Abstract
Alzheimer’s disease (AD) is a neurodegenerative disease with a complex and progressive pathological phenotype characterized first by hypometabolism and impaired mitochondrial bioenergetics followed by pathological burden. Increasing evidence indicates an antecedent and potentially causal role of mitochondrial bioenergetic deficits and brain hypometabolism coupled with increased mitochondrial oxidative stress in AD pathogenesis. Compromised aerobic glycolysis pathway coupled with oxidative stress is first accompanied by a shift toward a ketogenic pathway that eventually progresses into fatty acid oxidation (FAO) pathways and leads to white matter degeneration and overproduction and mitochondrial accumulation of β-amyloid.
Estrogen-induced signaling pathways converge upon the mitochondria to enhance mitochondrial function and to sustain aerobic glycolysis coupled with citric acid cycle-driven oxidative phosphorylation to potentiate ATP (Adenosine triphosphate) generation. In addition to potentiated mitochondrial bioenergetics, estrogen also enhances neural survival and health through maintenance of calcium homeostasis, promotion of antioxidant defense against free radicals, efficient cholesterol trafficking, and beta amyloid clearance.
Significantly, the convergence of E2 mechanisms of action onto mitochondria is also a potential point of vulnerability when activated in diseased neurons that exacerbates degeneration through increased load on dysregulated calcium homeostasis. The “healthy cell bias of estrogen action” hypothesis examines the role that regulating mitochondrial function and bioenergetics play in promoting neural health and the mechanistic crossroads that lead to divergent outcomes following estrogen exposure. As the continuum of neurological health progresses from healthy to unhealthy, so too do the benefits of estrogen or hormone therapy.
I. Introduction: Alzheimer’s Disease—Unlimited Cost/Limited Windows of Therapeutic Opportunity
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disease and the leading cause of dementia among the aged population. It is estimated that 5.4 million people are currently living with AD in the United States, and this number is projected to at least double by the year 2050 (Alzheimer’s Association, 2011). Additionally, the prevalence of AD increases exponentially with age in people aged 65 or older (Hansson et al., 2006). The majority of AD patients (about 67%) are women (Alzheimer’s Association, 2011) partially because there are more women than men in the oldest segment of the population (V. W. Henderson & Brinton, 2010). Additionally, loss of ovarian hormones associated with menopause in mid-to-late life has been linked to increased risk for AD in women (Brinton, 2008b; V. W. Henderson & Brinton, 2010).
The disease is symptomatically characterized by progressive memory deficits, cognitive impairments, and personality changes, which can be attributed to deteriorating synaptic function and the subsequent loss of neurons in vulnerable regions of the brain, including the neocortex, the limbic system, and the subcortical regions (Fassbender et al., 2001). From a histopathological view, AD is characterized by senile plaques and neurofibrillary tangles (NFTs) in the medial temporal lobe and cortical areas of the brain (Hansson et al., 2006). AD has been categorized into two major forms: familial AD (FAD) and late-onset AD (LOAD; also termed sporadic AD, or SAD) with the latter being the leading cause of dementia in the elderly. FAD is an autosomal dominant disorder with onset before 65 years of age. The majority of FAD cases have been attributed to mutations in three genes: amyloid precursor protein (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2) (K. Chen et al., 2007). In contrast, the complete etiology of LOAD has yet to be fully elucidated, although age has been recognized as the greatest risk factor.
Currently, no treatment exists to prevent, modify, or halt the progression of AD (Golde et al., 2011; Schneider et al., 2011). Available drugs approved by FDA only offer moderate and temporary symptom relief (Golde et al., 2011). Therapeutic developments for AD, particularly LOAD, have been largely impeded by limited understanding of disease etiology. The prevailing “amyloid cascade” hypothesis, which was first introduced by Hardy and Higgins in 1992 and has been enriched over the past decade, emphasizes the neurotoxic characteristics of β-amyloid (Aβ) as the main contributor to disease progression. This hypothesis proposes that the deposition of Aβ initiates a cascade of events, including the formation of NFTs, prolonged inflammatory responses, increased oxidative stress and mitochondrial dysfunction, which eventually lead to cell death and dementia (Armstrong, 2011; Hardy, 2006; Hardy & Higgins, 1992; Sommer, 2002). While this “amyloid cascade” hypothesis proposes a unified etiopathogenic mechanism for both FAD and LOAD, findings from both basic research and clinical observations indicate that a far more complex mechanism underlies LOAD. Recent studies indicate that in LOAD both Aβ deposition and NFTs, rather than being the cause of the disease, may be reactive products that arise from increased vulnerability to genetic and environmental risk factors as a function of aging (Armstrong, 2011; Gibson & Shi, 2010; Pimplikar, 2009; Simon et al., 2010). Moreover, candidates that directly target amyloid pathways, either through passive immunotherapy against Aβ (Bapineuzumab) (Prins et al., 2010) or via inhibition of pathways involved in Aβ generation (Tarenflurbil, Semagacestat, or Flurizan) (Imbimbo & Giardina, 2011), failed to achieve efficacy in recent clinical trials, indicating the therapeutic limitation of amyloid-specific strategies. Increasing evidence suggests that AD, particularly LOAD, is a multifaceted disease that could at least be partially attributed to a decline in mitochondrial function and altered brain metabolic activity.
II. Role of Mitochondrial Bioenergetics in Alzheimer’s Pathogenesis
A. Mitochondrial Dysfunction and β-Amyloid
The fundamental role of mitochondria in cellular bioenergetics and survival is well established (Brinton, 2008a; Magistretti, 2006; Wallace, 2005). In addition, the evidence for mitochondrial dysfunction as a pivotal factor in age-associated neurodegenerative diseases such as Alzheimer’s and Parkinson’s continues to mount (Brinton, 2008b; Moreira et al., 2006; Moreira et al., 2010, 2011; Mosconi, Mistur, Switalski, Brys et al., 2009; Swerdlow & Khan, 2009). Perturbations in mitochondrial function have long been observed in samples derived from clinically confirmed AD patients, including altered mitochondrial morphology, compromised enzyme complexes in the tricarboxylic acid cycle, and reduced cytochrome-c oxidase (COX) activity (Blass et al., 2000; Cardoso et al., 2004; Gibson et al., 1988; Parker, 1991; Perry et al., 1980; Sorbi et al., 1983). Later, the “cybrid model” of AD, generated by transferring mitochondrial DNA (mtDNA) from human AD patients into cell cultures that are devoid of endogenous mtDNA (ρ° cells), exhibited characteristics that recapitulated previous findings from clinical AD specimens. These findings included decreased mitochondrial mobility, increased oxidative stress, decreased COX activity, decreased mitochondrial membrane potential, and increased Aβ production, thereby providing further evidence for involvement of mitochondria and mtDNA in AD etiopathogenesis (Khan et al., 2000; Swerdlow, 2007). Increasing evidence indicates that mitochondria are direct targets of Aβ. Aβ has been demonstrated to accumulate within mitochondria and interact with a mitochondrial protein, Aβ-binding alcohol dehydrogenase (ABAD), resulting in decreased COX activity and increased oxidative stress (Lustbader et al., 2004; Reddy & Beal, 2008; Takuma et al., 2005). Further, the Aβ-induced neurotoxicity requires functional mitochondrial respiratory chain enzyme complexes (Cardoso et al., 2001) and is exacerbated in synergy with mitochondrial dysfunction in AD cybrid models (Cardoso et al., 2004).
While the neurotoxic mechanisms of Aβ converge upon mitochondria, compromised mitochondrial function, particularly a decline in mitochondrial bioenergetics and an increase in oxidative stress, propagates the degenerative process by further increasing Aβ generation. This creates a vicious cycle in which excessive Aβ accumulation and sustained mitochondrial dysfunction synergize to activate a cascade of neurodegenerative pathways (Cardoso, Santana et al., 2004; Silva et al., 2011; Swerdlow et al., 2010; Swerdlow & Khan, 2009).
B. Mitochondrial Bioenergetic Deficits in AD
Multiple levels of analysis and experimental paradigms, ranging from in vitro cell model systems and genomic analyses in animal models to postmortem autopsy of human brain and human brain imaging, indicate that dysfunction in glucose metabolism, bioenergetics, and mitochondrial function are consistent antecedents to development of Alzheimer’s pathology (Gibson & Shi, 2010; Hauptmann et al., 2009; Nicholson et al., 2010; Yao et al., 2009). A decline in brain glucose metabolism and mitochondrial function can appear decades prior to the onset of histopathological and/or clinical features and thus may serve as a biomarker of AD risk as well as a therapeutic target (Mosconi et al., 2008; Mosconi & McHugh, 2011; Mosconi et al., 2009; Mosconi et al., 2009; Reiman et al., 2004). Studies using multiple preclinical in vitro and in vivo AD models have demonstrated a decline in mitochondrial function prior to the development of Alzheimer’s pathology, including decreased mitochondrial respiration, decreased metabolic enzyme expression and activity, decreased cerebral glucose metabolism, increased oxidative stress, and increased mitochondrial Aβ load and ABAD expression (Chou et al., 2011; Diana et al., 2011; Du et al., 2010; Hauptmann et al., 2009; Nicholson et al., 2010; Yao et al., 2009). The decline in mitochondrial function deteriorates with AD progression (Lustbader et al., 2004; Takuma et al., 2005). Consistent with basic science findings, multiple positron emission tomography (PET) studies also report antecedent abnormality in cerebral glucose utilization decades prior to the onset of AD, particularly in the hippocampal and entorhinal cortical regions (De Santi et al., 2001; Ishii et al., 1997; Mosconi et al., 2008; Mosconi et al., 2009; Reiman et al., 2004; Rosenbloom et al., 2011; Spulber et al., 2008). This distinct pattern of brain hypometabolism predicted the cognitive decline in normal aging (Mosconi et al., 2008) or the progression of patients from mild cognitive impairment (MCI) to AD (Chetelat et al., 2003) with high accuracy. Recent clinical studies revealed a significant overlap between brain regions that exhibited abnormal glucose metabolism and regions that are most vulnerable to development of AD pathology (Bero et al., 2011; Vaishnavi et al., 2010; Vlassenko et al., 2010), providing further evidence of the association between disrupted glucose metabolism and AD pathogenesis.
C. Bioenergetic Deficits and Oxidative Stress
Impairment of mitochondrial bioenergetics and oxidative phosphorylation are closely associated with increased free radical production and consequent oxidative damage. As the major source for cellular reactive oxygen species, mitochondria generate free radicals (superoxide anion, O2−) and hydrogen peroxide (H2O2) as by-products of oxidative phosphorylation (Dumont et al., 2010; Lin & Beal, 2006). It is well documented that oxidative damage to mitochondrial membranes and proteins impairs mitochondrial oxidative phosphorylation efficiency and results in increased electron leak, increased H2O2 levels and higher oxidative stress (Beal, 2005; Reddy & Beal, 2008). Key enzymes involved in mitochondrial bioenergetics, such as pyruvate dehydrogenase (PDH) and α-ketoglutarate dehydrogenase (αKGDH), are often the targets of oxidative modifications. This leads to deceased enzyme activity, decreased efficiency of mitochondrial electron transport, and increased production of free radicals (Park et al., 1999; Starkov et al., 2004).
Higher oxidative stress is characteristic of AD brains (Atamna & Frey, 2007; Gibson & Shi, 2010): in AD patients, significant increases in lipid peroxides, 8-oxoguanine, and oxidized amino acids, have been identified in vulnerable brain regions (Nunomura et al., 2004; Reddy, 2006). In preclinical AD animal models, increased generation of H2O2 and elevated oxidative damage to cellular components has also been shown to precede the development of AD pathology (Nunomura et al., 2009; Pratico et al., 2001; Rhein et al., 2009; Trushina & McMurray, 2007; Wang et al., 2005; Yao et al., 2009). Interestingly, an increase in oxidative stress has been demonstrated to increase Aβ production in vitro and in vivo (Moreira et al., 2007; Nunomura et al., 2001; Zhang et al., 2007).
D. Alternative Fuel Sources and White Matter Degeneration in AD
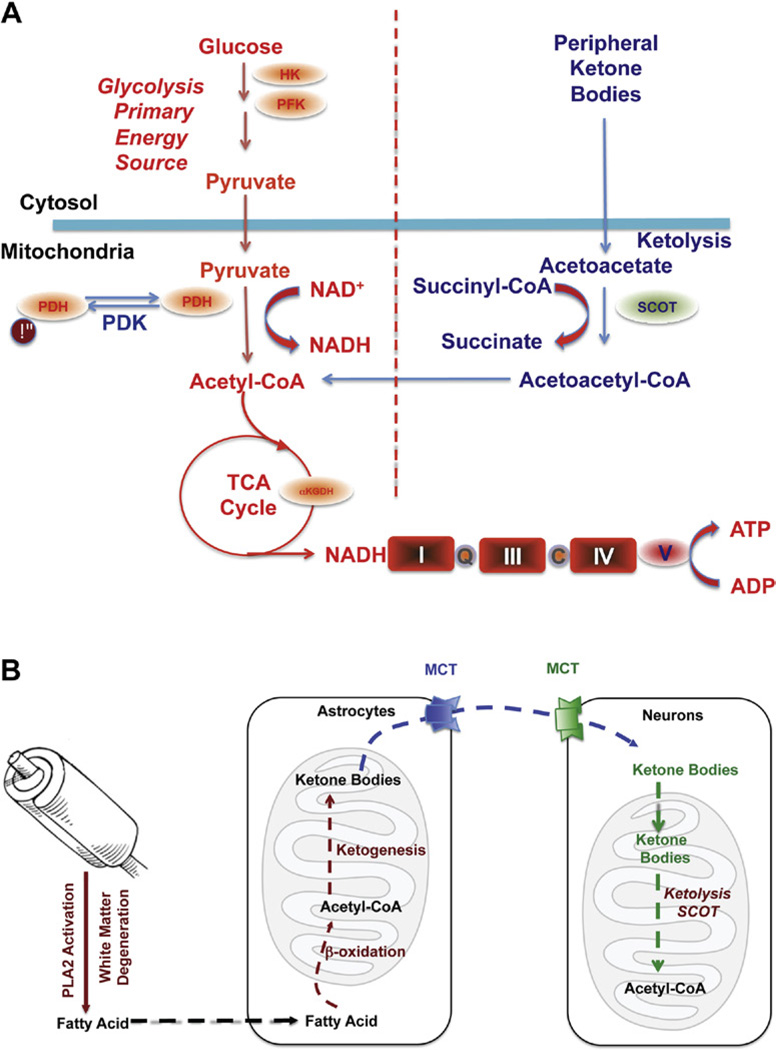
In parallel to the decline in brain glucose metabolism, white matter hyperintensities are also an early hallmark of AD (Kuczynski et al., 2010; Zhang et al., 2007). Defined as an alteration in white matter integrity, these hyperintensities are first observed in the cingulum bundle, uncinate fasciculus, and superior longitudinal fasciculus of MCI patients (O’Dwyer et al., 2011). These regions are integral structures in the brain’s default mode network, a system that is active when an individual is not engaged in goal-oriented activities or is at a resting state while awake. In addition to a loss in white matter integrity, patients with MCI have characteristic hypometabolism of the prefrontal and posterior cingulate cortices, and also major components of the default mode network (O’Dwyer et al., 2011; Villain et al., 2010; Vlassenko et al., 2010). Interestingly, the connectivity between these cortices is provided by the superior longitudinal fasiculus, a region where hyperintensity positively correlates with the observed hypometabolism (Kuczynski et al., 2010). This loss in white matter integrity could be a direct result of the bioenergetic shift in these two cortices, indicating a switch from the use of ketone bodies supplied from the peripheral ketogenic organ, the liver, to ketone bodies resulting from local myelin breakdown via fatty acid oxidation (FAO) by astroglia (Morris, 2005) (Fig. 1). Alternately, lesions in white matter integrity may be caused by inadequate lipid synthesis due to competition between consumption of ketones/acetyl-CoA for bioenergetics and lipid synthesis (Morris, 2005).
