Abstract
Malassezia (Pityrosporum) folliculitis is a fungal acneiform condition commonly misdiagnosed as acne vulgaris. Although often associated with common acne, this condition may persist for years without complete resolution with typical acne medications. Malassezia folliculitis results from overgrowth of yeast present in the normal cutaneous flora. Eruptions may be associated with conditions altering this flora, such as immunosuppression and antibiotic use. The most common presentation is monomorphic papules and pustules, often on the chest, back, posterior arms, and face. Oral antifungals are the most effective treatment and result in rapid improvement. The association with acne vulgaris may require combinations of both antifungal and acne medications. This article reviews and updates readers on this not uncommon, but easily missed, condition.
A 22-year-old African American woman returned to the clinic for a follow-up appointment regarding her acne. She presented with an eight-month history of oily skin and pruritic papules on her forehead, chest, back, arms, and neck after previously being well-controlled on typical acne medications. She described the lesions as extremely itchy with an additional burning sensation, which is relieved by placing a cool wet towel over the affected areas. She was otherwise in good health and reported no additional medical conditions. This patient is a full-time student and denies frequent sweating. She does not exercise regularly and does not have an outdoor or high temperature working environment. She did notice an improvement in her skin while at school in the northern part of the country and worsening upon returning home in the south.
She has struggled with acne since the age of 15, but was previously well-controlled and stable using azelaic acid gel twice a day and an over-the-counter salicylic acid wash until two years ago. At this time, several products were utilized over the course of a year, including tretinoin 0.1% gel, trichloroacetic acid peels, and 2.5% adapalene/benzoyl peroxide gel. The patient reported no improvement with any of these agents and has even tried her own remedies. These included a diet change of decreased soda and increased water intake as well as a better sleep cycle and washing her pillow cases and sheets frequently, also with no improvement.
Oily, monomorphic follicular papules were noted on the forehead, cheeks, chest, and upper back on physical exam (Figures 1 and 2). A shave biopsy of the left upper back was done at this time. Histopathology confirmed a suppurative folliculitis with Pityrosporum species visualized with Periodic Acid-Schiff (PAS) stain. A dense collection of neutrophils was noted within the infundibulum, along with a perifollicular lymphohistiocytic neutrophilic infiltrate. This confirmed the diagnosis of Pityrosporum folliculitis, and the patient was started on oral fluconazole 100mg daily, topical clotrimazole cream applied twice daily, and tretinoin 0.0375% cream. She noticed drastic improvement within 24 hours, remained on this treatment regimen for two months, and is currently attempting a tapering of the oral antifungal to every other day.
Figure 1.
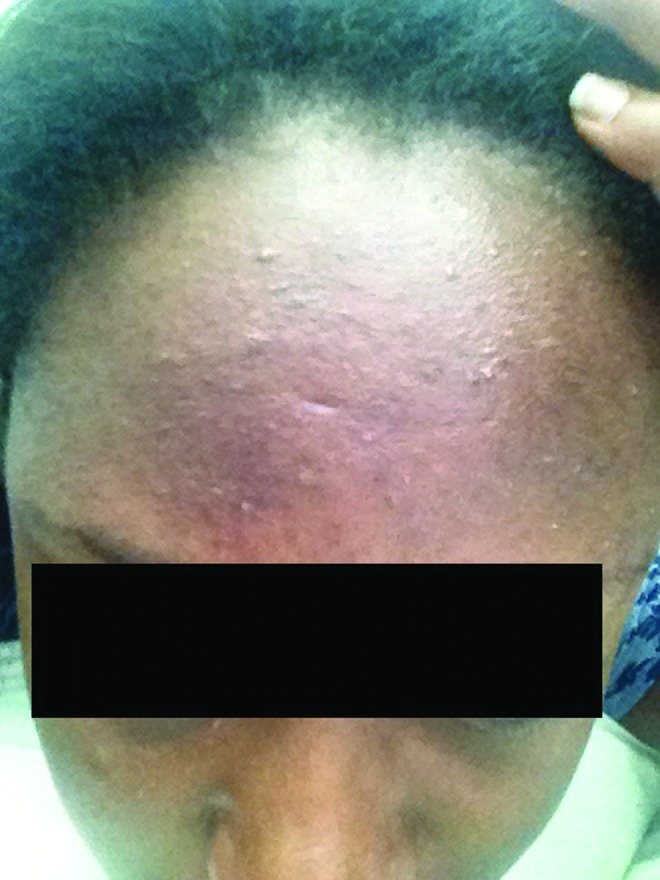
Monomorphic papules and pustules on the forehead.
