Abstract
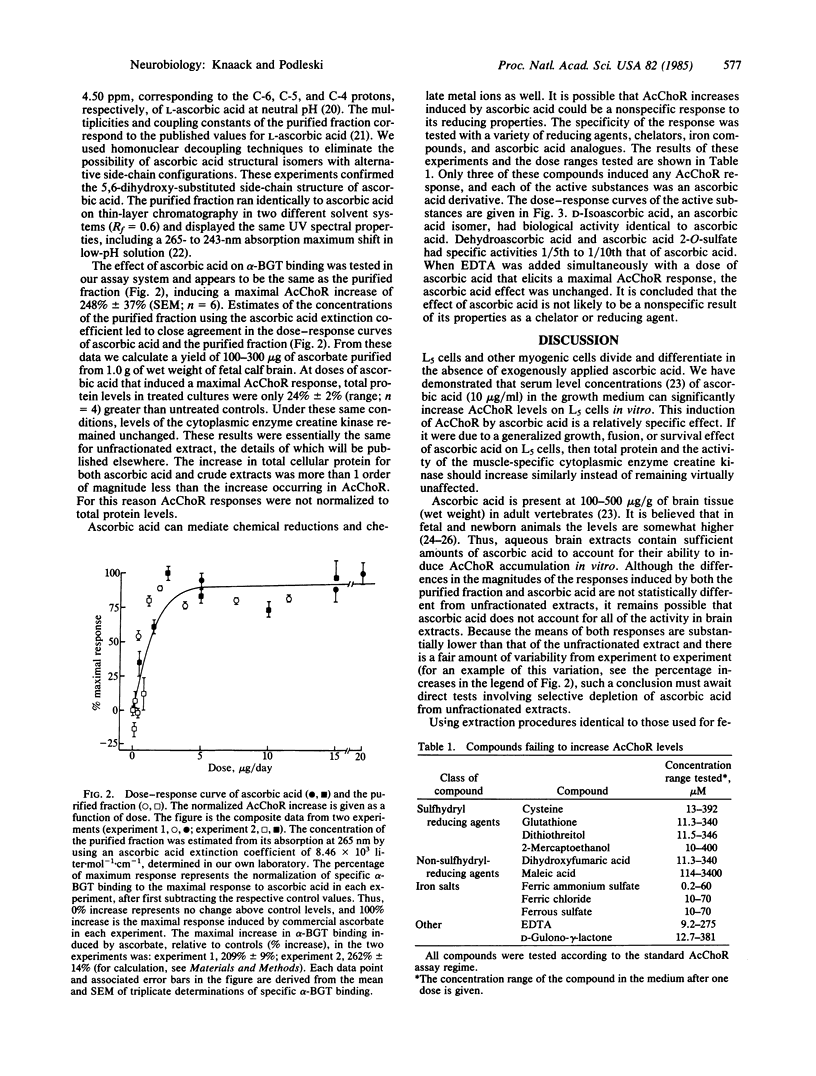
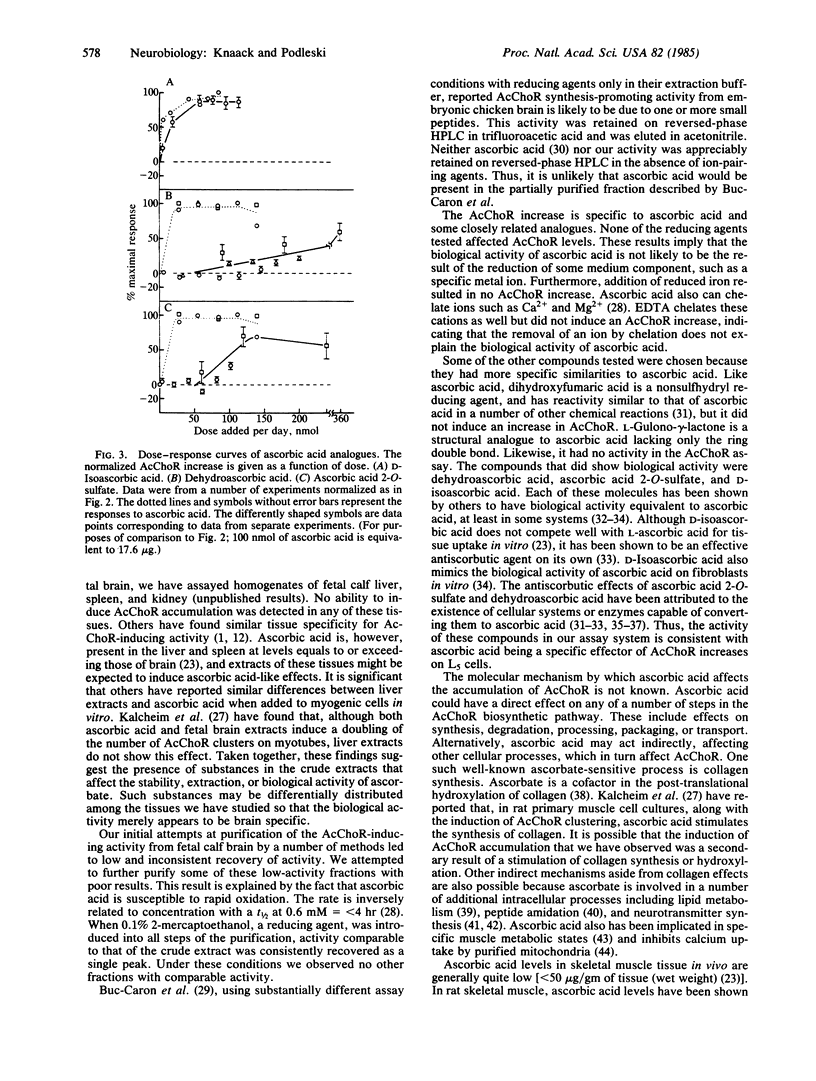
Extracts of fetal calf brain cause a 3- to 5-fold increase in acetylcholine receptors (AcChoR) on cultured myogenic L5 cells. Purification of the substance causing the major portion of this receptor increase has been completed. Ultraviolet spectral characteristics, nuclear magnetic resonance, mass spectra, and AcChoR induction by the active factor are the same as those of commercially available ascorbic acid. The biological activity of ascorbic acid is not mimicked by reducing agents with or without sulfhydryl groups. Compounds related to ascorbic acid were tested for their ability to induce AcChoR increases on L5 cells. D-Isoascorbic acid is the only substance with identical biological activity to ascorbic acid. Dehydroascorbic acid and ascorbic acid 2-O-sulfate also induce AcChoR increases but with lower specific activity. These data show that ascorbic acid can play a role in regulating AcChoR expression in myogenic tissue, and the presence of ascorbic acid in the purified fraction from fetal calf brain accounts for its ability to increase AcChoR in L5 cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlard B. P., de Souza S. W., Moon S. The effect of age, growth retardation and asphyxia on ascorbic acid concentrations in developing brain. J Neurochem. 1973 Oct;21(4):877–881. doi: 10.1111/j.1471-4159.1973.tb07532.x. [DOI] [PubMed] [Google Scholar]
- Allison J. H., Stewart M. A. Myo-inositol and ascorbic acid in developing rat brain. J Neurochem. 1973 Jun;20(6):1785–1788. doi: 10.1111/j.1471-4159.1973.tb00296.x. [DOI] [PubMed] [Google Scholar]
- Barnes M. J. Function of ascorbic acid in collagen metabolism. Ann N Y Acad Sci. 1975 Sep 30;258:264–277. doi: 10.1111/j.1749-6632.1975.tb29287.x. [DOI] [PubMed] [Google Scholar]
- Benitez L. V., Halver J. E. Ascorbic acid sulfate sulfohydrolase (C2 sulfatase): the modulator of cellular levels of L-ascorbic acid in rainbow trout. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5445–5449. doi: 10.1073/pnas.79.18.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S., Steinbach J. H. The distribution of alpha-bungarotoxin binding sites of mammalian skeletal muscle developing in vivo. J Physiol. 1977 May;267(1):195–213. doi: 10.1113/jphysiol.1977.sp011808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond A. D. Ascorbic-2-sulfate metabolism by human fibroblasts. Ann N Y Acad Sci. 1975 Sep 30;258:307–313. doi: 10.1111/j.1749-6632.1975.tb29290.x. [DOI] [PubMed] [Google Scholar]