FIGURE 1. Bioenergetic substrate and catalytic compensatory adaptations to sustain metabolic demand of the brain.
(A) Compensatory bioenergetic adaptation I: Glucose, the primary fuel source of brain metabolism, is converted via glycolysis to pyruvate which is further converted into acetyl-CoA to initiate and sustain the TCA cycle. Under metabolically challenging conditions (i.e., starvation, aging, and neurodegeneration) neurons can utilize peripheral ketone bodies (β-hydroxybutyrate and acetoacetate generated by the liver) through ketolysis to generate acetyl -CoA. (B) Compensatory bioenergetic adaptation II: local consumption of white matter for bioenergetics. With disease progression, peripheral ketone bodies are exhausted and the brain has to consume local white matter for energy production. Degradation of white matter via activation of PLA2 generates fatty acids that are further metabolized into acetyl-CoA through β-oxidation in the astrocytes. Acetyl-CoA is further converted into ketone bodies and transported into neurons by monocarboxylate transporters (MCTs) where ketone bodies are converted back into acetyl-CoA by SCOT and other important enzymes in ketolysis and further utilized toward ATP generation. For color version of this figure, the reader is referred to the online version of this book.
Considering the role of the cingulum bundle in connecting the hippocampal formation to both the prefrontal cortex and the posterior cingulate cortex, the degeneration of this white matter tract in addition to the hypometabolism of the prefrontal and posterior cingulate cortices results in the early atrophy of the hippocampus (Risacher et al., 2009; Whitwell et al., 2007) as well as impaired memory symptomatic of AD (Villain et al., 2010).
The default mode network’s heavy reliance on glucose to perform aerobic glycolysis makes synaptic transmission especially susceptible to bioenergetic deficits (Vaishnavi et al., 2010; Vlassenko et al., 2010). Recently, it has been found that amyloid beta deposition and abnormal aerobic glycolysis are present in AD in a strikingly similar pattern, specifically in the default mode network (Vlassenko et al., 2010). Mitochondrial dysfunction results in a series of changes that contribute to Aβ accumulation in mitochondria, including impaired oxidative phosphorylation, uncoupled electron transport chain, compromised ATP synthase, and COX inhibition (Manczak et al., 2006; Readnower et al., 2011; Young & Bennett, 2010). The fact that white matter degeneration is also selectively localized to the default mode network converges on a mechanistic pathway that links Aβ localization and activation of phospholipase A2 (PLA2). PLA2 subsequently activates sphingomyelinase, which in turn breaks down the myelin sheath to generate fatty acids that can be used in ketogenic energy production. The region-specific association between white matter degeneration, brain hypometabolism and Aβ accumulation provides compelling evidence in support of a bioenergetic mechanism that unifies both compromised glucose metabolism and white matter catabolism in AD pathogenesis.
In parallel with the decline in glucose metabolism in AD, there is a generalized shift away from glucose-derived energy production, which is associated with a decrease in the expression of glycolytic enzymes coupled to a decrease in the activity of the PDH complex (Blass et al., 2000). Alterations in the brain metabolic profile in AD are further evidenced by concomitant activation of compensatory pathways that promote the usage of alternative substrates, such as ketone bodies, to compensate the decline in glucose-driven ATP generation. We have previously reported that in the female 3xTgAD mouse model, prepathological decreases in PDH expression and mitochondrial bioenergetics were paralleled by increased expression of suc-cinyl-CoA:3-ketoacid coenzyme A transferase (SCOT) and hydroxyacyl-Coenzyme A dehydrogenase (HADHA) at a young age (3 months). HADHA is a subunit of the mitochondrial trifunctional protein, which catalyzes the last three steps of mitochondrial β-oxidation of long chain fatty acids to generate acetyl-CoA, whereas SCOT is the key enzyme that converts ketone bodies into acetyl-CoA. The increase in HADHA and SCOT expression indicates early activation of ketolytic and/or FAO pathways to compensate for compromised PDH capacity, and to provide alternative sources of ace-tyl-CoA, to sustain ATP generation (Yao et al., 2010). Consistent with these mechanistic analyses, clinical observations have also reported a substrate switch that parallels AD progression. While there is a 100:0 ratio of glucose to other substrates utilization in young controls, there is a 2:1 ratio in AD patients compared to a ratio of 29:1 in healthy elderly controls (Hoyer et al., 1991).
E. A Bioenergetic-Centric Hypothesis of AD
We have discussed four different but important pathogenic factors of AD, including decreased mitochondrial bioenergetics, increased oxidative stress, compromised white matter, and elevated Aβ generation. While each individual aspect focuses on a specific perspective of AD pathogenesis, the unique temporal-spatial association between these components indicates a common mitochondrial-centric mechanism that unifies these aspects into a bioenergetic compensatory network. In healthy aging, the brain exhibits a glucose-driven metabolic phenotype. The energy-redox axis is tightly coupled and physiological concentrations of H2O2 are maintained by the coordinated activity of endogenous antioxidant systems. In contrast, in prodromal AD brains, glucose metabolism is compromised early in the disease process and creates a bioenergetic crisis, switching the brain from efficient glucose-driven energy production to less efficient ketone bodydriven energy production. The compromised bioenergetic state is accompanied and further exacerbated by elevated oxidative stress, which is associated with increased expression of enzymes involved in ketogenesis and FAO, such as SCOT and HADHA, as well as mitochondrial Aβ accumulation (Young & Bennett, 2010). Further aiding this switch toward inefficiency is the elevated H2O2 production that results from decreased mitochondrial efficiency and increased oxidative stress. H2O2 leads to activation of PLA2, which degrades the myelin sheath so that it may be used as an additional source of fatty acids in ketogenesis. Consequently, the release and enrichment of free cholesterol resulting from white matter degeneration leads to impairment of the lipid-protein bilayer and contributes to hyperactivation of y-secretase and Aβ overproduction (Burns et al., 2003; Petanceska et al., 2002; Vetrivel & Thinakaran, 2010). Cleavage of APP by y-secretase leads to intraneuronal Aβ production and translocation of Aβ to mitochondria (Manczak et al., 2006; Readnower et al., 2011; Young & Bennett, 2010).
While we posit a stepwise progression of bioenergetic compensatory adaptations, it is more likely that there is a vicious cycle of exacerbating interactions. Mitochondrial accumulation of Aβ would exacerbate the bioenergetic deficits by contributing to decline in the energy-transducing efficiency. Mitochondrial accumulation of Aβ would also induce an increase in ABAD expression, perpetuating the activation of the FAO pathway and the degeneration of myelin, thereby propagating the transition of brain metabolism into a ketogenic/FAO phenotype.
III. Estrogen Action in the Brain-Convergence upon Mitochondrial Bioenergetics and Brain Metabolism
A. Estrogen-Induced Activation of Signaling Pathways: Convergence upon Mitochondria
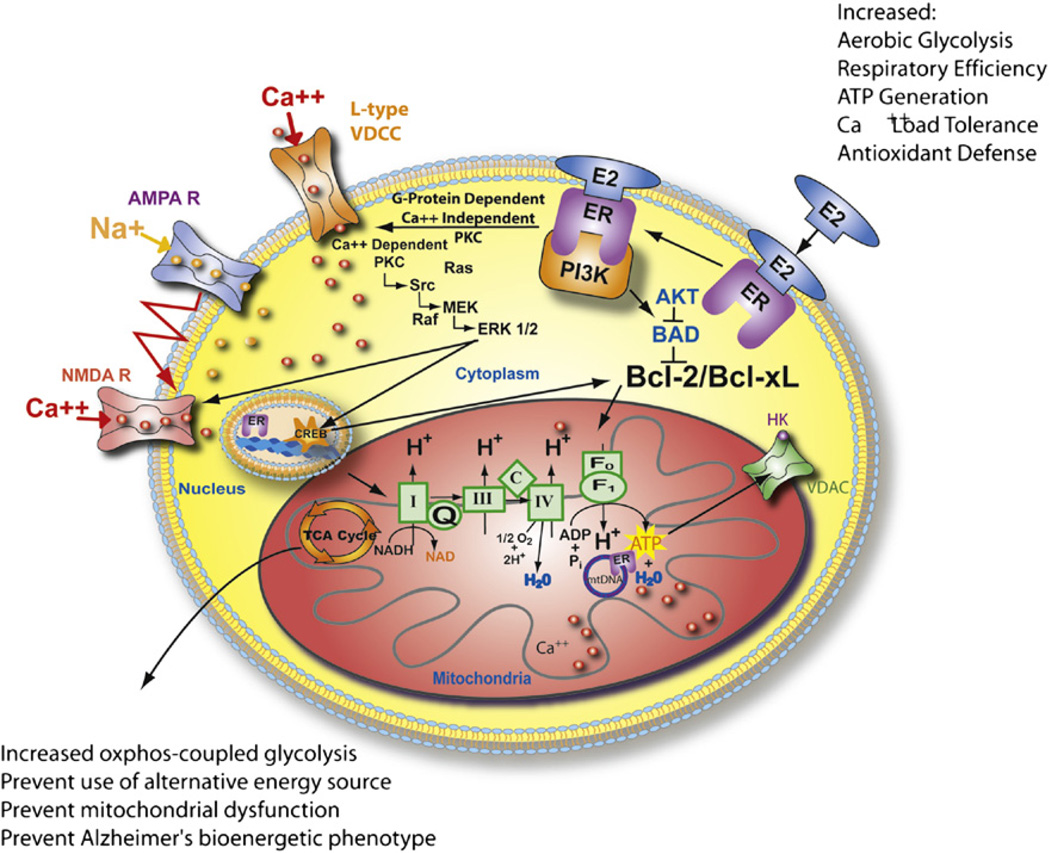
Our investigation of estrogen regulation of mitochondrial function was stimulated by our findings that 17β-estradiol (E2) prevented dysregulation of Ca2+ homeostasis by increasing mitochondrial sequestration of Ca2+ while simultaneously sustaining mitochondrial respiration (Morrison et al., 2006; Nilsen & Brinton, 2003, 2004). Further, we serendipitously observed years earlier that estrogens increased ATP generation in healthy hippocampal neurons and sustained ATP generation in hippocampal neurons following exposure to Aβ1–42 (Brinton et al., 2000). More recently, we demonstrated that in vitro, E2 increased maximal mitochondrial respiration in neurons and basal and maximal respiration in glia (Yao et al., 2011). E2 pretreatment protected against inhibitors of mitochondrial electron transport chain in cultured primary neurons (Yao et al., 2011). In addition, in mice ovariectomy (OVX)-induced loss of estrogen led to significant deficits in mitochondrial bioenergetics and accumulation of mitochondrial Aβ, whereas E2 treatment initiated at time of OVX prevented the OVX-induced deficits (Yao, Irwin et al., 2011). These findings coupled with our increasing awareness that estrogen-induced signaling pathways converged upon the mitochondria (Mannella & Brinton, 2006; Nilsen & Brinton, 2003, 2004; Nilsen et al., 2006), led us to the directly investigate mitochondria as a pivotal convergence point of estrogen action in neurons (Fig. 2).
FIGURE 2. Estrogen mechanisms of action converge upon the mitochondria.
Estrogen (17β-estradiol, E2) binding to a membrane associated estrogen receptor (ER) undergoes a protein-protein interaction with the regulatory subunit of PI3K, p85, to activate the divergent but coordinated activation of the Akt and MAPk signaling cascades. These E2-induced signaling pathways in hippocampal and cortical neurons converge upon the mitochondria to enhance glucose uptake and metabolism, aerobic glycolysis, pyruvate dehydrogenase to couple aerobic glycolysis to acetyl-CoA production and tricarboxylic acid cycle (TCA)-coupled oxidative phosphorylation and ATP generation. In parallel, E2 increases antioxidant defense and antiapoptotic mechanisms. Estrogen receptors at the membrane, in mitochondria, and within the nucleus are well positioned to regulate coordinated mitochondrial and nuclear gene expression required for optimal bioenergetics. Enhancing and sustaining glycolysis, aerobic metabolism, and mitochondrial function would be predicted to prevent the shift to alternative fuel sources and the hypometabolism characteristic of Alzheimer’s disease. Figure modified from (Morrison, et al., 2006). For color version of this figure, the reader is referred to the online version of this book.
In neurons and brain, 17β-estradiol (E2) activates a system of signaling cascades, including mitogen-activated protein kinase (MAPK) (Arevalo et al., 2011; Nilsen & Brinton, 2003; Singh, Setalo, Guan, Frail, & Toran-Allerand, 2000), phosphatidylinositol-3-kinase (PI3K) (Brinton, 2008a; Cheskis et al., 2008; Spencer-Segal et al., 2011), G protein regulated signaling, c-fos, protein kinase C (PKC) (Cordey et al., 2003), and Ca2+ influx (T. W. Wu et al., 2005). Each of the pathways has been associated with E2 regulation of neuronal function and survival. Further, of these E2-inducible signaling pathways, PI3K has the potential for simultaneously activating the MAPK, PKC, Ca2+ influx, and Akt signaling pathways (Mannella & Brinton, 2006; Simoncini et al., 2000). The outcome of activating these pathways is the coordinated neuroprotective responses that involve immediate, intermediate, and long-term responses. Immediate responses involve PKC mediated phosphorylation events that rapidly open L-type calcium channels to active the Src/ERK/CREB signaling pathway. In parallel, activation of the PI3K pathway leads to phosphorylation of Akt to inactivate proapoptotic proteins (Mannella & Brinton, 2006). Intermediate responses are characterized by translocation of Ca2+ pERK and pAKT to the nucleus and activation of the transcription factor CREB.
B. Estrogen Regulation of Glucose Metabolism
Earlier work from the Simpkins group demonstrated that E2 increased expression of glucose transporter subunits and increased glucose transport in blood–brain barrier endothelium (Shi & Simpkins, 1997). Later work by Bondy and colleagues confirmed E2 regulation of glucose transporter proteins and that regulation of glucose transporters occurs in neurons in the nonhuman primate brain (Cheng et al., 2001). In the frontal cortex of ovariectomized nonhuman primates, E2 treatment induced two- to fourfold increases in glucose transporter proteins Glut3 and Glut4 mRNA and protein (Cheng et al., 2001). Analysis of cellular localization indicated that E2-induced a marked rise in neuronal Glutl mRNA levels with no appreciable effect on vascular Glutl gene expression. Collectively, these data indicate that E2 regulate metabolic functions sustaining the energetic demands of neuronal activation (Bishop & Simpkins, 1995; Nilsen & Brinton, 2003, 2004; Nilsen et al., 2006; Simpkins & Dykens, 2008; Simpkins et al., 2005).