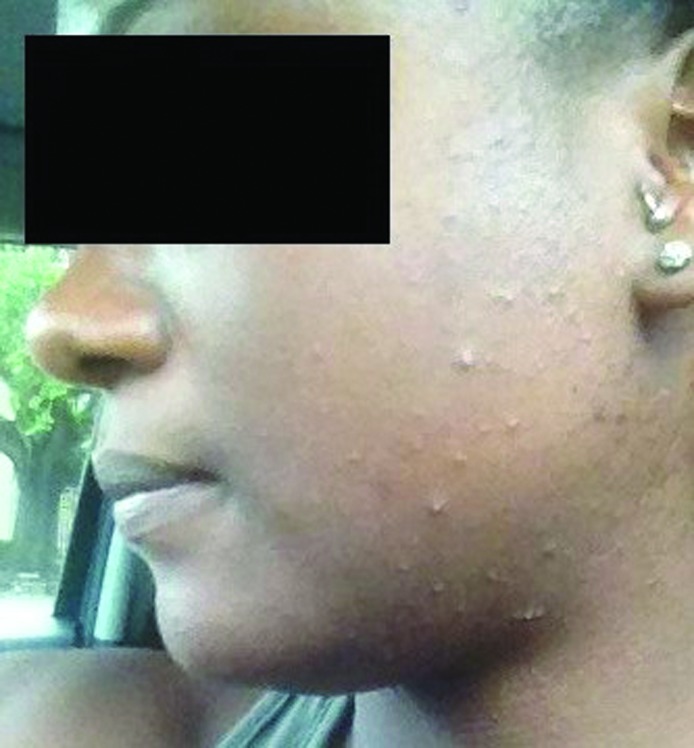
Figure 2.
Monomorphic papules and pustules on the cheek.
DISCUSSION
Malassezia (Pityrosporum) folliculitis (MF) is an acneiform eruption, described first by Weary et al in 1969 and recognized by Potter in 1973 specific disease.1-4 Often misdiagnosed as acne vulgaris, it is easy to miss and thus is likely underdiagnosed.3-7 MF is a benign disorder that results from an overgrowth of the Malassezia yeast present in the normal cutaneous flora, secondary to occlusion of the follicle or disturbance of normal cutaneous flora.7-8 The yeast is primarily found in the infundibulum of the sebaceous glands, as it thrives on the lipid composition of sebum.3,6,8
It presents as intensely pruritic, 1 to 2mm, monomorphic follicular papules and pustules, often on the upper back, chest, and shoulders.4,9 Durdu et al10 found that 71.4 percent of MF lesions in a study of 49 patients were found in more than one region. The most common location was the face (57.1%), followed by the back (53%), extensor surfaces of the arms (38.8%), chest (36.7%), and neck (18.3%). Furthermore, on the face, the chin and sides of the face were most commonly affected compared with the central facial lesions of acne vulgaris. Pruritus was a component of 79.6 percent of patients’ symptoms, and 10.2 percent even presented with excoriations.
MF is common in adolescents, likely due to increased sebaceous gland activity2,11 Marcon et al12 found that the frequency and density of colonization of the yeast is related to age and sebaceous gland activity. It is commonly found in people living in hot, humid climates, particularly those affected by excessive sweating, and is reported to be more common in males.9-10,13 Other predisposing factors include topical or oral antibiotic use, particularly tetracyclines, oral corticosteroid use, and immunosuppression.10,14 Malassezia furfur, made up of Pityrosporum orbiculare and ovale, have been detected in follicular contents of steroid acne.15 A study of 49 patients in Turkey, performed by Durdu et al,10 found the incidence of MF to be four percent of patients attending their dermatology clinic, with an average age of 26 (range 12-62) years.