- Brockes J. P., Hall Z. W. Synthesis of acetylcholine receptor by denervated rat diaphragm muscle. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1368–1372. doi: 10.1073/pnas.72.4.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buc-Caron M. H., Nystrom P., Fischbach G. D. Induction of acetylcholine receptor synthesis and aggregation: partial purification of low-molecular-weight activity. Dev Biol. 1983 Feb;95(2):378–386. doi: 10.1016/0012-1606(83)90039-8. [DOI] [PubMed] [Google Scholar]
- Burden S. Development of the neuromuscular junction in the chick embryo: the number, distribution, and stability of acetylcholine receptors. Dev Biol. 1977 Jun;57(2):317–329. doi: 10.1016/0012-1606(77)90218-4. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Activation and inhibition of the action potential Na+ ionophore of cultured rat muscle cells by neurotoxins. Biochem Biophys Res Commun. 1976 Jan 12;68(1):136–142. doi: 10.1016/0006-291x(76)90020-6. [DOI] [PubMed] [Google Scholar]
- Chinoy N. J. Source of electron energy for animal metabolism. I. Role of ascorbic acid in the metabolism of avian muscle. Histochemie. 1969;19(2):125–128. doi: 10.1007/BF00281091. [DOI] [PubMed] [Google Scholar]
- Cohen S. A., Fischbach G. D. Clusters of acetylcholine receptors located at identified nerve-muscle synapses in vitro. Dev Biol. 1977 Aug;59(1):24–38. doi: 10.1016/0012-1606(77)90237-8. [DOI] [PubMed] [Google Scholar]
- Devreotes P. N., Fambrough D. M. Turnover of acetylcholine receptors in skeletal muscle. Cold Spring Harb Symp Quant Biol. 1976;40:237–251. doi: 10.1101/sqb.1976.040.01.025. [DOI] [PubMed] [Google Scholar]
- Diliberto E. J., Jr, Allen P. L. Mechanism of dopamine-beta-hydroxylation. Semidehydroascorbate as the enzyme oxidation product of ascorbate. J Biol Chem. 1981 Apr 10;256(7):3385–3393. [PubMed] [Google Scholar]
- Diliberto E. J., Jr, Dean G., Carter C., Allen P. L. Tissue, subcellular, and submitochondrial distributions of semidehydroascorbate reductase: possible role of semidehydroascorbate reductase in cofactor regeneration. J Neurochem. 1982 Aug;39(2):563–568. doi: 10.1111/j.1471-4159.1982.tb03982.x. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E., Glembotski C. C. Identification in pituitary tissue of a peptide alpha-amidation activity that acts on glycine-extended peptides and requires molecular oxygen, copper, and ascorbic acid. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5144–5148. doi: 10.1073/pnas.80.16.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrough D. M. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979 Jan;59(1):165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- Fox F. W., Levy L. F. Experiments confirming the antiscorbutic activity of dehydroascorbic acid and a study of its storage and that of ascorbic acid by the guinea-pig at different levels of intake. Biochem J. 1936 Feb;30(2):211–217. doi: 10.1042/bj0300211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff G. L., Hudson A. J., Strickland K. P. Changes in the relative amounts of ascorbic acid and glycogen in denervated rat gastrocnemius muscle. Can J Biochem. 1965 Jun;43(6):705–710. doi: 10.1139/o65-081. [DOI] [PubMed] [Google Scholar]
- HEWITT E. J., DICKES G. J. Spectrophotometric measurements on ascorbic acid and their use for the estimation of ascorbic acid and dehydroascorbic acid in plant tissues. Biochem J. 1961 Feb;78:384–391. doi: 10.1042/bj0780384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halver J. E., Smith R. R., Tolbert B. M., Baker E. M. Utilization of ascorbic acid in fish. Ann N Y Acad Sci. 1975 Sep 30;258:81–102. doi: 10.1111/j.1749-6632.1975.tb29270.x. [DOI] [PubMed] [Google Scholar]
- Hornig D. Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann N Y Acad Sci. 1975 Sep 30;258:103–118. doi: 10.1111/j.1749-6632.1975.tb29271.x. [DOI] [PubMed] [Google Scholar]
- Jessell T. M., Siegel R. E., Fischbach G. D. Induction of acetylcholine receptors on cultured skeletal muscle by a factor extracted from brain and spinal cord. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5397–5401. doi: 10.1073/pnas.76.10.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalcheim C., Vogel Z., Duksin D. Embryonic brain extract induces collagen biosynthesis in cultured muscle cells: involvement in acetylcholine receptor aggregation. Proc Natl Acad Sci U S A. 1982 May;79(10):3077–3081. doi: 10.1073/pnas.79.10.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy R. V., Satyam P. Changes in ascorbic acid content in denervated frog gastrocnemius muscle. Experientia. 1969 Mar 15;25(3):267–268. doi: 10.1007/BF02034383. [DOI] [PubMed] [Google Scholar]
- LEVIN E. Y., LEVENBERG B., KAUFMAN S. The enzymatic conversion of 3,4-dihydroxyphenylethylamine to norepinephrine. J Biol Chem. 1960 Jul;235:2080–2086. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LeVine H., 3rd, Bronson D. D., Khouri R., Sahyoun N. E. Ascorbic acid is an endogenous cytosolic inhibitor of ATP-supported rat liver mitochondrial calcium transport. J Biol Chem. 1983 Dec 25;258(24):14954–14959. [PubMed] [Google Scholar]