In addition to facilitating glucose transport into the brain and into neural cells, E2 simultaneously promotes aerobic glycolysis. Evidence derived from rat brain indicates that, in vivo, E2 significantly increased glycolytic enzyme activity of hexokinase (soluble and membrane-bound), phosphofructokinase and pyruvate kinase within 4 h (Kostanyan & Nazaryan, 1992). The neuroprotective effect of E2 is mediated by the coordinated and near simultaneous activation of both the MAPK and Akt signaling pathways through activation of PI3K in hippocampal neurons (Mannella & Brinton, 2006) (Fig. 2). Remarkably, the anti-apoptotic effect of Akt is dependent upon hexokinase association with the voltage-dependent anion channel (VDAC) of mitochondria (Gottlob et al., 2001). Hexokinases are known to bind to VDAC to directly couple intramitochondrial ATP synthesis to glucose metabolism (Miyamoto et al., 2008). Moreover, of the four hexokinase isoforms, only HKI and HKII are known to associate with mitochondria where they associate with the mitochondrial outer membrane and bind to VDAC (Gottlob et al., 2001). While it is known that E2 activates Akt (Mannella & Brinton, 2006; Singh, 2001; Znamensky et al., 2003) and increases HKII activity (Kostanyan & Nazaryan, 1992), it remains to be determined whether E2 is promoting the association of HKII and VDAC in neural cells.
Functional impact of estrogen-induced glucose transporter protein would require a concomitant change in factors regulating glucose metabolism which in turn suggests a role for insulin or its brain homologue insulin growth factor-1 (IGF1) and its cognate receptor, IGF-1R. Bondy and colleagues found that IGF-1R mRNA was concentrated in cortical neurons in a distribution similar to Glut3 and Glut4 (Cheng et al., 2001). In the nonhuman primate frontal cortex, E2-treated animals showed a significant increase in IGF1 mRNA without a concomitant rise IGF1 receptor mRNA (Cheng, Cohen, Tseng et al., 2001). These investigators went on to elucidate the molecular mechanisms whereby IGF1 regulated neuronal metabolism by demonstrating that the active, phosphorylated form of Akt/PKB was selectively co-localized with the “insulin-sensitive” glucose transporter, Glut4, in IGFl-expressing neurons. Akt is a major target of insulin signaling in the regulation of glucose transport via the facilitative glucose transporter (Glut4) and glycogen synthesis in peripheral tissues. In parallel to these studies of glucose transport and metabolism, Garcia-Segura and colleagues have for many years demonstrated the synergistic coupling between ERs and IGF-1R (Arevalo et al., 2011; Cardona-Gomez et al., 2002; Garcia-Segura et al., 2010; Garcia-Segura et al., 2000; Mendez & Garcia-Segura et al., 2006; Mendez et al., 2006; Spencer-Segal et al., 2011). Results of their analyses provide substantial evidence for the role of IGF-1, PI3K to Akt signaling pathway and ER in estrogen-inducible neuroprotection (Cardona-Gomez et al., 2002; Garcia-Segura et al., 2000; Mendez et al., 2003). Findings of the neuroprotective actions of the synergy between the ER and insulin/IGF-1 signaling cascades are particularly relevant to prevention of neurodegenerative diseases. In fact, in AD patients, compromised brain insulin regulation in brain regions that are vulnerable to AD pathology, including decreased expression of both insulin and insulin receptors, and impaired insulin signaling pathways, have been suggested to at least partially account for the cognitive deficits associated with this disease (Schioth et al., 2011; W. Q. Zhao et al., 2008). Torres-Aleman and coworkers have demonstrated that low circulating IGF-1 in brain is associated with greater accumulation of beta amyloid whereas Aβ burden can be reduced by increasing serum IGF-I (Carro et al., 2002). The inverse relationship between serum IGF-I and brain Aβ levels reflects the ability of IGF-I to induce clearance of β amyloid from brain, likely by enhancing transport of Aβ carrier proteins such as albumin and transthyretin into the brain (Carro et al., 2002).
C. Estrogen Regulation of Mitochondrial Function: Bioenergetic Survival for the Brain
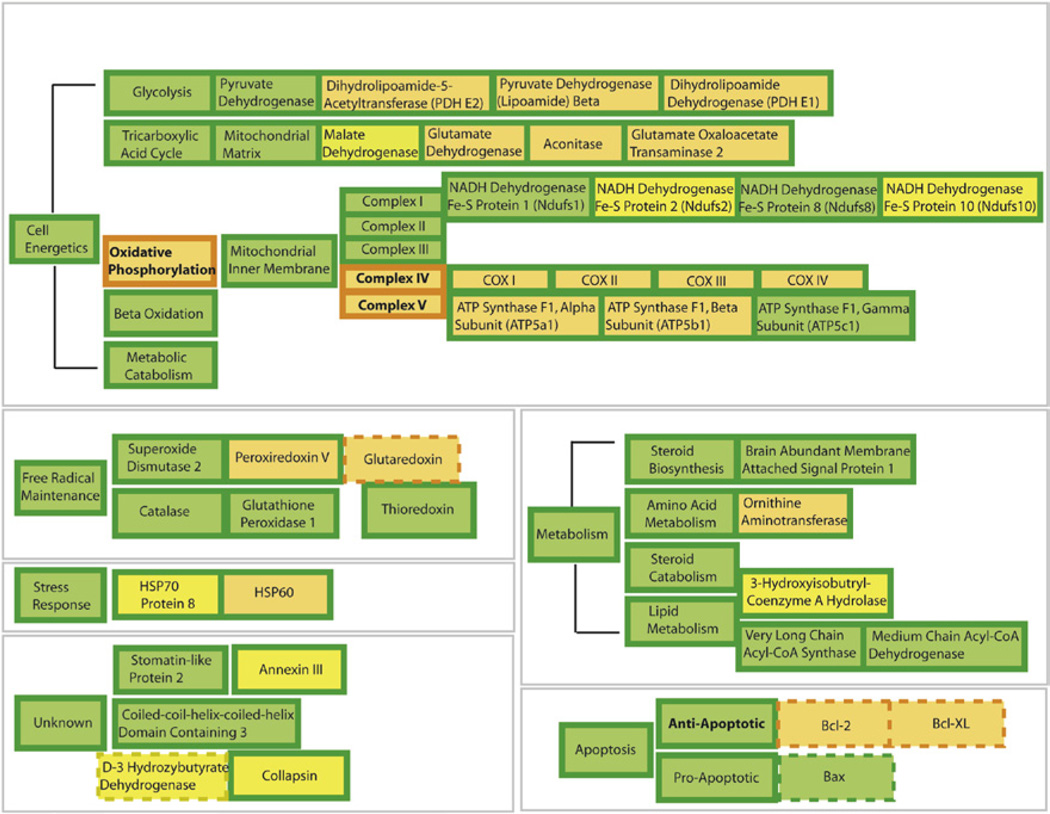
Estrogen-mediated up-regulation of glucose transport and aerobic glycolysis is further enhanced by estrogen-induced potentiation of mitochondrial bioenergetic function. We previously conducted a proteomic analysis of brain mitochondria from female rats treated with E2. Mitochondria, by some estimates, contain up to 1500 proteins (Lopez et al., 2007), a number that is amenable to examination by two-dimensional gel electrophoresis coupled with LC-MS/MS protein identification. Results of our proteomic analyses indicated that of the 499 detected proteins, 66 proteins had a twofold or greater change in expression (Nilsen, et al., 2007). Of these, 28 proteins were increased in expression following E2 treatment, whereas 38 proteins decreased in expression relative to control. E2 regulated protein expression and activity of key metabolic enzymes including PDH, aconitase, and ATP-synthase (Nilsen et al., 2007). Overall, E2-induced marked changes in proteins involved in cellular energetics, free radical maintenance, metabolism, stress response, and cell survival (Fig. 3).
FIGURE 3. Overview of 17β-estradiol (E2) regulation of female rat brain mitoproteome in vivo.
Results of the functional proteomic analysis of the brain mitoproteome were combined with a bioinformatic assessment of the brain mitoproteome regulated by E2. Proteins with known responses to E2 were separated into functional subgroups based on common mitochondrial ontology. Orange represents upregulation and yellow represents downregulation. Filled boxes are based on results of Nilsen, Irwin et al.,(Nilsen, et al., 2007). Dashed boxes are derived from published literature [reviewed in Nilsen, et al.,(2007)]. Bold lettering represents altered activity. E2 significantly increased key components of cellular energetic machinery including proteins involved in the tricarboxylic acid cycle and oxidative phosphorylation. Further, E2 increased expression of antioxidant enzymes and antiapoptotic proteins. Collectively, the data indicates a comprehensive regulation of mitochondrial function by E2, which increases key elements in the tricarboxylic acid cycle, pyruvate metabolism, mitochondrial oxidative phosphorylation, respiratory efficiency, and ATP generation while reducing free radical leak and oxidative damage. For interpretation of the references to color in this figure legend, the reader is referred to the online version of this book.
In cellular energetics, E2 induced twofold increases in key enzymes required for glycolysis. E2 has been reported to increase activity of the key cytosolic glycolytic enzymes hexokinase, phosphofructokinase, and phosphoglycerate kinase in rodent brain (Kostanyan & Nazaryan, 1992). In coordination with up-regulated substrate influx from increased glycolysis, E2 increased expression of multiple subunits of the PDH enzyme complex. PDH is a key regulatory enzyme complex linking the glycolytic metabolism to the citric acid cycle by transforming pyruvate into acetyl-CoA. In brain, PDH is further responsible for directing acetyl-CoA either to the tricarboxylic acid cycle (TCA, aka citric acid cycle) or to the acetylcholine synthesis (Holmquist et al., 2007). The mitoproteome profile induced by E2 is reflective of enhanced glycolytic activity coupled with increased glutamatergic turnover (increased glutamate dehydrogenase and glutamate oxaloacetate transaminase-2) (Nilsen et al., 2007). Together, these findings indicate that E2 promotes enhanced mitochondrial utilization of glucose, the main energy source for the brain (Fig. 3).
In parallel with increased PDH expression and activity, estrogen further increased expression and activity of proteins required for oxidative phosphorylation and electron transfer, a result that was consistent with a coordinated response that optimizes glucose metabolism in brain (Nilsen et al., 2007). E2-induced significant increased activity of Complex IV (Nilsen et al., 2007; Yao, Irwin et al., 2011)and the protein expression of its subunits I-IV (Nilsen et al., 2007), a finding consistent with previous reports (Bettini & Maggi, 1992; Stirone et al., 2005). The E2-induced increase is particularly relevant given that reduction in Complex IV is an early marker of AD (Lin & Beal, 2006). E2 also increased expression of ATP synthase Flα and β (Nilsen et al., 2007), which is consistent with the increase in proteins required for mitochondrial respiration and with our previous report of estrogen-induced increases in ATP levels in primary neuronal cultures (Brinton et al., 2000; Yao, Irwin et al., 2011).
E2-induced enhancement of energetic efficiency was paralleled by an increase in free radical defense systems. Many components of the mitochondrial bioenergetic network are vulnerable to oxidative stress, which can impair mitochondrial and cellular function as well as increasing apoptotic vulnerability (Lin & Beal, 2006; Yao et al., 2004). Damaged electron transport chain complexes compromise ATP synthesis and accelerate the generation of free radicals, which could cause or exacerbate neuronal degeneration (Lin & Beal, 2006; Yao et al., 2004). Free radical balance is maintained by reduction of the superoxide anion to hydrogen peroxidase by superoxide dismutases with the resulting hydrogen peroxide can then be removed by various peroxidases (Cadenas, 2004). Reduction in reactive oxygen species contributes to neuroprotection and can reduce the overall stress response. E2 treatment has been demonstrated to protect against H202 and arachidonic acid induced DNA damage in vitro (Moor et al., 2004; Tang & Subbiah, 1996). In rodent models, both short-term and long-term E2 treatments prevented the OVX-induced increase in lipid peroxides (Irwin et al., 2008; Yao, Irwin et al., 2011). Mechanistically, estrogen induces increase in the expression of a variety of antioxidant enzymes, including peroxiredoxin-V, glutaredoxin, and MnSOD (Nilsen & Brinton, 2004; Nilsen et al., 2007). In addition, we identified significant alterations in the expression of two mitochondrial heat-shock proteins (HSPs), Hsp70 and Hsp60, which are important in the correct import of nascent proteins to the mitochondrial matrix. The estrogen-induced increase in antioxidants, reduction in free radicals and substantially lower oxidative damage to mtDNA has been posited to be a major contributor to the greater longevity of females relative to males. (Borras et al., 2007; Vina, Borras, Gambini, Sastre, & Pallardo, 2005; Vina, Sastre, Pallardo, Gambini, & Borras, 2006).
Remarkably, E2 regulation of mitochondrial function in neural tissue is closely paralleled in the vasculature (Duckles, Krause, Stirone, & Procaccio, 2006; Stirone, Duckles et al., 2005). In vascular endothelium, chronic estrogen treatment increased mitochondrial capacity for oxidative phosphorylation while simultaneously decreasing production of reactive oxygen species. In contrast to the emerging data regarding ERβ regulation of neural mitochondrial function, E2 regulation of mitochondrial function in cerebral blood vessels is mediated by ERα (Razmara et al., 2008). Estrogen regulation of mitochondrial function in both neural and vascular tissue has functional importance for coordinated responses between neural activity and vascular integrity on the one hand and sustaining neural viability on the other.
E2 regulated both mitochondrial and nuclear encoded gene products, requires coordinated control of mitochondrial and nuclear encoded gene transcription (Nilsen et al., 2007; Stirone, Boroujerdi, Duckles, & Krause, 2005). Neuronal estrogen receptors have been detected in mitochondria (McEwen et al., 2001; T. A. Milner et al., 2005; Stirone, Duckles et al., 2005; Yager & Chen, 2007; Yang et al., 2004). Further, both ERa and ERβ can promote neuroprotection, activate MAPK pathways, and differentially potentiate brain mitochondrial function in vitro and in vivo (Irwin et al., 2012). In addition to classical ERs, membrane sites of estrogen action (mER), which activate the PBK/PKC/Src/MEK/ERK signaling pathway, activating CREB, have been identified as required for E2-inducible neuroprotection (Levin, 2001; Mannella & Brinton, 2006; T.W. Wu & Brinton, 2004; X. Zhao et al., 2005). While the mechanisms whereby ERs coordinate the complex signaling pathway between the three main compartments: membrane, mitochondria, and nucleus, remain to be determined (Wagner et al., 2008), it is striking that ERs are perfectly positioned to coordinate events at the membrane with events in the mitochondria and nucleus (McEwen et al., 2001; T. A. Milner et al., 2005; T. A. Milner et al., 2008; T. A. Milner et al., 2001; Yang et al., 2004).