As mentioned earlier, MF is commonly misdiagnosed as acne vulgaris, but also may be confused with bacterial folliculitis. Differentiating MF from acne vulgaris is important as antibiotic treatment may alter cutaneous flora and exacerbate the disease. The two can be differentiated by the lack of response to oral and topical antibiotics, absence of comedones and the often pruritic nature of the lesions.2,3,9 Diagnosis and treatment may be missed for long periods of time as different acne medications are tried without relief.
Worth noting is the association between MF and steroid acne, as the two often share a similar clinical presentation.16,17 A study conducted by Yu et al5 identified large numbers of Pityrosporum ovale within the follicles of patients on systemic steroids with acneiform eruptions. Sixty-one total patients were examined with steroid acne or other acneiform eruptions. Clinical diagnoses of these patients included steroid acne, MF, and acne vulgaris, and of these, 66 percent had a confirmed MF diagnosis based on direct microscopy. This indicates a possible need for antifungal treatments among steroid acne patients.
Diagnostic studies include microscopic evaluation of the presence of yeast, cultures, and biopsies. Additionally, Wood’s lamp can be used to illuminate the lesions, which portray a yellow-green fluorescence. This particular diagnostic tool was observed to be positive in 66.7 percent of 49 MF patients.10 A general KOH with Parker blue ink shows numerous spores and short curved hyphae. However, this can be misleading as Malassezia yeast are present as a normal part of the cutaneous flora in 75 to 98 percent of healthy individuals.2,7,10,18 Use of a comedo extractor is recommended by the authors of Yu et al5 rather than a simple skin scraping for KOH preparation as this will reveal levels of yeast within the follicle rather than the stratum corneum. Calcofluor white can be used in replacement of KOH allowing for easier visualization; however this method does require additional ultraviolet (UV) light microscopy.7 In the study done by Durdu et al,10 May-Grunwald-Giemsa (MGG) smears showed higher positivity (100%) compared to KOH (81.6%) as confirmed by fungal culture. Fungal cultures may be useful; however, different growth rates and culture requirements between specific Malassezia species make them more difficult.19 Malassezia species require C12, C13, and C14 fatty acids, which are not obtained by standard Sabouraud medium.11,20 This requirement can be obtained by adding olive oil to the medium or using MDA or Leeming and Notman agars as well as modified Dixon medium. A temperature of 31 to 35°C and placing cultures in plastic bags to enhance humidity also aids in the growth of Malassezia species.7 Lastly, shave or punch biopsies are useful for histopathological diagnosis. Histologically, these specimens show dilated follicles plugged with keratinous material, amorphous cellular debris, and inflammatory cells. The follicle contains numerous round yeast forms and demonstrates positivity with PAS stain.7,21,22 When performing a biopsy, as with the KOH preparation, it is important to obtain the follicle as yeast are often normally present on the skin’s surface. Performing a KOH slide or shave biopsy is more practical, as cultures can be difficult to perform in clinical settings.