- Loring R. H., Jones S. W., Matthews-Bellinger J., Salpeter M. M. 125I-alpha-bungarotoxin. Effects of radiodecomposition on specific activity. J Biol Chem. 1982 Feb 10;257(3):1418–1423. [PubMed] [Google Scholar]
- Murad S., Grove D., Lindberg K. A., Reynolds G., Sivarajah A., Pinnell S. R. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981 May;78(5):2879–2882. doi: 10.1073/pnas.78.5.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachla L. A., Kissinger P. T. Analysis of ascorbic acid by liquid chromatography with amperometric detection. Methods Enzymol. 1979;62:15–24. doi: 10.1016/0076-6879(79)62183-3. [DOI] [PubMed] [Google Scholar]
- Patrick J., Heinemann S. F., Lindstrom J., Schubert D., Steinbach J. H. Appearance of acetylcholine receptors during differentiation of a myogenic cell line. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2762–2766. doi: 10.1073/pnas.69.10.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podleski T. R., Axelrod D., Ravdin P., Greenberg I., Johnson M. M., Salpeter M. M. Nerve extract induces increase and redistribution of acetylcholine receptors on cloned muscle cells. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2035–2039. doi: 10.1073/pnas.75.4.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter M. M., Spanton S., Holley K., Podleski T. R. Brain extract causes acetylcholine receptor redistribution which mimics some early events at developing neuromuscular junctions. J Cell Biol. 1982 May;93(2):417–425. doi: 10.1083/jcb.93.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankova L., Rigas D. A., Bigley R. H. Dehydroascorbate uptake and reduction by human blood neutrophils, erythrocytes, and lymphocytes. Ann N Y Acad Sci. 1975 Sep 30;258:238–242. doi: 10.1111/j.1749-6632.1975.tb29284.x. [DOI] [PubMed] [Google Scholar]
- Tolbert B. M., Downing M., Carlson R. W., Knight M. K., Baker E. M. Chemistry and metabolism of ascorbic acid and ascorbate sulfate. Ann N Y Acad Sci. 1975 Sep 30;258:48–69. doi: 10.1111/j.1749-6632.1975.tb29267.x. [DOI] [PubMed] [Google Scholar]
- Vogel Z., Sytkowski A. J., Nirenberg M. W. Acetylcholine receptors of muscle grown in vitro. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3180–3184. doi: 10.1073/pnas.69.11.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]