D. Clinical Evidence of Estrogen Regulation of Brain Metabolism In Vivo
As the evidence of estrogen-mediated enhancement in mitochondrial bioenergetics and brain metabolism continues to mount from basic science discoveries (Lopez-Grueso et al., 2010), multiple clinical observations further corroborate the critical role of estrogen in sustaining brain metabolism in human. As part of a 9-year study in the Baltimore Longitudinal Study of Aging, Resnick and colleagues conducted positron emission topography (PET) to assess regional cerebral blood flow in a small cohort of women who were estrogen therapy (ET) users versus women who were not. Results of this analysis showed that ET users and nonusers showed significant differences in PET-regional cerebral blood flow relative activation patterns during the memory tasks. ET users showed better performance on neuropsychological tests of figural and verbal memory and on some aspects of the PET activation tests (Resnick & Henderson, 2002; Resnick et al., 1998). In a follow-up longitudinal study from the same cohort of healthy menopausal women, Maki and Resnick (Maki & Resnick, 2000) found that regional cerebral blood flow was increased in ET users relative to nonusers in the hippocampus, parahippocampal gyrus, and temporal lobe, regions that form a memory circuit and that are sensitive to preclinical AD (Maki & Resnick, 2000). Further these investigators found that the increase in regional cerebral blood flow was associated with higher scores on a battery of cognitive tests (Maki & Resnick, 2000). In a 2-year follow-up analysis, Rasgon and colleagues detected a significant decrease in metabolism of the posterior cingulate cortex among postmenopausal women at 2-year follow-up who did not receive estrogen whereas those women who were estrogen users did not exhibit significant metabolic change in the posterior cingulate (Rasgon et al., 2005). In addition, short-term use of estrogen has been demonstrated to enhance regional blood flow during cognitive tasks (Joffe et al., 2006; Shaywitz et al., 1999) and to enhance prefrontal-hippocampal as well as the amygdalar–cortical network connectivity (Ottowitz, Derro et al., 2008; Ottowitz, Siedlecki et al., 2008). Eberling and colleagues compared regional metabolism between healthy older women that are hormone users and nonhormone users and women with AD and found that hormone users exhibited the highest regional metabolic rate whereas AD patients exhibited the lowest metabolic rate with nonhormone users exhibit intermediate profile (Eberling et al., 2000). The same group in a follow-up study further identified that compared to the nonhormone users hormone users exhibited higher metabolic rate in the inferior frontal cortex and temporal cortex (Eberling et al., 2004). These findings that estrogen use may preserve regional cerebral metabolism and protect against metabolic decline in postmenopausal women, especially in posterior cingulate and prefrontal cortex, is particularly important given that metabolism in this region of the brain decline in the earliest stages of AD (Liang et al., 2008; Rasgon et al., 2005).
In fact, multiple clinical and epidemiological analyses clearly indicate that hormone therapy (HT) prior to or at the menopause transition is associated with enhanced memory and hippocampal function (Maki et al., 2011) and can reduce the risk of AD in postmenopausal women (Berent-Spillson et al., 2010; V. W. Henderson & Brinton, 2010) whereas women not receiving HT following surgically induced menopause are at increased risk for neurodegenerative diseases, including AD and Parkinsonism (Rocca et al., 2007; Rocca et al., 2010).
IV. Healthy Cell Bias of Estrogen Action: Consolidation of Clinical Observations and Basic Mechanistic Discoveries
Clinically, the impact of hormone interventions and the association with risk of AD has been fraught with controversy. However, this state of controversy is diminishing as a clearer understanding of the neurobiological mechanisms underlying the disparities in response to hormone therapies.
A. Prevention versus Treatment Paradigm of Estrogen Intervention
As discussed earlier, decades of basic science investigation of estrogen action in brain and subsequent observational and clinical trials indicated the benefit of estrogen-based therapies (Brinton, 2005, 2008a, 2008b; V. W. Henderson & Brinton, 2010; Morrison et al., 2006; Singh et al., 2008; Wise, 2006; Yao, Chen et al., 2011; Yao, Irwin et al., 2011). Embedded among these reports were suggestions that the beneficial effects of estrogen were conditional (Brinton, 2008a, 2008b; S. Chen, Nilsen, & Brinton, 2006; Nilsen & Brinton, 2002; Resnick & Henderson, 2002; Sherwin & Henry, 2008; Sohrabji, 2005; K Yaffe, 2003; Zandi et al., 2002). Results of the widely publicized Women’s Health Initiative Memory Study (WHIMS) clinical trial drew substantial attention to how conditional ET and HT can be (Shumaker et al., 2004; Shumaker et al., 2003). Analysis of the model systems used across the basic to clinical research continuum separate into two broad classes: those that use prevention interventions in healthy organisms and those that use hormone interventions in organisms with compromised neurological function (Brinton, 2005, 2008a, 2008b). Basic science analyses that led to elucidation of the neurotrophic and neuroprotective effects of estrogen and the underlying mechanisms of action typically used a prevention experimental paradigm (Brinton, 2005, 2008a, 2008b; Yao, Irwin et al., 2011). The prevention paradigm relies on healthy neurons/brains/animals/humans as the starting foundation followed by exposure to estrogen/hormone followed by exposure to neurodegenerative insult. The prevention paradigm of basic science analyses parallels the human studies of Sherwin and colleagues who investigated the cognitive impact of ET in women with surgical or pharmacological-induced menopause (Sherwin, 2009, 2011; Sherwin & McGill, 2003). Observational, retrospective and prospective, studies are also consistent with the outcomes of basic science analyses (Brinton, 2005). For the most part, the epidemiological observational data indicate reduction in risk of AD in women who began ET or HT at the time of the menopause (Brinton, 2005, 2008b; V. W. Henderson & Brinton, 2010; K. Yaffe et al., 1998; Zandi et al., 2002). The comparable benefit observed in most observational studies and basic science analyses suggest that for the most part, the data within the observational studies were derived from women with healthy neurological status.
In contrast, studies that fall within the second class, hormone intervention in women with compromised neurological function, that is, a treatment paradigm, exhibit a mixed profile (Brinton, 2005, 2008b). This was first evident in the results from the Cache County in which risk of AD varied with age of HT initiation and duration of use (Zandi et al., 2002). A woman’s sex-specific increase in risk disappeared entirely with more than 10 years of treatment with most of the HT-related reduction in incidence reflecting former use. There was no effect with current hormone replacement therapy (HRT) use unless duration of treatment exceeded 10 years (Zandi et al., 2002). Efficacy of ET which observed in the early AD treatment trials which lasted 1.5–2 months (Fillit et al., 1986) was not sustained when ET for an extended period of time (V.W. Henderson et al., 2000; Mulnard et al., 2000). In a randomized double-blind clinical trial of ET in a cohort in aged women, >72 years, diagnosed with AD, ET resulted in a modest benefit of ET in the short term (2 months) and adverse progression of disease in the long term (12 months) (V.W. Henderson et al., 2000; Mulnard et al., 2000). In the WHIMS cohort of women, 65–79 years of age, with no indicators of neurological disease but with variable health status, no statistically significant increase in AD risk occurred in the ET/CEE arm of the trial (Shumaker et al., 2004). However, there was no benefit of ET and there was a clear decline in cognitive performance over time (Shumaker et al., 2004). In contrast, the combination of CEE + MPA for 5 years increased the risk of developing AD by twofold (Shumaker et al., 2003) and when the results of the ET and HT data were combined there was a twofold increase in the risk of AD (Shumaker et al., 2003). Subsequent post hoc analyses of the WHIMS data suggested that women who had reported prior hormone user had a significantly lower risk of AD disease and all-cause dementia during the WHIMS trials (Henderson et al., 2007).
B. “Healthy Cell Bias” Hypothesis of Estrogen Action in Brain and the “Critical Window” for Estrogen-Based Therapy: Underlying Mechanisms
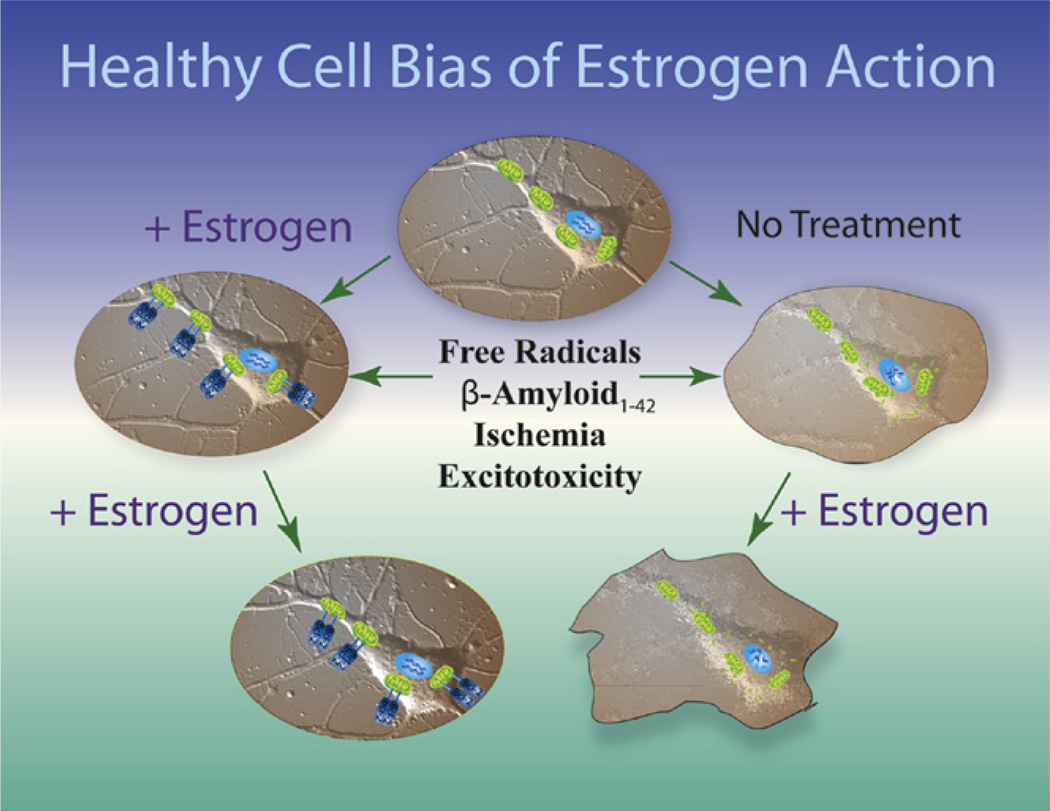
Collectively, the data suggest that as the continuum of neurological health progresses from healthy to unhealthy so too do the benefits of ET or HT (Brinton, 2005, 2008b). If neurons are healthy at the time of estrogen exposure, their response to estrogen is beneficial for both neurological function and survival. In contrast, if neurological health is compromised, estrogen exposure over time exacerbates neurological demise. Based on the analyses reviewed herein, the hypothesis of a “healthy cell bias of estrogen action” is proposed (Fig. 4).
FIGURE 4. Healthy cell bias of estrogen action.
Evidence from basic to clinical science indicates that neurons and women treated with estrogen prior to the exposure to neurodegenerative insult prevent neural demise. In stark contrast, basic and clinical evidence further indicate that exposure to estrogen following neurodegenerative insult can result in an exacerbation of neurological demise. Estrogen regulation of calcium signaling and mitochondrial function play key roles in determining the outcome of estrogen exposure. Figure modified from T. W. Wu, et al.,(2005). For color version of this figure, the reader is referred to the online version of this book.
The healthy cell bias of estrogen action hypothesis predicts that ET if initiated at the time of peri-to menopause when neurological health is not yet comprised, will be of benefit as manifested as reduced risk for age-associated neurodegenerative diseases such as Alzheimer’s and Parkinson’s. Further, E2 promotion of glycolysis and glycolytic coupled citric acid function, mitochondrial oxidative phosphorylation and ATP generation, antioxidant and antiapoptotic mechanisms serves as the pivotal pathway by which estrogen sustains neurological health and defense. In contrast, when activated in diseased neurons, addition of estrogen, while of modest benefit initially, an effect likely mediated by neurons not yet affected by the disease, adds to the Ca2+ homeostatic challenge with predictable exacerbation of the degenerative process (Chen et al., 2006). Similar to the “healthy cell bias” model in basic discoveries, the “critical window or timing hypothesis” has been proposed in clinical to interpret the disparity in outcomes between studies using the preinsult prevention paradigm and studies adopting the postinsult treatment paradigm. This hypothesis posit that the benefits and efficacy of estrogen-based HT depends stringently on the time of treatment initiation and that estrogen is most efficacious in terms of preserving cognitive function when administered prior or in the peri-to early menopausal period whereas estrogen treatment initiated years after menopause has no benefits and may even pose adverse effect (Sherwin, 2007, 2009, 2011).
Although stated differently, the healthy cell bias of estrogen action and the critical window hypothesis of clinical estrogen treatment consolidate into a unified underlying mechanism that it is the dependency upon Ca2+ signaling and the requirement for optimal Ca2+ homeostatic mechanisms that we believe is the Achilles heel of estrogen action. Through activating the PI3 kinase signaling pathway, E2 promotes influx of Ca2+ via L-type Ca2+ channels that in turns activates the Src/ERK/CREB cascade (Fig. 2) (Mannella & Brinton, 2006; T. W. Wu et al., 2005; X. Zhao et al., 2005). Estrogen-induction of this Ca2+-dependent signaling cascade leads to activation of mechanisms of learning and memory and neural defense (Brinton, 2001; Morrison et al., 2006; Woolley, 2007). Our studies of E2 regulation of intracellular Ca2+ dynamics and homeostasis originated in an attempt to resolve the paradox of dual regulation of [Ca2+]i by E2 in hippocampal neurons after nontoxic and excitotoxic glutamate exposure (Nilsen et al., 2002). Analyses of [Ca2+]i dynamics between the cytosolic and mitochondrial compartments revealed that E2 caused an increase in mitochondrial sequestration of [Ca2+]i when neurons were exposed to excitotoxic glutamate, which was paralleled by attenuation of cytoplasmic [Ca2+]i (Nilsen & Brinton, 2003). E2-induced attenuation was correlated with an increase in Bcl-2 expression, which could provide a mechanism by which neurons are protected against deleterious effects of increased mitochondrial [Ca2+] (Murphy et al., 1996; Nilsen & Brinton, 2003). Further, the increased mitochondrial sequestration of Ca2+ induced by E2 protected neurons against adverse consequences of excess cytoplasmic Ca2+ and subsequent dysregulation of Ca2+ homeostasis. Despite an increased mitochondrial Ca2+ load, E2 preserved mitochondrial respiratory capacity (Nilsen & Brinton, 2003).
