nelson-Rees W. A. Specific chromosome loss associated with the expression of tumorigenicity in human cell hybrids. Somatic Cell Genet. 1981 Nov;7(6):699–712. doi: 10.1007/BF01538758. [DOI] [PubMed] [Google Scholar]
- van Someren H., Beijersbergen van Henegouwen H., Los W., Wurzer-Figurelli E., Doppert B., Vervloet M., Meera Khan P. Enzyme electrophoresis on cellulose acetate gel. II. Zymogram patterns in man-Chinese hamster somatic cell hybrids. Humangenetik. 1974;25(3):189–201. doi: 10.1007/BF00281426. [DOI] [PubMed] [Google Scholar]