Another indication of this disease is the drastic improvement following use of antifungal medications. The most effective treatment is oral antifungal medication, particularly in the beginning as the yeast is located deep within the hair follicle.10,19 Topical antifungals are useful as adjunctive therapy as well as maintenance and prophylactic therapy, especially as recurrence is common.2,9,21 One study, by Levy et al, studied three different treatment regimens among patients.23 The first group received topical ketoconazole alone, the second received oral ketoconazole, and the third group received both oral ketoconazole with topical ketoconazole. They report that these treatments promoted cure in 12, 75, and 75 percent of 26 patients, respectively, indicating the increased efficacy of oral antifungals. Furthermore, this indicates that the addition of topical antifungal while on an oral medication may not have any increased efficacy, although further studies are required to determine recurrence rates in each group. Antifungal medications are also useful for their anti-inflammatory mechanisms.19
Many investigators have studied the efficacy of itraconazole, as this antifungal is excreted in high concentrations in sebum.24 Itraconazole is a broad-spectrum triazole, which is highly lipophilic and keratophilic with good oral absorption and extensive tissue distribution.13 Parsad et al13 utilized 200mg itraconazole for seven days in 13 patients. Of these 13 patients, 11 showed negative mycological exam at Week 5, compared to one in the placebo group, made up of 12 subjects. In one case report, 200mg itraconazole daily for two months resulted in nearly complete disappearance of lesions, promoting apparent cure; however, the patient did relapse after 12 months.22 Two weeks of 200mg itraconazole daily resulted in complete recovery of 79.6 percent of patients.10 Furthermore, itraconazole appears to delay relapses.25
Adverse effects associated with oral antifungal medications include nausea, vomiting, diarrhea, abdominal pain, and hepatotoxicity; for this reason, some authors are proposing alternative treatments, including photodynamic therapy (PDT).2 Other reasons for alternative therapies include infection relapses and possible drug resistance.26 Lee et al21 did a pilot study using topical PDT with methyl aminolevulinate (MAL) cream as a photosensitizer treatment for MF. Patients underwent three sessions at two-week intervals with assessment at one month following the last treatment. Minimal side effects were noted, including a mild burning sensation after each treatment, which disappeared within 12 hours and slight hyperpigmentation, which disappeared within a couple of months. Out of the six patients included in this study, three presented with strong improvement, one with moderate improvement, one with mild improvement, and one with no improvement. However, this patient was an athlete and reported frequent sweating. The study reported no recurrence after four months. Proposed mechanisms for the effectiveness of this treatment include the complete destruction of fungal hyphae and inactivation of spores, which remain and survive on the skin following medical treatment, thus enabling recurrence, destruction of the pilosebaceous unit, and anti-inflammatory properties of red light, which influences cytokine release from macrophages.27-29
Associations indicated in patients with Malassezia folliculitis include seborrheic dermatitis and tinea versicolor in 40 and six percent, respectively5,16 This is intuitive as both associated diseases also result from the Malassezia species. Acne vulgaris is also associated with MF, with various incidence; 27 percent as reported by Back et al, and 12.2 percent reported by Durdu et al.10,16 This association indicates that acne medications may be necessary adjuncts along with antifungal medications. However, antibiotic medications are not useful as they alter the normal cutaneous flora, allowing for overgrowth of the yeast. Some authors suggest discontinuing all acne medications when starting the antifungal medication, allowing for visualization of the extent of acne vulgaris.2 The authors have hypothesized a possible benefit from treatment with topical tretinoin, which may combat both diseases because of its anti-inflammatory and keratolytic properties.
MALASSEZIA
Malassezia folliculitis was originally assumed to be caused by Pityrosporum ovale, thus leading to the name Pityrosporum folliculitis.30 In fact, Potter et al confirmed the link of Pityrosporum orbiculare and P. ovale, which make up Malassezia furfur, to this disease, which was confirmed by Back et al.8,16,21,31-34 Malassezia furfur is an oval, monopolar budding yeast.7,35 It is a polymorphic, lipophilic micro-organism with a thick, multilayered cell wall.11
This yeast is found in the stratum corneum and pilar folliculi where it uses its own lipases and phospholipases to hydrolyse triglycerides from sebum into free fatty acids for their own nutritive lipid source, thus leading to proliferation.36 It is an opportunistic organism, which changes from the saprophytic phase to the pathogenic mycelian phase under certain conditions, such as increased temperature, greasy skin, sweating and immunosuppression.37 Mokronosova et al38 relate this to a change in the composition of fatty acids of the sebaceous gland due to an increase in androgen concentration.
The inflammatory component of MF has many possible mechanisms. One possibility being Malassezia’s in vitro ability to induce keratinocyte production of inflammatory cytokines via Toll-like receptor 2 (TLR 2).39 Among these inflammatory cytokines are interleukin (IL)-lα, IL-6, IL-8, IL-12, and tumor necrosis factor (TNF)-α along with anti-inflamatory cytokines IL-4 and IL-10.40 Malassezia activate complement cascades by both the classic and alternative pathways.11 Other possible mechanisms leading to inflammation include damage to the epithelial barrier function due to lipase and phospholipase activity of Malassezia, sensitization to cross-reactive allergens produced by Malassezia, and an irritant, nonimmunogenic stimulation of the immune system.3,39,41 This last mechanism is supported by the presence of an increased number of NK1+ and CD16+ cells within biopsies from lesional skin.41 While no difference was identified between the number of IL-associated cells between lesional and nonlesional skin, increased intensity was seen intercellularly in lesional skin.