The above mechanistic studies were conducted in healthy neurons derived from embryonic hippocampus, we therefore sought to determine whether E2 regulation of Ca2+ homeostasis extended to neurons derived from middle-aged and aged rodent hippocampus (Brewer, Reichensperger, & Brinton, 2006). Results of these analyses were both striking and consistent with earlier observations. Age-associated dysregulation of [Ca2+]i homeostasis was prevented by 48 h of prior exposure to E2 , a time frame consistent with E2-induced Bcl-2 expression (Nilsen & Brinton, 2003; T. W. Wu et al., 2005). Embryonic neurons exhibited the greatest capacity to regulate Ca2+ homeostasis followed by middle-age neurons (Brewer et al., 2006). In neurons derived from aged rat hippocampus, the first peak of [Ca2+]i was substantially greater than at other ages and the return to baseline Ca2+ rapidly dysregulated with an inability to restore [Ca2+]i following the first glutamate pulse that persisted throughout the 20 pulses. E2 pretreatment of aged neurons profoundly attenuated the peak [Ca2+]i rise and delayed the age-associated dysregulation of baseline [Ca2+]i, normalizing responses to those of middle-age neurons treated with E2 (Brewer et al., 2006). In a series of experiments designed to address controversies of ET, we conducted in vitro experiments designed to simulate the WHIMS trial in a dish. We hypothesized that E2 exposure of healthy neurons in a prevention mode would promote Ca2+ homeostasis to prevent Aβ1–42-induced neurodegeneration whereas E2 exposure of degenerating neurons in a treatment mode would exacerbate Aβ1–42-induced Ca2+ homeostatic dysregulation (S. Chen et al., 2006). Results of those analyses indicated that in a prevention mode of exposure, E2 was most effective when present prior to and during Aβ1–42 insult. In contrast, E2 treatment following Aβ1–42 exposure was ineffective in reversing Aβ-induced degeneration and exacerbated Aβ1–42 -induced cell death. We further found that low E2 significantly prevented Aβ1–42-induced rise in [Ca2+]i whereas high E2 significantly increased [Ca2+] i and did not prevent Aβ1–42-induced [Ca2+]i dysregulation (S. Chen et al., 2006). Therapeutic benefit resulted only from low dose E2 exposure prior to, but not following, Aβ1–42-induced neurodegeneration. Collectively, these data support a role of low dose E2 in promoting Ca2+ homeostasis in healthy embryonic, middle-aged and aged neurons. Further, the data indicate that once dysregulation of Ca2+ homeostasis has occurred, as in the case of Aβ1–42-induced Ca2+ dysregulation, exposure to low-dose E2 is of no benefit and exposure to high-dose E2 is deleterious and exacerbates neural demise. In addition to the preclinical investigations, multiple clinical studies further confirmed that the benefit of estrogen-based HT is indeed at least partially dependent on the time of initiation. Recent analyses by Whitmer and colleagues in a large clinical database revealed that use of HT in midlife may protect against cognitive impairment whereas HT initiation in late life could have deleterious effects (Whitmer et al., 2011). In a separate study, Smith and colleagues reported that early initiation of HT in menopausal women is associated with increased hippocampal and posterior cingulate cholinergic activity (Smith et al., 2011). Similarly, Gorenstein et al evaluated the effect of estrogen replacement therapy on verbal cognitive performance of middleaged postmenopausal women and reported better performance of the estrogen group on digit span-forward and on the recall of the easy stimuli on the verbal-paired associates tests despite the magnitude of benefits is moderate (Gorenstein et al., 2011).
V. Clinical Implications for Biomarker Identification and Therapeutic Development for Alzheimer’s Disease
Investigating mechanisms of estrogen action in parallel to identifying events antecedent to the development of Alzheimer’s pathology that have mechanistic plausibility provide insights into the basis for disparities between basic science discovery and clinical outcomes. More generally, results of these investigations raise questions regarding applying preventive strategies to treatment modalities in the clinical realm and the reliance of healthy model systems that are abruptly exposed to neurodegenerative insults that typically develop incrementally, slowly and accumulate over time in the preclinical discovery realm. This is particularly true for ageassociated neurodegenerative diseases in which the normal aging brain undergoes dramatic changes that are either unrelated to or are the earliest signs of neurodegenerative vulnerability (Blalock et al., 2003; Blalock et al., 2004; Miller et al., 2008; Rowe et al., 2007; Toescu, Verkhratsky, & Landfield, 2004). Efforts to bridge these gaps in women’s cognitive health are emerging and hold the promise to serve as a model for mechanistic and translational neuroscience research at the bench and the bedside (Asthana et al., in press and http://www.nia.nih.gov/ResearchInformation/ExtramuralPrograms/NeuroscienceOfAging/NNA_Conferences/BenchtoB edside.htm).
The real and perceived risks of HT remain and were amplified by results of both the WHI and WHIMS trials. It is clear that many, but not all, women could potentially benefit from ET or HT intervention. Biomarkers to identify women appropriate for and which type of hormone regimen remains largely undeveloped beyond the hot flash (Gleason, Dowling, Friedman, Wharton, & Asthana, 2011; Yao, Rettberg et al., 2011).
Considering the central role of mitochondrial bioenergetics and brain metabolism in AD pathogenesis and in estrogen action in the brain, it may well serve as a valid target for both biomarker development for early identification of the at AD risk population and for therapeutic development for AD prevention and treatment.
A. Development of Bioenergetic-Centric Biomarkers for AD
Recently, the clinical phases of AD have been expanded to include presymptomatic AD, during which time an individual appears cognitively normal but is beginning to exhibit some of the pathological changes of AD (Sperling et al., 2011). Defining this presymptomatic phase was particularly important for explaining why some individuals have no cognitive deficits but, upon autopsy, amyloid plaques and NFTs are present in the brain (De Meyer et al., 2010; Jack et al., 2008; Knopman et al., 2003; Mintun et al., 2006; Price & Morris, 1999). This provides further confirmation that a successful therapeutic intervention for AD will require very early identification of prodromal AD patients. Therefore, an area of concentrated focus within the Alzheimer’s research community is the identification and validation of biomarkers–biospecimen or neuroimaging variables that can be used to reliably predict individuals at risk of developing AD. Development of a biomarker profile of AD would be of great benefit both to clinicians and the drug development community; clinicians so that accurate diagnoses could be made antemortem, and pharmaceutical companies so that the efficacy of new drug formulations could be tested (Williams, 2011).
The criteria for a biomarker of AD were proposed in 1998 by the Working Group on Molecular and Biochemical Markers of AD, and have since become standards for the field. The Working Group specified: “the ideal biomarker for AD should detect a fundamental feature of neuropathology and be validated in neuropathologically confirmed cases; it should have a diagnostic sensitivity >80% for detecting AD and a specificity of >80% for distinguishing other dementias; it should be reliable, reproducible, noninvasive, simple to perform, and inexpensive.” The challenge of developing biomarkers that measure preclinical AD is that they must be able to discriminate between individuals who have AD pathology and those who do not, but all while the individuals are still at a cognitively intact stage so there is adequate time for prevention. As the prodromal stage of AD is known to exist decades prior to the manifestation of clinical symptoms, this would imply that preventative measures will require a method for routine screening of all patients in the age range of 50–65 years. Thus, a useful biomarker would need to be not only specific and sensitive but also cost-effective, so it would be broadly accessible.
Currently, the most thoroughly studied biomarkers of AD are the levels of three proteins measured in the cerebrospinal fluid (CSF): amyloid β1–42 (Aβ42), total tau protein, and p-tau, a phosphorylated form of tau protein. CSF levels of Aβ42 are decreased in AD, which is predicted to be due to the incorporation of Aβ42 into amyloid plaques (Blennow, Vanmechelen, & Hampel, 2001). CSF levels of both total tau and p-tau are increased, likely due to degenerating neurons releasing these proteins into the CSF (Jack et al., 2010). All three of these biomarkers have been validated, but changes in CSF levels of these proteins are likely occurring far downstream of the initial mitochondrial bioenergetic crisis; this implies that by the time they are measurable, the window for disease prevention may have already passed.
A recent development in the biomarker field has been the use of neuroimaging. Magnetic resonance imaging (MRI) can be used to visualize changes in brain structure that are associated with AD. Longitudinal MRI studies conducted by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) showed a pattern of temporal lobe atrophy that was significantly greater in patients who converted from MCI to AD than those who did not convert (Trojanowski et al., 2010). In addition, loss of hippocampal volume proved indicative of AD pathology and correlated with the APOε4 allele in FAD (Trojanowski et al., 2010). Unfortunately, by the time volumetric changes are quantifiable, substantial loss of grey and white matter has already occurred. Thus, while MRI detection of atrophy is useful as a diagnostic tool, its utility for prevention of AD is limited.
PET scanning is another neuroimaging method that has been used to study the development of AD. One type of PET imaging uses radiolabeled molecules which bind and label amyloid in the living brain. The most thoroughly studied examples of these compounds are Pittsburgh Compound B (PiB) (Klunk et al., 2004) and 18F-AV-45 (florbetapir) (Wong et al., 2010). Studies conducted using brain tissue from autopsy-confirmed AD patients show that PiB binds only to fibrillar amyloid, particularly plaques that are immunoreactive for Aβ40 or Aβ42 (Ikonomovic et al., 2008). 18F-AV-45 has also been shown to bind selectively to Aβ plaques in the postmortem AD brain (Choi et al., 2009). Unfortunately, amyloid neuroimaging techniques suffer from the same limitations as measurements of CSF Aβ42 and tby the time Aβ42 is aggregated into plaques, the pathogenesis of AD is established in brain. Additionally, PiB binding is not a 100% reliable biomarker, as there are cases of autopsy-confirmed AD that failed to show PiB labeling in the brain (Rosen et al., 2010).
Using fluorodeoxyglucose PET (FDG-PET), a significant body of research indicates that abnormalities in cerebral glucose utilization appear decades prior to the onset of clinical AD (de Leon et al., 2001; Mosconi, Mistur, Switalski, Tsui et al., 2009; Reiman et al., 2004). Further, the decrease in brain metabolism precedes the atrophy detected by MRI (De Santi et al., 2001) and predicts a decline in cognitive function (de Leon et al., 2001; Jagust et al., 2006; Mosconi et al., 2008). A decline in glucose metabolism could be simply due to decrease in brain mass; however, deficits in brain metabolism exceeded the magnitude of cortical atrophy (Ibanez et al., 1998). Based on a bioenergetic perspective of the etiology of AD, brain hypometabolism represents a response to an antecedent shift from utilizing glucose to requiring the alternative fuels of fatty acids and derived ketone bodies. Thus, hypometabolism still may be too late in the etiological cascade of events to be used as a biomarker for AD prevention, but could be applicable to identifying prodromal AD. Indeed, hypometabolism measured by FDG-PET has been identified as a “gold standard” for early-stage diagnosis of AD, although this method is hampered by high cost and relative inaccessibility of the scanning equipment.
Considering the central and antecedent role of mitochondrial bioenergetics in AD pathogenesis, a biomarker that reliably detects a shift to inefficient mitochondrial bioenergetics in the brain could provide the earliest indication that an individual is at risk for AD. Based on the Working Group recommendations that a biomarker be simple to measure and inexpensive, and our requirement that it be broadly accessible, the most desirable biomarker would be measurable in blood samples. One such marker could be plasma levels of ketone bodies. In preclinical models, for example, the 3xTgAD mouse, brain mitochondrial levels of enzymes involved in ketone body catabolism are upregulated very early in the disease process (Chou et al., 2011), suggesting a compensatory response that may be indicative of a shift toward the use of an alternate fuel source due to ineffective glucose metabolism in brain. Elevated levels of ketone bodies in the plasma would be then expected to indicate increased ketone generation by the liver in response to the disrupted brain glucose metabolism (Fig. 1).
Mitochondrial enzyme activity also holds promise as a potential biomarker of preclinical AD. It is well established that complex IV activity is decreased in platelet mitochondria isolated from individuals with AD (Bosetti et al., 2002; Cardoso, Proenca et al., 2004). Additionally, Valla et al reported a decrease in platelet mitochondrial complex IV that was present in MCI patients as well as patients with AD, suggesting that changes in mitochondrial complex IV activity may be occurring early in AD pathogenesis (Valla et al., 2006). Interestingly, it was recently reported that platelet mitochondrial complex IV activity is reduced in young, cognitively normal individuals who have a maternal history of LOAD (Mosconi et al., 2011). Mitochondrial DNA is maternally inherited and codes for the proteins which make up complex IV, suggesting that some forms of LOAD may result from a maternally transmitted mitochondrial deficit. Reduced platelet mitochondrial COX activity has potential as an early marker for individuals with a maternally inherited risk of LOAD.
Activity of mitochondrial enzyme complexes has also been investigated in lymphocytes, with varying degrees of success. Some studies have found no effect of AD status on lymphocyte mitochondrial activity (Molina et al., 1997), whereas a recent study showed increased activity of mitochondrial respiratory chain complexes II and IV in lymphocytes isolated from patients with AD when compared with controls (Feldhaus et al., 2011). Another study showed that although there was no baseline difference in lymphocyte mitochondrial enzyme activities between controls and AD patients, those patients who were treated with the cholinesterase inhibitor rivastigmine showed increased activity of complexes II, III, and IV, indicating that increased mitochondrial efficiency might be associated with better disease outcome.
B. Therapeutics Targeting Mitochondria and Bioenergetics for AD Treatment and Prevention
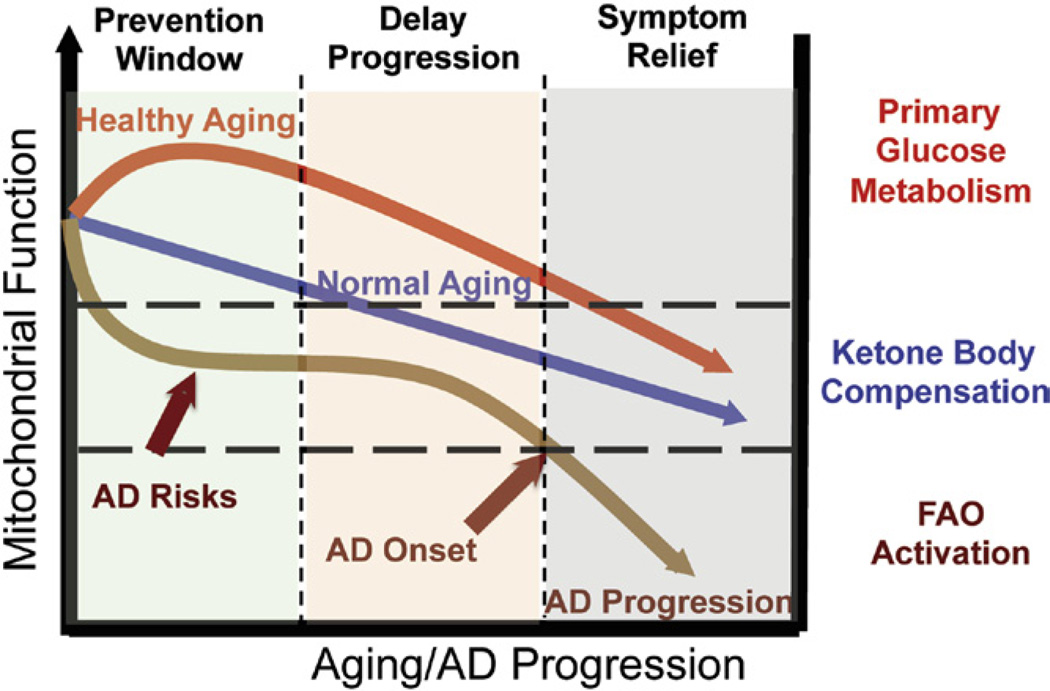
Alzheimer’s is a neurodegenerative disease with a complex and progressive pathological phenotype characterized first by hypometabolism and impaired mitochondrial bioenergetics followed by pathological burden. The progressive and multifaceted degenerative phenotype of Alzheimer’s suggests that successful treatment strategies need to be equally multifaceted and stage specific. Increasing evidence indicates an antecedent and potentially causal role of mitochondrial bioenergetic deficits and brain hypometabolism coupled with increased mitochondrial oxidative stress in AD pathogenesis. Mitochondrial deficits have been demonstrated to activate a cassette of neurotoxic events that all contribute to synaptic dysfunction, pathology development and eventually neuronal loss and cognitive impairment (Beal, 2005; Reddy & Beal, 2008). Further, deficits in mitochondrial bioenergetics and brain metabolism exhibit a stage-specific trajectory with disease progression, which was first evidenced by the decline in glucose uptake and utilization that takes place decades prior to AD onset, followed by parallel activation of pathways to use alternative fuel substrates, ketone bodies, to compensate for the decline in glucose metabolism (Yao et al., 2010; Yao et al., 2009). As disease progresses, exacerbated decline in glucose utilization and exhaustion of available ketone reservoir leads to further disturbance of mitochondrial function and activation of FAO pathway that eventually results in white matter degeneration and neuronal death observed in AD (Bartzokis et al., 2004; Brinton, 2008a; Carmichael et al., 2010; Kuczynski et al., 2010). This unique trajectory of glucose-ketone-FAO progression of brain mitochondrial metabolic alteration provides an ideal therapeutic target that is both disease modifying and stage specific (Fig. 5).
FIGURE 5. Trajectory of mitochondrial function, substrate utilization during AD progression and therapeutic strategy.
At young age or in healthy aging, brain metabolic activity is supported by glucose, the primary fuel source, whereas in prodromal and incipient AD the antecedent decline in glucose metabolism is first paralleled by compensatory activation of ketogenic pathways, which later diminishes and progresses into local fatty acid oxidation and white matter degeneration. The prevention strategy aims to enhance the glucose-driven mitochondrial bioenergetics to promote healthy aging and prevent AD. Alternatively, in prodromal and incipient AD, sustained activation of ketogenesis provides prolonged supplement of the alternative fuel source, ketone bodies, and therefore sustains mitochondrial bioenergetic function and prevents/delays further progression of the disease. At the middle to late stage of AD, rather than modifying disease progression, treatments merely offer symptom relief. For color version of this figure, the reader is referred to the online version of this book.
Candidates that potentiate mitochondrial bioenergetics and enhance brain glucose metabolism are expected to prevent the antecedent decline in brain glucose metabolism, promote healthy aging and therefore prevent AD. Interestingly, many candidates within this category are naturally occurring herbals and small cofactors, which often are on the GRAS (generally recognized as safe) list. R-α-lipoic acid, an important cofactor for key mitochondrial metabolic enzymes, including PDH, αKGDH, and branched chain α-ketoacid dehydrogenase (BCKDH), has been demonstrated to up-regulate mitochondrial bioenergetics, promote glucose metabolism, and suppress oxidative stress due to its potent antioxidant capacity (Packer & Cadenas, 2011). Resveratrol, a redox active ingredient in grapes and wine, improves brain energy metabolism and reduces amyloid accumulation in preclinical animal models (Karuppagounder et al., 2009; Marambaud et al., 2005; O’Dwyer et al., 2011; Vingtdeux et al., 2008). Both R-a-lipoic acid and resveratrol are currently under clinical trials for their efficacy in AD prevention and treatment (Packer & Cadenas, 2011; Wollen, 2010). Other important regulators of mitochondrial metabolic activity include B-vitamins which are also cofactors of key metabolic/mitochondrial enzymes.
Another class of natural products that are of great potential for AD prevention is the isoflavones, naturally rich in soy and soy-based diets. These plant derived phytoestrogens are a class of naturally occurring polyphenolic molecules that structurally resemble the mammalian estrogen (L. Zhao & Brinton, 2007; L. Zhao et al., 2009) but have a binding preference for ERβ with weaker affinities. High intake of soy-derived phytoestrogens has been linked to the low prevalence rate of AD in Asia (L. Zhao & Brinton, 2007). Further, multiple studies demonstrated that phytoestrogens, particularly various forms of isoflavones, regulate mitochondrial function by modulating mitochondrial oxidative stress (Huang & Zhang, 2010), activating the Akt signaling pathway, promoting expression of anti-apoptotic proteins (Xing et al., 2011), and potentiating mitochondrial bioenergetic capacity (L. Zhao et al., 2009).
Due to their structural properties, many of these naturally occurring compounds can often act as free radical scavengers directly. All together, these compounds exhibit potential to promote brain glucose utilization, potentiate brain metabolic activity, and simultaneously suppress oxidative damage with relatively low toxicity, which make them ideal candidates for development of nutraceutical cocktails to promote brain metabolism during healthy aging and therefore prevent AD.
While the preventive strategy focuses heavily on the enhancement of brain glucose metabolism, the shift toward an alternative fuel source, ketone bodies, observed in both preclinical AD models and in AD patients provides a second therapeutic window that targets the specific glucose–ketone transition stage to sustain brain metabolic activity and therefore prevent or delay further exacerbation in brain bioenergetic deficits. Ketone bodies are mainly synthesized in the liver through FAO and are well documented to serve as alternative energy substrates for the heart, muscle, and brain. Ketogenic pathways have been demonstrated to exist in astrocytes (Auestad et al., 1991; Guzman & Blazquez, 2004). Epidemiological analyses indicate a positive association between dietary intake of ketones/consumption of ketogenic diets and reduced risk for AD (S. T. Henderson, 2008; Morris, 2005). The switch from glucose as the primary fuel to the alternative of ketone bodies in the AD brain was the basis for Accera to develop Ketasyn, which is converted to ketone bodies in the liver for subsequent use by the brain. This approach capitalizes on the brain’s relative inability to utilize glucose and its dependency on ketone bodies. Phase II clinical trial in AD patients and in individuals suffering from age-associated memory impairment has been completed and both groups showed improvement in memory function using the ketone body alternative fuel source (http://www.accerapharma.com).
While increasing ketone body supply provides more substrate to the brain to utilize as an alternative fuel, the therapeutic efficacy could be limited due to a diminished brain capacity to utilize ketone bodies. To address the issue of deficits in the ketogenic metabolic pathway, our group investigated the efficacy of the ketogenic modulator, 2-deoxy-D-glucose (2-DG) to increase brain capacity to utilize ketone bodies as fuel. Results of these analyses demonstrated that dietary 2-DG intake induced ketogenesis, sustained mitochondrial bioenergetics, and reduced pathology in the triple transgenic Alzheimer’s (3xTgAD) mouse model (Jia Yao, 2011). Based on these clinical and preclinical findings, a combination of nutraceutical and pharmaceutical modulators that simultaneously enhance mitochondrial bioenergetics while sustaining availability and utilization of an alternative fuel substrate (ketone bodies), could prevent further decline in brain metabolism and to delay progression of AD.
VI. Conclusion
Alzheimer’s disease is a complex disease with a prolonged trajectory of etiopathogenic changes in brain bioenergetics decades prior to the clinical onset of the disease. Although it remains to be clinically confirmed, the trajectory of alterations in brain metabolic profile provides the foundation upon which to develop an array of bioenergetic-centric biomarkers to predict AD risk at the preclinical stage and therefore provide the best opportunity to prevent and/or delay the onset of AD. From a therapeutic perspective, this unique trajectory of alterations in brain metabolic capacity enable a bioenergetic-centric strategy that targets disease-stage specific profile of brain metabolism for disease prevention and treatment. A combination of nutraceutical and pharmaceutical interventions that enhances glucose-driven metabolic activity and potentiate mitochondrial bioenergetic function could prevent the antecedent decline in brain glucose metabolism, promote healthy aging, and prevent AD. Alternatively, during the prodromal incipient phase of AD, sustained activation of ketogenic metabolic pathways coupled with supplement of the alternative fuel source, ketone bodies, could sustain mitochondrial bioenergetic function to prevent or delay further progression of the disease.
In healthy brain and neural cells, estrogen coordinates activation of signaling pathways that converge upon the mitochondria to sustain aerobic glycolysis and enhance citric acid-driven oxidative phosphorylation and ATP generation. E2-induced potentiation of aerobic glycolysis and mitochondrial glucose utilization would be predicted to prevent conversion of the brain to using alternative sources such as ketone bodies and the subsequent ketone-FAO progression. Such convergence of estrogen-induced signaling onto mitochondria is also a point of vulnerability when activated in diseased neurons, which exacerbates degeneration through increased load on dysregulated calcium homeostasis. As the continuum of neurological health progresses from healthy to unhealthy, so too do the benefits of estrogen-based HT. The diversity of estrogen-inducible outcomes requires advances in biomarkers to identify women who will benefit from vs those who should not receive estrogen therapy. Identification of early stage changes in bioenergetic capacity of brain that are preventable or reversible by estrogen therapy holds promise to prevent or reduce the risk of developing Alzheimer’s disease.
Acknowledgment
Support of the NIA 2R01AG032236 (to RDB), NIA 5P01AG026572 (to RDB), Norris Research Foundation (to RDB) is gratefully acknowledged.
Abbreviations
- Aβ
amyloid beta
- ABAD
Aβ-binding-alcohol-dehydrogenase
- AD
Alzheimer’s disease
- E2 17β
estradiol
- FAD
familial AD
- LOAD
late-onset Alzheimer’s disease
- MRI
magnetic resonance imaging
- OXPHOS
oxidative phosphorylation
- PET
positron emission tomography
- SAD
sporadic AD
Footnotes
Conflict of Interest Statement: Patent pending on 2-deoxy-D-glucose formulations for prevention and treatment of Alzheimer’s disease. The pending patent entitled “2-Deoxy-D-Glucose Formulations for Prevention or Treatment of Neurodegenerative Diseases” (serial number: 61/452,463) was filed on March 14, 2011 subsequent to all data collection and analysis.
References
- Alzheimer’s Association. Alzheimer’s Disease Facts and Figures. 2011:12–13. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Arevalo MA, Ruiz-Palmero I, Scerbo MJ, Acaz-Fonseca E, Cambiasso MJ, Garcia-Segura LM. Molecular mechanisms involved in the regulation of neuritogenesis by estradiol: Recent advances. The Journal of Steroid Biochemistry and Molecular Biology. 2012;131(1–2):52–56. doi: 10.1016/j.jsbmb.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Armstrong RA. The pathogenesis of Alzheimer’s disease: A reevaluation of the “amyloid cascade hypothesis.”. International Journal of Alzheimer’s Disease. 2011 doi: 10.4061/2011/630865. 2011, 630865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asthana S, Brinton RD, Henderson VW, McEwen BS, Morrison JH, Schmidt PJ. Frontiers proposal. National Institute on Aging “bench to bedside: estrogen as a case study” AGE. 2009;31(3):199–210. doi: 10.1007/s11357-009-9087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atamna H, Frey WH., 2nd Mechanisms of mitochondrial dysfunction and energy deficiency in Alzheimer’s disease. Mitochondrion. 2007;7(5):297–310. doi: 10.1016/j.mito.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Auestad N, Korsak RA, Morrow JW, Edmond J. Fatty acid oxidation and ketogenesis by astrocytes in primary culture. Journal of Neurochemistry. 1991;56(4):1376–1386. doi: 10.1111/j.1471-4159.1991.tb11435.x. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiology of Aging. 2004;25(7):843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Annals of Neurology. 2005;58(4):495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- Berent-Spillson A, Persad CC, Love T, Tkaczyk A, Wang H, Reame NK, et al. Early menopausal hormone use influences brain regions used for visual working memory. Menopause. 2010;17(4):692–699. doi: 10.1097/gme.0b013e3181cc49e9. [Comparative Study Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, et al. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nature Neuroscience. 2011;14(6):750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettini E, Maggi A. Estrogen induction of cytochrome c oxidase subunit III in rat hippocampus. Journal of Neurochemistry. 1992;58(5):1923–1929. doi: 10.1111/j.1471-4159.1992.tb10070.x. [DOI] [PubMed] [Google Scholar]
- Bishop J, Simpkins JW. Estradiol enhances brain glucose uptake in ovariectomized rats. Brain Research Bulletin. 1995;36(3):315–320. doi: 10.1016/0361-9230(94)00208-i. [DOI] [PubMed] [Google Scholar]
- Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, et al. Gene microarrays in hippocampal aging: Statistical profiling identifies novel processes correlated with cognitive impairment. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23(9):3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW. Incipient Alzheimer’s disease: Microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(7):2173–2178. doi: 10.1073/pnas.0308512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blass J, Sheu R, Gibson G. Inherent abnormalities in energy metabolism in Alzheimer disease. Interaction with cerebrovascular compromise. Annals of the New York Academy of Sciences. 2000;903:204–221. doi: 10.1111/j.1749-6632.2000.tb06370.x. [DOI] [PubMed] [Google Scholar]
- Blennow K, Vanmechelen E, Hampel H. CSF total tau, Abeta42 and phosphorylated tau protein as biomarkers for Alzheimer’s disease. Molecular Neurobiology. 2001;24(1–3):87–97. doi: 10.1385/MN:24:1-3:087. [Research Support, Non-U.S. Gov’t Review] [DOI] [PubMed] [Google Scholar]
- Borras C, Gambini J, Vina J. Mitochondrial oxidant generation is involved in determining why females live longer than males. Frontiers in Bioscience. 2007;12:1008–1013. doi: 10.2741/2120. [DOI] [PubMed] [Google Scholar]
- Bosetti F, Brizzi F, Barogi S, Mancuso M, Siciliano G, Tendi EA, et al. Cytochrome c oxidase and mitochondrial FIFO-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer’s disease. Neurobiology of Aging. 2002;23(3):371–376. doi: 10.1016/s0197-4580(01)00314-1. [Comparative Study Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Reichensperger JD, Brinton RD. Prevention of age-related dysregulation of calcium dynamics by estrogen in neurons. Neurobiology of Aging. 2006;27(2):306–317. doi: 10.1016/j.neurobiolaging.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Cellular and molecular mechanisms of estrogen regulation of memory function and neuroprotection against Alzheimer’s disease: Recent insights and remaining challenges. Learning & Memory. 2001;8(3):121–133. doi: 10.1101/lm.39601. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: Evidence in support of a healthy cell bias of estrogen action. Annals of the New York Academy of Sciences. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Estrogen regulation of glucose metabolism and mitochondrial function: Therapeutic implications for prevention of Alzheimer’s disease. Advanced Drug Delivery Reviews. 2008a;60(13–14):1504–1511. doi: 10.1016/j.addr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. The healthy cell bias of estrogen action: Mitochondrial bioenergetics and neurological implications. Trends in Neurosciences. 2008b;31(10):529–537. doi: 10.1016/j.tins.2008.07.003. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD, Chen S, Montoya M, Hsieh D, Minaya J, Kim J, et al. The women’s health initiative estrogen replacement therapy is neurotrophic and neuroprotective. Neurobiology of Aging. 2000;21(3):475–496. doi: 10.1016/s0197-4580(00)00109-3. [DOI] [PubMed] [Google Scholar]
- Burns M, Gaynor K, Olm V, Mercken M, LaFrancois J, Wang L, et al. Preseni-lin redistribution associated with aberrant cholesterol transport enhances beta-amyloid production in vivo. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23(13):5645–5649. doi: 10.1523/JNEUROSCI.23-13-05645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E. Mitochondrial free radical production and cell signaling. Molecular Aspects of Medicine. 2004;25(1–2):17–26. doi: 10.1016/j.mam.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of estrogen and insulin-like growth factor-I in the brain: molecular mechanisms and functional implications. The Journal of Steroid Biochemistry and Molecular Biology. 2002;83(1–5):211–217. doi: 10.1016/s0960-0760(02)00261-3. [DOI] [PubMed] [Google Scholar]
- Cardoso SM, Proenca MT, Santos S, Santana I, Oliveira CR. Cytochrome c oxidase is decreased in Alzheimer’s disease platelets. Neurobiology of Aging. 2004;25(1):105–110. doi: 10.1016/s0197-4580(03)00033-2. [Comparative Study] [DOI] [PubMed] [Google Scholar]