Since the original description of Pityrosporum folliculitis, reclassifications of the Malassezia species and many studies on the most common associated species have been done. Although different species may be involved, all species have the same clinical presentation.10 Although minor discrepancies occurred on the particular order of most to least common, the main species identified on lesional skin were M. globosa, M. restricta, and M. sympodialis.7,23,34,42-43 M. globosa was formerly known as P. orbiculare and M. restricta resembles P. ovale.3,19 These particular species were not only identified as most common on lesional skin, but also nonlesional skin of the same patient as well as healthy controls.40 Durdu et al10 in Turkey identified the most common species in lesional samples to be M. globosa (69.4%) based on recombinant deoxyribonucleic acid (rDNA) analysis. This was followed by M. sympodialis, M. restricta, and M. furfur in order of most to least common. Furthermore, the same species were again identified on lesional and nonlesional samples of the same patient in 72 percent of cases. These results point toward the hypothesis that MF indeed results from an overgrowth of the normal cutaneous flora and not an exogenous species.34 These studies were done in different geographical locations and thus it is possible that this resulted in differences in commonality of identified species.42
Antifungal medications have been studied to determine in vitro sensitivity of various Malassezia species, which can be identified through fungal cultures. In the study done by Nakamura et al,44 all species were found to be sensitive to oral ketoconazole, which was also the only drug to inhibit growth of M. furfur strains isolated from systemic infection, indicating that this medication may be a good initial choice. Fluconazole was found to be very active against M. sympodialis and M. slooffiae, although to a lesser extent, but inactive against M. globosa and M. restricta. Meanwhile, itraconazole had high activity against M. globosa. This study indicates that resistance to various antifungals exists between Malassezia species and initial medications may need to be changed if they lack clinical improvement. Hammer et al45 found ketoconazole to be more active against M. furfur than econazole and miconazole, but M. sympodialis, M. slooffiae, M. globosa, and M. obtuse showed similar efficacies to all.
Conclusion
Malassezia folliculitis may persist for many years if it is misdiagnosed as acne vulgaris. For this reason, it is important to consider this diagnosis in patients failing to respond to typical acne medications, in particular those with pruritic, 1 to 2mm monomorphic papules and pustules. The diagnosis of Malassezia (Pityrosporum) folliculitis can be identified via the usual clinical presentation, direct microscopy and culture, histopathological examination, and rapid efficacy of oral antifungal treatments.16,46-47 This disease occurs more commonly in hot, humid environments, especially in individuals with excessive sweating or occlusions of the skin.
When deciding treatment regimens, it is important to observe what triggers flares. Treatments may be required during summer months as the weather is more hot and humid or during periods of increased sweating, such as intense exercise or outdoor work. Occlusive clothing and topical products, such as make-up, lotion, or sunscreens may also promote flares. As acne vulgaris and MF coexist in 12.2 to 27 percent of cases, it may be necessary to combine antifungal treatments along with typical acne medications.10,16 Use of antibiotics; however, may alter normal flora and lead to the yeast’s overgrowth. For this reason, other anti-acne medications are preferred over antibiotics, as antibiotics are counterproductive.
Footnotes
DISCLOSURE:The authors report no relevant conflicts of interest.
REFERENCES
- 1.Weary PE, Russell CM, Butler HK, et al. Acneform eruption resulting from antibiotic administration. Arch Dermatol. 1969;100:179–183. [PubMed] [Google Scholar]
- 2.Ayers K, Sweeney SM, Wiss K. Pityrosporum folliculitis: diagnosis and management in six female adolescents with acne vulgaris. Arch Pediatr Adolesc Med. 2005;159:64–67. doi: 10.1001/archpedi.159.1.64. [DOI] [PubMed] [Google Scholar]
- 3.Gaitanis G, Velegraki A, Mayser P, et al. Skin diseases associated with Malassezia yeasts: facts and controversies. Clin Dermatol. 2013;31:455–463. doi: 10.1016/j.clindermatol.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Potter BS, Burgoon CF, Johnson WC. Pityrosporum folliculitis. Report of seven cases and review of the Pityrosporum organism. Arch Dermatol. 1973;107:388–391. doi: 10.1001/archderm.107.3.388. [DOI] [PubMed] [Google Scholar]
- 5.Yu HJ, Lee SK, Son SJ, et al. Steroid acne vs Pityrosporum folliculitis: the incidence of Pityrosporum ovale and the effect of antifungal drugs in steroid acne. Int J Dermatol. 1998;37:772–777. doi: 10.1046/j.1365-4362.1998.00229.x. [DOI] [PubMed] [Google Scholar]
- 6.Abdel-Razek M, Fadaly G, Abdel-Raheim M, et al. Pityrosporum (Malassezia) folliculitis in Saudi Arabia: diagnosis and therapeutic trials. Clin Exp Dermatol. 1995;20:406–409. doi: 10.1111/j.1365-2230.1995.tb01358.x. [DOI] [PubMed] [Google Scholar]
- 7.Erchiga VC, Florencio VD. Malassezia species in skin diseases. Curr Opin Infect Dis. 2002;15:133–142. doi: 10.1097/00001432-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Hill MK, Goodfield MJ, Rodgers FG, et al. Skin surface electron microscopy in Pityrosporum folliculitis: the role of follicular occlusion in disease and the response to oral ketoconazole. Arch Dermatol. 1990;126:1071–1074. doi: 10.1001/archderm.126.8.1071. [DOI] [PubMed] [Google Scholar]
- 9.Poli F Differential diagnosis of facial acne on black skin. Int J Dermatol. 2012;51(Sl):24–26. doi: 10.1111/j.1365-4632.2012.05559.x. [DOI] [PubMed] [Google Scholar]
- 10.Durdu M, Guran M, Ilkit M. Epidemiological characteristics of Malassezia folliculitis and use of the May-Grunwald-Giemsa stain to diagnose the infection. Diagn Microbiol Infect Dis. 2013 doi: 10.1016/j.diagmicrobio.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Ljubojevic S, Skerley M, Lipozencic J, et al. The role of Malassezia furfur in dermatology. Clin Dermatol. 2002;20:179–182. doi: 10.1016/s0738-081x(01)00240-1. [DOI] [PubMed] [Google Scholar]
- 12.Marcon MJ, Powell DA. Human infections due to. Malassezia spp. Clin Microbiol Rev. 1992;5:101–119. doi: 10.1128/cmr.5.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsad D, Saini R, Negi KS. Short-term treatment of Pityrosporurm folliculitis: a double blind placebo-controlled study. J Eur Acad Dermatol Venereol. 1998;11(2):188–190. doi: 10.1111/j.1468-3083.1998.tb00781.x. [DOI] [PubMed] [Google Scholar]
- 14.Alves EV, Martins JE, Ribeiro EB, et al. Pityrosporurm folliculitis: renal transplantation case report. J Dermatol. 2000;27:49–51. doi: 10.1111/j.1346-8138.2000.tb02118.x. [DOI] [PubMed] [Google Scholar]
- 15.Katoh T, Irimajiri J. Pityriasis versicolor and Malassezia folliculitis. Nippon Ishinkin Gakkai Zasshi. 1999;40:69–71. doi: 10.3314/jjmm.40.69. [DOI] [PubMed] [Google Scholar]
- 16.Back 0, Faergemann J, Hornqvist R. Pityrosporurm folliculitis: a common disease of the young and middle aged. J Am Acad Dermatol. 1985;12:56–61. doi: 10.1016/s0190-9622(85)70009-6. [DOI] [PubMed] [Google Scholar]
- 17.Hurwitz RM. Steroid acne. J Am Acad Dermatol. 1989;21:1179–1181. doi: 10.1016/s0190-9622(89)70325-x. [DOI] [PubMed] [Google Scholar]
- 18.McGinley KJ, Leyden JJ, Marples RR. Quantitative microbiology of the scalp in non-dandruff, dandruff and seborrheic dermatitis. ■J Invest Dermatol. 1975;64:401–405. doi: 10.1111/1523-1747.ep12512335. [DOI] [PubMed] [Google Scholar]
- 19.Gupta AK, Batra R, Bluhm R, et al. Skin diseases associated with Malassezia species. J Am Acad Dermatol. 2004;51(5):785–798. doi: 10.1016/j.jaad.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 20.Rupke SJ. Fungal skin disorders. Prim Care. 2000;27:407–421. doi: 10.1016/s0095-4543(05)70203-7. [DOI] [PubMed] [Google Scholar]
- 21.Lee JW, Lee HI, Kim MN, et al. Topical photodynamic therapy with methyl aminolevulinate may be an alternative therapeutic option for the recalcitrant Malassezia folliculitis. Int J Dermatol. 2011;50:485–496. doi: 10.1111/j.1365-4632.2009.04377.x. [DOI] [PubMed] [Google Scholar]
- 22.Marques SA, Pires de Camargo RM, Marques MEA, et al. Exuberant clinical presentation of probable Malassezia folliculitis in a young nonimmunosuppressed patient. An Bras Dermatol. 2012;87(3):459–462. doi: 10.1590/s0365-05962012000300016. [DOI] [PubMed] [Google Scholar]
- 23.Nakabayashi A, Sei Y, Guillot J. Identification of Malassezia species isolated from patients with seborrheic dermatitis, atopic dermatitis, pityriasis versicolor and normal subjects. Med Mycol. 2000;38:337–341. doi: 10.1080/mmy.38.5.337.341. [DOI] [PubMed] [Google Scholar]
- 24.Faergemann J. In vitro and in vivo activities of ketoconazole and itraconazole against Pityrosporurm orbiculare. Antimicrob Agents Chemother. 1984;26:773–774. doi: 10.1128/aac.26.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caputo R, Barbareschi M. Itraconazole: new horizons. G Ital Dermatol Venereol. 2002;137:1–7. [Google Scholar]
- 26.Lee JW, Kim BJ, Kim MN. Photodynamic therapy: new treatment for recalcitrant. Malassezia. doi: 10.1002/lsm.20857. folliculitis. Laser Surg Med. 2010 ;42: 192-196. [DOI] [PubMed] [Google Scholar]
- 27.Smijs TG, van der Haas RN, Lugtenburg J, et al. Photodynamic treatment of the dermatophyte Trichophyton rubrum and its microconidia with prophyrin photosensitizers. Photochem Photobiol. 2004;80:197–202. doi: 10.1562/2004-04-22-RA-146. [DOI] [PubMed] [Google Scholar]
- 28.Horn M, Wolf P. Topical methyl aminolevulinate photodynamic therapy for the treatment of folliculitis. Photodermatol Photoimmunol Photomed. 2007;23:145–147. doi: 10.1111/j.1600-0781.2007.00290.x. [DOI] [PubMed] [Google Scholar]
- 29.Yu W, Nairn JO, Lanzafame RJ. Effects of photostimulation on wound healing in diabetic mice. Lasers Surg Med. 1997;20:56–63. doi: 10.1002/(sici)1096-9101(1997)20:1<56::aid-lsm9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 30.Graham JH. Pityrosporurn ovale-in the discussion. Arch Dermatol. 1968;98:421. [Google Scholar]
- 31.Potter BS, Burgoon CF, Johnson WC. Pityrosporurn folliculitis. Arch Dermatol. 1973;107:388–391. doi: 10.1001/archderm.107.3.388. [DOI] [PubMed] [Google Scholar]
- 32.Faergemann J, Johansson S, Back O, et al. An immunologic and cultural study of Pityrosporurn folliculitis. J Am Acad Dermatol. 1986;14:429–433. doi: 10.1016/s0190-9622(86)70053-4. [DOI] [PubMed] [Google Scholar]
- 33.Roberts SO. Pityrosporurn orbiculare: incidence and distribution on clinically normal skin. Br J Dermatol. 1969;81:264–269. doi: 10.1111/j.1365-2133.1969.tb13978.x. [DOI] [PubMed] [Google Scholar]
- 34.Akaza N, Akamatsu H, Sasaki Y, et al. Malassezia folliculitis is caused by cutaneous resident Malassezia species. Med Mycol. 2009;47:618–624. doi: 10.1080/13693780802398026. [DOI] [PubMed] [Google Scholar]
- 35.Takeo K, Nakai E. Mode of cell growth of Malassezia (Pityrosporurm) as revealed by using plasma membrane configuration as natural makers. Can J Microbiol. 1986;32:389–394. doi: 10.1139/m86-074. [DOI] [PubMed] [Google Scholar]
- 36.Nazzaro-Porro M, Passi S, Caprilli F, et al. Growth requirements and lipid metabolism of Pityrosporurn orbiculare. J Invest Dermatol. 1976;60:178–182. doi: 10.1111/1523-1747.ep12481919. [DOI] [PubMed] [Google Scholar]
- 37.Roberts SOB. Pityriasis versicolor: a clinical and mycological investigation. Br J Dermatol. 1996;81:315–326. doi: 10.1111/j.1365-2133.1969.tb13990.x. [DOI] [PubMed] [Google Scholar]
- 38.Mokronosova MA, Arzumanian VG, Gervazieva VB. Yeast-like fungi Malassezia (Pityrosporurn): clinical and immunological aspects of the study. Vestn Ross Akad Med Nauk. 1998;5:47–50. [PubMed] [Google Scholar]
- 39.Baroni A, Orlando M, Donnarumma G, et al. Toll-like receptor 2 (TLR-2) mediates intracellular signaling in human keratinocytes in response to. Malassezia furfur. Arch Dermatol Res. 2006;297:280–288. doi: 10.1007/s00403-005-0594-4. [DOI] [PubMed] [Google Scholar]
- 40.Gaitanis G, Magiatis P, Hantschke M, et al. The Malassezia genus in skin and systemic diseases. Clin Microbiol Rev. 2012;25:106–141. doi: 10.1128/CMR.00021-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faergemann J, Bergbrant I-M, Dohse M, et al. Seborrhoeic dermatitis and Pityrosporurn (Malassezia) folliculitis:characterization of inflammatory cells and mediators in the skin by immunohistochemistry. Br J Dermatol. 2001;144:549–556. doi: 10.1046/j.1365-2133.2001.04082.x. [DOI] [PubMed] [Google Scholar]
- 42.Ko JH, Lee YW, Choe YB, Ahn KJ. Epidemiologic study of Malassezia yeasts in patients with Malassezia folliculitis by 26S rDNA PCR-RFLP analysis. Ann Dermatol. 2011;23:177–184. doi: 10.5021/ad.2011.23.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crespo EV, Ojeda MA, Vera CA, et al. Mycology of pityriasis versicolor. J Mycol Med. 1999;9:143–148. [Google Scholar]
- 44.Nakamura Y, Kano R, Murai T, et al. Susceptibility testing of Malassezia species using the urea broth microdilution method. Antimicrob Agents Chemother. 2000;44:2185–2186. doi: 10.1128/aac.44.8.2185-2186.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammer KA, Carson CF, Riley TV. In vitro activities of ketoconazole, econazole, miconazole and Melaleuca alternifolia (tea tree) oil against Malassezia species. Antimicrob Agents Chemother. 2000;44:467–469. doi: 10.1128/aac.44.2.467-469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jillson OF. Pityrosporurn folliculitis. Cutis. 1985;12:56–61. [Google Scholar]
- 47.Jacinto-Jamora S, Tamesis J, Katigbak ML. Pityrosporurn folliculitis in the Philippines: diagnosis, prevalence, and management. J Am Acad Dermatol. 1991;24:693–696. doi: 10.1016/0190-9622(91)70104-a. [DOI] [PubMed] [Google Scholar]