- Cardoso SM, Santana I, Swerdlow RH, Oliveira CR. Mitochondria dysfunction of Alzheimer’s disease cybrids enhances Abeta toxicity. Journal of Neurochemistry. 2004;89(6):1417–1426. doi: 10.1111/j.1471-4159.2004.02438.x. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- Cardoso SM, Santos S, Swerdlow RH, Oliveira CR. Functional mitochondria are required for amyloid beta-mediated neurotoxicity. The FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2001;15(8):1439–1441. doi: 10.1096/fj.00-0561fje. [DOI] [PubMed] [Google Scholar]
- Carmichael O, Schwarz C, Drucker D, Fletcher E, Harvey D, Beckett L, et al. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Archives of Neurology. 2010;67(11):1370–1378. doi: 10.1001/archneurol.2010.284. [Clinical Trial Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nature Medicine. 2002;8(12):1390–1397. doi: 10.1038/nm1202-793. [DOI] [PubMed] [Google Scholar]
- Chen K, Reiman EM, Alexander GE, Caselli RJ, Gerkin R, Bandy D, et al. Correlations between apolipoprotein E epsilon4 gene dose and whole brain atrophy rates. American Journal of Psychiatry. 2007;164(6):916–921. doi: 10.1176/ajp.2007.164.6.916. [DOI] [PubMed] [Google Scholar]
- Chen S, Nilsen J, Brinton RD. Dose and temporal pattern of estrogen exposure determines neuroprotective outcome in hippocampal neurons: Therapeutic implications. Endocrinology. 2006;147(11):5303–5313. doi: 10.1210/en.2006-0495. [DOI] [PubMed] [Google Scholar]
- Cheng CM, Cohen M, Tseng V, Bondy CA. Endogenous IGF1 enhances cell survival in the postnatal dentate gyrus. Journal of Neuroscience Research. 2001;64(4):341–347. doi: 10.1002/jnr.1084. [DOI] [PubMed] [Google Scholar]
- Cheng CM, Cohen M, Wang J, Bondy CA. Estrogen augments glucose transporter and IGF1 expression in primate cerebral cortex. The FASEB journal: Official Publication of the Federation of American Societies for Experimental Biology. 2001;15(6):907–915. doi: 10.1096/fj.00-0398com. [DOI] [PubMed] [Google Scholar]
- Cheskis BJ, Greger J, Cooch N, McNally C, McLarney S, Lam HS, et al. MNAR plays an important role in ERa activation of Src/MAPK and PI3K/Akt signaling pathways. Steroids. 2008;73(9–10):901–905. doi: 10.1016/j.steroids.2007.12.028. [Review] [DOI] [PubMed] [Google Scholar]
- Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60(8):1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- Choi SR, Golding G, Zhuang Z, Zhang W, Lim N, Hefti F, et al. Preclinical properties of 18F–AV-45: A PET agent for Abeta plaques in the brain. Journal of Nuclear Medicine: Official Publication, Society of Nuclear Medicine. 2009;50(11):1887–1894. doi: 10.2967/jnumed.109.065284. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou JL, Shenoy DV, Thomas N, Choudhary PK, Laferla FM, Goodman SR, et al. Early dysregulation of the mitochondrial proteome in a mouse model of Alzheimer’s disease. Journal of Proteomics. 2011;74(4):466–479. doi: 10.1016/j.jprot.2010.12.012. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- Cordey M, Gundimeda U, Gopalakrishna R, Pike CJ. Estrogen activates protein kinase C in neurons: Role in neuroprotection. Journal of Neurochemistry. 2003;84(6):1340–1348. doi: 10.1046/j.1471-4159.2003.01631.x. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, et al. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET) Proceedings of the National Academy of Sciences of the United States of America. 2001;98(19):10966–10971. doi: 10.1073/pnas.191044198. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, De Deyn PP, et al. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Archives of Neurology. 2010;67(8):949–956. doi: 10.1001/archneurol.2010.179. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santi S, de Leon MJ, Rusinek H, Convit A, Tarshish CY, Roche A, et al. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiology of Aging. 2001;22(4):529–539. doi: 10.1016/s0197-4580(01)00230-5. [DOI] [PubMed] [Google Scholar]
- Diana FF, Silva Esteves AR, Oliveira CR, Cardoso SM. The common upstream driver of Abeta and tau pathology in Alzheimer s disease. Current Alzheimer Research Mitochondria. 2011;8(5):563–572. doi: 10.2174/156720511796391872. [DOI] [PubMed] [Google Scholar]
- Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(43):18675–18670. doi: 10.1073/pnas.1006586107. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckles SP, Krause DN, Stirone C, Procaccio V. Estrogen and mitochondria: A new paradigm for vascular protection? Molecular Interventions. 2006;6(1):26–35. doi: 10.1124/mi.6.1.6. [DOI] [PubMed] [Google Scholar]
- Dumont M, Lin MT, Beal MF. Mitochondria and antioxidant targeted therapeutic strategies for Alzheimer’s disease. Journal of Alzheimer’s Disease: JAD. 2010;2(Suppl. 20):S633–S643. doi: 10.3233/JAD-2010-100507. [Research Support, N.I.H., Extramural Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberling JL, Reed BR, Coleman JE, Jagust WJ. Effect of estrogen on cerebral glucose metabolism in postmenopausal women. Neurology. 2000;55(6):875–877. doi: 10.1212/wnl.55.6.875. [Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- Eberling JL, Wu C, Tong-Turnbeaugh R, Jagust WJ. Estrogen- and tamoxi-fen-associated effects on brain structure and function. Neuroimage. 2004;21(1):364–371. doi: 10.1016/j.neuroimage.2003.08.037. [Comparative Study Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- Fassbender K, Masters C, Beyreuther K. Alzheimer’s disease: Molecular concepts and therapeutic targets. Naturwissenscbaften. 2001;88(6):261–267. doi: 10.1007/s001140100237. [DOI] [PubMed] [Google Scholar]
- Feldhaus P, Fraga DB, Ghedim FV, De Luca RD, Bruna TD, Heluany M, et al. Evaluation of respiratory chain activity in lymphocytes of patients with Alzheimer disease. Metabolic Brain Disease. 2011;26(3):229–236. doi: 10.1007/s11011-011-9253-y. [DOI] [PubMed] [Google Scholar]
- Fillit H, Weinreb H, Cholst I, Luine V, McEwen B, Amador R, et al. Observations in a preliminary open trial of estradiol therapy for senile dementia-Alzheimer’s type. Psychoneuroendocrinology. 1986;11(3):337–345. doi: 10.1016/0306-4530(86)90019-3. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Arevalo MA, Azcoitia I. Interactions of estradiol and insulin-like growth factor-I signalling in the nervous system: New advances. Progress in Brain Research. 2010;181:251–272. doi: 10.1016/S0079-6123(08)81014-X. [Research Support, Non-U.S. Gov’t Review] [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Cardona-Gomez GP, Chowen JA, Azcoitia I. Insulinlike growth factor-I receptors and estrogen receptors interact in the promotion of neuronal survival and neuroprotection. Journal of Neurocytology. 2000;29(5–6):425–437. doi: 10.1023/a:1007125626308. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Sheu KF, Blass JP, Baker A, Carlson KC, Harding B, et al. Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer’s disease. Archives of Neurology. 1988;45(8):836–840. doi: 10.1001/archneur.1988.00520320022009. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- Gibson GE, Shi Q. A mitocentric view of Alzheimer’s disease suggests multi-faceted treatments. Journal of Alzheimer’s disease: JAD. 2010;2(Suppl. 20):S591–S607. doi: 10.3233/JAD-2010-100336. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason CE, Dowling NM, Friedman E, Wharton W, Asthana S. Using predictors of hormone therapy use to model the healthy user bias: How does healthy user status influence cognitive effects of hormone therapy? Menopause. 2011;19(l5):524–533. doi: 10.1097/gme.0b013e318238ff2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde TE, Schneider LS, Koo EH. Anti-abeta therapeutics in Alzheimer’s disease: The need for a paradigm shift. Neuron. 2011;69(2):203–213. doi: 10.1016/j.neuron.2011.01.002. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenstein C, Renno J, Jr, Vieira Filho AH, Gianfaldoni A, Goncalves MA, Halbe HW, et al. Estrogen replacement therapy and cognitive functions in healthy postmenopausal women: A randomized trial. Archives of Women’s Mental Health. 2011;14(5):367–373. doi: 10.1007/s00737-011-0230-6. [Randomized Controlled Trial Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes & Development. 2001;15(11):1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman M, Blazquez C. Ketone body synthesis in the brain: Possible neuroprotective effects. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2004;70(3):287–292. doi: 10.1016/j.plefa.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurology. 2006;5(3):228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- Hardy J. Alzheimer’s disease: The amyloid cascade hypothesis: An update and reappraisal. Journal of Alzheimer’s Disease. 2006;9(Suppl. 3):151–153. doi: 10.3233/jad-2006-9s317. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer’s disease: The amyloid cascade receive hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [Review] [DOI] [PubMed] [Google Scholar]
- Hauptmann S, Scherping I, Drose S, Brandt U, Schulz KL, Jendrach M, et al. Mitochondrial dysfunction: An early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiology of Aging. 2009;30(10):1574–1586. doi: 10.1016/j.neurobiolaging.2007.12.005. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- Henderson ST. Ketone bodies as a therapeutic for Alzheimer’s disease. Neurotherapeutics: The Journal of the American Society for Experimental NeuroThera-peutics. 2008;5(3):470–480. doi: 10.1016/j.nurt.2008.05.004. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Brinton RD. Menopause and mitochondria: Windows into estrogen effects on Alzheimer’s disease risk and therapy. Progress in Brain Research. 2010;182:77–96. doi: 10.1016/S0079-6123(10)82003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Espeland MA, Hogan PE, Rapp SR, Stefanick ML, Wactawski-Wende J, Johnson K, et al. Prior use of hormone therapy and incident Alzheimer’s disease in the Women’s Health Initiative Memory Study. Neurology. 2007;6S(Suppl. 1):A205. [Google Scholar]
- Henderson VW, Paganini-Hill A, Miller BL, Elble RJ, Reyes PF, Shoupe D, et al. Estrogen for Alzheimer’s disease in women: randomized, double-blind, placebo-controlled trial. Neurology. 2000;54(2):295–301. doi: 10.1212/wnl.54.2.295. [DOI] [PubMed] [Google Scholar]
- Holmquist L, Stuchbury G, Berbaum K, Muscat S, Young S, Hager K, et al. Lipoic acid as a novel treatment for Alzheimer’s disease and related dementias. Pharmacology & Therapeutics. 2007;113(1):154–164. doi: 10.1016/j.pharmthera.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Hoyer S, Nitsch R, Oesterreich K. Predominant abnormality in cerebral glucose utilization in late-onset dementia of the Alzheimer type: A cross-sectional comparison against advanced late-onset and incipient early-onset cases. Journal of Neural TransmissionParkinson’s Disease and Dementia Section. 1991;3(1):1–14. doi: 10.1007/BF02251132. [DOI] [PubMed] [Google Scholar]
- Huang YH, Zhang QH. Genistein reduced the neural apoptosis in the brain of ovariectomised rats by modulating mitochondrial oxidative stress. The British Journal of Nutrition. 2010;104(9):1297–1303. doi: 10.1017/S0007114510002291. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- Ibanez V, Pietrini P, Alexander GE, Furey ML, Teichberg D, Rajapakse JC, et al. Regional glucose metabolic abnormalities are not the result of atrophy in Alzheimer’s disease. Neurology. 1998;50(6):1585–1593. doi: 10.1212/wnl.50.6.1585. [DOI] [PubMed] [Google Scholar]
- Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131(Pt 6):1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbimbo BP, Giardina GA. Gamma-secretase inhibitors and modulators for the treatment of Alzheimer’s disease: Disappointments and Hopes. Current topics in Medicinal Chemistry. 2011;11(12):1555–1570. doi: 10.2174/156802611795860942. [DOI] [PubMed] [Google Scholar]
- Irwin RW, Yao J, Hamilton R, Cadenas E, Brinton RD, Nilsen J. Progesterone and estrogen regulate oxidative metabolism in brain mitochondria. Endocrinology. 2008;149(6):3167–3175. doi: 10.1210/en.2007-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RW, Yao J, To J, Hamilton RT, Cadenas E, Brinton RD. Selective oestrogen receptor modulators differentially potentiate brain mitochondrial function. Journal of Neuroendocrinology. 2012;24(1):236–248. doi: 10.1111/j.1365-2826.2011.02251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Sasaki M, Kitagaki H, Yamaji S, Sakamoto S, Matsuda K, et al. Reduction of cerebellar glucose metabolism in advanced Alzheimer’s disease. Journal of Nuclear Medicine. 1997;38(6):925–928. [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurology. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain: A Journal of Neurology. 2008;131(Pt 3):665–680. doi: 10.1093/brain/awm336. [Comparative Study Evaluation Studies Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W, Gitcho A, Sun F, Kuczynski B, Mungas D, Haan M. Brain imaging evidence of preclinical Alzheimer’s disease in normal aging. Annals of Neurology. 2006;59(4):673–681. doi: 10.1002/ana.20799. [DOI] [PubMed] [Google Scholar]
- Jia Yao SC, Mao, Zisu, Cadenas, Enrique, Brinton, Roberta Diaz 2-Deoxy-D-Glucose Treatment Induces Ketogenesis, Sustains Mitochondrial Function, and Reduces Pathology in Female Mouse Model of Alzheimer’s Disease. PLoS One. 2011;6(7):e21788. doi: 10.1371/journal.pone.0021788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe H, Hall JE, Gruber S, Sarmiento IA, Cohen LS, Yurgelun-Todd D, et al. Estrogen therapy selectively enhances prefrontal cognitive processes: A randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13(3):411–422. doi: 10.1097/01.gme.0000189618.48774.7b. [Randomized Controlled Trial Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochemistry International. 2009;54(2):111–118. doi: 10.1016/j.neuint.2008.10.008. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SM, Cassarino DS, Abramova NN, Keeney PM, Borland MK, Trimmer PA, et al. Alzheimer’s disease cybrids replicate beta-amyloid abnormalities through cell death pathways. Annals of Neurology. 2000;48(2):148–155. [Research Support, U.S. Gov’t, P.H.S.] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Annals of Neurology. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, et al. Neuropathology of cognitively normal elderly. Journal of Neuropathology and Experimental Neurology. 2003;62(11):1087–1095. doi: 10.1093/jnen/62.11.1087. [Comparative Study Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- Kostanyan A, Nazaryan K. Rat brain glycolysis regulation by estradiol-17 beta. Biochimica et Biophysica Acta. 1992;1133(3):301–306. doi: 10.1016/0167-4889(92)90051-c. [DOI] [PubMed] [Google Scholar]
- Kuczynski B, Targan E, Madison C, Werner M, Zhang Y, Reed B, et al. White matter integrity and cortical metabolic associations in aging and dementia. Alzbeimers & Dementia. 2010;6(1):54–62. doi: 10.1016/j.jalz.2009.04.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER. Cell localization, physiology, and nongenomic actions of estrogen receptors. Journal of Applied Physiology. 2001;91(4):1860–1867. doi: 10.1152/jappl.2001.91.4.1860. [DOI] [PubMed] [Google Scholar]
- Liang WS, Reiman EM, Valla J, Dunckley T, Beach TG, Grover A, et al. Alzheimer’s disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(11):4441–4446. doi: 10.1073/pnas.0709259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Beal M. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lopez JR, Lyckman A, Oddo S, Laferla FM, Querfurth HW, Shtifman A. Increased intraneuronal resting [Ca(2+)] in adult Alzheimer’s disease mice. Journal of Neurochemistry. 2007;105(1):262–271. doi: 10.1111/j.1471-4159.2007.05135.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Grueso R, Borras C, Gambini J, Vina J. Aging and ovariectomy cause a decrease in brain glucose consumption in vivo in Wistar rats. Revista Espanola de Geriatria y Gerontologia. 2010;45(3):136–140. doi: 10.1016/j.regg.2009.12.005. [Research Support, Non-US. Gov’t] [DOI] [PubMed] [Google Scholar]
- Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304(5669):448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ. Neuron-glia metabolic coupling and plasticity. The Journal of Experimental Biology. 2006;209(Pt 12):2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- Maki PM, Dennerstein L, Clark M, Guthrie J, LaMontagne P, Fornelli D, et al. Perimenopausal use of hormone therapy is associated with enhanced memory and hippocampal function later in life. Brain Research. 2011;1379:232–243. doi: 10.1016/j.brainres.2010.11.030. [Comparative Study Randomized Controlled TrialResearch Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Resnick SM. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiology of Aging. 2000;21(2):373–383. doi: 10.1016/s0197-4580(00)00123-8. [DOI] [PubMed] [Google Scholar]
- Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: Implications for free radical generation and oxidative damage in disease progression. Human Molecular Genetics. 2006;15(9):1437–1449. doi: 10.1093/hmg/ddl066. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] [DOI] [PubMed] [Google Scholar]
- Mannella P, Brinton RD. Estrogen receptor protein interaction with phosphati-dylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signalregulated kinase 1/2 in the same population of cortical neurons: A unified mechanism of estrogen action. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26(37):9439–9447. doi: 10.1523/JNEUROSCI.1443-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. The Journal of Biological Chemistry. 2005;280(45):37377–37382. doi: 10.1074/jbc.M508246200. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- McEwen B, Akama K, Alves S, Brake WG, Bulloch K, Lee S, et al. Tracking the estrogen receptor in neurons: Implications for estrogen-induced synapse formation. Proceedings of the National Academy of Sciences of the United States of America. 2001;95(13):7093–7100. doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez P, Azcoitia I, Garcia-Segura LM. Estrogen receptor alpha forms estrogen-dependent multimolecular complexes with insulin-like growth factor receptor and phosphatidylinositol 3-kinase in the adult rat brain. Brain Research. Molecular Brain Research. 2003;112(1–2):170–176. doi: 10.1016/s0169-328x(03)00088-3. [DOI] [PubMed] [Google Scholar]
- Mendez P, Garcia-Segura LM. Phosphatidylinositol 3-kinase and glycogen synthase kinase 3 regulate estrogen receptor-mediated transcription in neuronal cells. Endocrinology. 2006;147(6):3027–3039. doi: 10.1210/en.2005-1224. [DOI] [PubMed] [Google Scholar]
- Mendez P, Wandosell F, Garcia-Segura LM. Cross-talk between estrogen receptors and insulin-like growth factor-I receptor in the brain: Cellular and molecular mechanisms. Frontiers in Neuroendocrinology. 2006;27(4):391–403. doi: 10.1016/j.yfrne.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Miller JA, Oldham MC, Geschwind DH. A systems level analysis of transcriptional changes in Alzheimer’s disease and normal aging. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28(6):1410–1420. doi: 10.1523/JNEUROSCI.4098-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, et al. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. The Journal of Comparative Neurology. 2005;491(2):81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Milner TA, Lubbers LS, Alves SE, McEwen BS. Nuclear and extranuclear estrogen binding sites in the rat forebrain and autonomic medullary areas. Endocrinology. 2008;149(7):3306–3312. doi: 10.1210/en.2008-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultra-structural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. Journal of Comparative Neurology. 2001;429(3):355–371. [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, et al. [11 C]PIB in a nondemented population: Potential antecedent marker of Alzheimer disease. Neurology. 2006;67(3):446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death and Differentiation. 2008;15(3):521–529. doi: 10.1038/sj.cdd.4402285. [DOI] [PubMed] [Google Scholar]
- Molina JA, de Bustos F, Jimenez-Jimenez FJ, Benito-Leon J, Gasalla T, Orti-Pareja M, et al. Respiratory chain enzyme activities in isolated mitochondria of lymphocytes from patients with Alzheimer’s disease. Neurology. 1997;48(3):636–638. doi: 10.1212/wnl.48.3.636. [Clinical Trial Controlled Clinical Trial Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- Moor AN, Gottipati S, Mallet RT, Sun J, Giblin FJ, Roque R, et al. A putative mitochondrial mechanism for antioxidative cytoprotection by 17beta-estra-diol. Experimental Eye Research. 2004;78(5):933–944. doi: 10.1016/j.exer.2004.01.001. [Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- Moreira PI, Cardoso SM, Santos MS, Oliveira CR. The key role of mitochondria in Alzheimer’s disease. Journal of Alzheimer’s Disease: JAD. 2006;9(2):101–110. doi: 10.3233/jad-2006-9202. [Review] [DOI] [PubMed] [Google Scholar]
- Moreira PI, Santos MS, Seica R, Oliveira CR. Brain mitochondrial dysfunction as a link between Alzheimer’s disease and diabetes. Journal of the Neurological Sciences. 2007;257(1–2):206–214. doi: 10.1016/j.jns.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Zhu X, Wang X, Lee HG, Nunomura A, Petersen RB, et al. Mitochondria: A therapeutic target in neurodegeneration. Biochimica et Biopbysica Acta. 2010;1802(1):212–220. doi: 10.1016/j.bbadis.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AA. Cerebral ketone body metabolism. Journal of Inherited Metabolic Disease. 2005;28(2):109–121. doi: 10.1007/s10545-005-5518-0. [Review] [DOI] [PubMed] [Google Scholar]
- Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: How basic neuroscience can inform hormone therapy in women. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26(41):10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, de Leon M, Murray J, E L, Lu J, Javier E, et al. Reduced mitochondria cytochrome oxidase activity in adult children of mothers with Alzheimer’s disease. Journal of Alzheimer’s Disease: JAD. 2011;27(3):483–490. doi: 10.3233/JAD-2011-110866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, De Santi S, Li J, Tsui WH, Li Y, Boppana M, et al. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiology of Aging. 2008;29(5):676–692. doi: 10.1016/j.neurobiolaging.2006.12.008. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, McHugh PF. FDG-and amyloid-PET in Alzheimer’s disease: Is the whole greater than the sum of the parts? The Quarterly Journal of Nuclear Medicine and Molecular Imaging: Official Publication of the Italian Association of Nuclear Medicine. 2011;55(3):250–264. [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Mistur R, Switalski R, Brys M, Glodzik L, Rich K, et al. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology. 2009;72(6):513–520. doi: 10.1212/01.wnl.0000333247.51383.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Mistur R, Switalski R, Tsui WH, Glodzik L, Li Y, et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. European Journal of Nuclear Medicine and Molecular Imaging. 2009;36(5):811–822. doi: 10.1007/s00259-008-1039-z. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulnard RA, Cotman CW, Kawas C, van Dyck CH, Sano M, Doody R, et al. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: A randomized controlled trial. Alzheimer’s Disease Cooperative Study. JAMA: The Journal of the American Medical Association. 2000;283(8):1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- Murphy AN, Bredesen DE, Cortopassi G, Wang E, Fiskum G. Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(18):9893–9898. doi: 10.1073/pnas.93.18.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson RM, Kusne Y, Nowak LA, LaFerla FM, Reiman EM, Valla J. Regional cerebral glucose uptake in the 3xTG model of Alzheimer’s disease highlights common regional vulnerability across AD mouse models. Brain Research. 2010;1347:179–185. doi: 10.1016/j.brainres.2010.05.084. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Impact of progestins on estradiol potentiation of the glutamate calcium response. Neuroreport. 2002;13(6):825–830. doi: 10.1097/00001756-200205070-00018. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Mechanism of estrogen-mediated neuroprotection: Regulation of mitochondrial calcium and Bcl-2 expression. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(5):2842–2847. doi: 10.1073/pnas.0438041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Mitochondria as therapeutic targets of estrogen action in the central nervous system. Current Drug Targets. CNS and Neurological Disorders. 2004;3(4):297–313. doi: 10.2174/1568007043337193. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Chen S, Brinton RD. Dual action of estrogen on glutamate-induced calcium signaling: mechanisms requiring interaction between estrogen receptors and src/ mitogen activated protein kinase pathway. Brain Research. 2002;930(1–2):216–234. doi: 10.1016/s0006-8993(02)02254-0. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Chen S, Irwin RW, Iwamoto S, Brinton RD. Estrogen protects neuronal cells from amyloid beta-induced apoptosis via regulation of mitochondrial proteins and function. BMC Neuroscience. 2006;7:74. doi: 10.1186/1471-2202-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Irwin RW, Gallaher TK, Brinton RD. Estradiol in vivo regulation of brain mitochondrial proteome. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27(51):14069–14077. doi: 10.1523/JNEUROSCI.4391-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A, Chiba S, Lippa CF, Cras P, Kalaria RN, Takeda A, et al. Neuronal RNA oxidation is a prominent feature of familial Alzheimer’s disease. Neurobiology of Disease. 2004;17(1):108–113. doi: 10.1016/j.nbd.2004.06.003. [Comparative Study Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- Nunomura A, Hofer T, Moreira PI, Castellani RJ, Smith MA, Perry G. RNA oxidation in Alzheimer disease and related neurodegenerative disorders. Acta Neuropathologica. 2009;118(1):151–166. doi: 10.1007/s00401-009-0508-1. [Review] [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, et al. Oxidative damage is the earliest event in Alzheimer disease. Journal of Neuropathology and Experimental Neurology. 2001;60(8):759–767. doi: 10.1093/jnen/60.8.759. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- O’Dwyer L, Lamberton F, Bokde AL, Ewers M, Faluyi YO, Tanner C, et al. Multiple indices of diffusion identifies white matter damage in mild cognitive impairment and Alzheimer’s disease. PLoS One. 2011;6(6):e21745. doi: 10.1371/journal.pone.0021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottowitz WE, Derro D, Dougherty DD, Lindquist MA, Fischman AJ, Hall JE. FDG-PET analysis of amygdalar-cortical network covariance during pre-versus post-menopausal estrogen levels: potential relevance to resting state networks, mood, and cognition. Neuro Endocrinology Letters. 2008;29(4):467–474. [Research Support, N.I.H., Extramural] [PMC free article] [PubMed] [Google Scholar]
- Ottowitz WE, Siedlecki KL, Lindquist MA, Dougherty DD, Fischman AJ, Hall JE. Evaluation of prefrontal-hippocampal effective connectivity following 24 hours of estrogen infusion: An FDG-PET study. Psychoneuroendocrinology. 2008;33(10):1419–1425. doi: 10.1016/j.psyneuen.2008.09.013. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer L, Cadenas E. Lipoic acid: Energy metabolism and redox regulation of transcription and cell signaling. Journal of Clinical Biochemistry and Nutrition. 2011;48(1):26–32. doi: 10.3164/jcbn.11-005FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park LC, Zhang H, Sheu KF, Calingasan NY, Kristal BS, Lindsay JG, et al. Metabolic impairment induces oxidative stress, compromises inflammatory responses, and inactivates a key mitochondrial enzyme in microglia. Journal of Neurochemistry. 1999;72(5):1948–1958. doi: 10.1046/j.1471-4159.1999.0721948.x. [Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- Parker WD., Jr Cytochrome oxidase deficiency in Alzheimer’s disease. Annals of the New York Academy of Sciences. 1991;640:59–64. doi: 10.1111/j.1749-6632.1991.tb00191.x. [Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- Perry EK, Perry RH, Tomlinson BE, Blessed G, Gibson PH. Coenzyme A-acetylating enzymes in Alzheimer’s disease: Possible cholinergic ‘compartment’ of pyruvate dehydrogenase. Neuroscience Letters. 1980;18(1):105–110. doi: 10.1016/0304-3940(80)90220-7. [DOI] [PubMed] [Google Scholar]
- Petanceska SS, DeRosa S, Olm V, Diaz N, Sharma A, Thomas-Bryant T, et al. Statin therapy for Alzheimer’s disease: Will it work? Journal of Molecular Neuroscience. 2002;19(1–2):155–161. doi: 10.1007/s12031-002-0026-2. [DOI] [PubMed] [Google Scholar]
- Pimplikar SW. Reassessing the amyloid cascade hypothesis of Alzheimer’s disease. The International Journal of Biochemistry & Cell Biology. 2009;41(6):1261–1268. doi: 10.1016/j.biocel.2008.12.015. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2001;21(12):4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Annals of Neurology. 1999;45(3):358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- Prins ND, Visser PJ, Scheltens P. Can novel therapeutics halt the amyloid cascade? [ Alzheimer’s Research & Therapy. 2010;2(2):5. doi: 10.1186/alzrt28. Editorial] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon NL, Silverman D, Siddarth P, Miller K, Ercoli LM, Elman S, et al. Estrogen use and brain metabolic change in postmenopausal women. Neurobiology of Aging. 2005;26(2):229–235. doi: 10.1016/j.neurobiolaging.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Razmara A, Sunday L, Stirone C, Wang X, Krause D, Duckies S, et al. Mitochondrial effects of estrogen are mediated by ER(alpha) in brain endothelial cells. The Journal of Pharmacology and Experimental Therapeutics. 2008;325(3):782–790. doi: 10.1124/jpet.107.134072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Readnower RD, Sauerbeck AD, Sullivan PG. Mitochondria, amyloid beta, and Alzheimer’s disease. International Journal of Alzheimer’s Disease. 2011 doi: 10.4061/2011/104545. 2011, 104545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH. Mitochondrial oxidative damage in aging and Alzheimer’s disease: Implications for mitochondrially targeted antioxidant therapeutics. Journal of Biomedicine & Biotechnology. 2006;2006(3):31372. doi: 10.1155/JBB/2006/31372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: Implications for cognitive decline in aging and Alzheimer’s disease. Trends in Molecular Medicine. 2008;14(2):45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(1):284–289. doi: 10.1073/pnas.2635903100. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Henderson VW. Hormone therapy and risk of Alzheimer disease: A critical time. JAMA: The Journal of the American Medical Association. 2002;288(17):2170–2172. doi: 10.1001/jama.288.17.2170. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Maki PM, Golski S, Kraut MA, Zonderman AB. Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Hormones and Behavior. 1998;34(2):171–182. doi: 10.1006/hbeh.1998.1476. [DOI] [PubMed] [Google Scholar]
- Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, et al. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(47):20057–20062. doi: 10.1073/pnas.0905529106. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risacher SL, Saykin AJ, West JD, Shen L, Firpi HA, McDonald BC. Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Current Alzheimer Research. 2009;6(4):347–361. doi: 10.2174/156720509788929273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2007;70(3):200–209. doi: 10.1212/01.wnl.0000280573.30975.6a. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen, and cognitive aging: The timing hypothesis. Neuro-Degenerative Diseases. 2010;7(1–3):163–166. doi: 10.1159/000289229. [Research Support, N.I.H., Extramural Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RF, Ciliax BJ, Wingo TS, Gearing M, Dooyema J, Lah JJ, et al. Deficient high-affinity binding of Pittsburgh compound B in a case of Alzheimer’s disease. Acta Neuropathologica. 2010;119(2):221–233. doi: 10.1007/s00401-009-0583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom MH, Alkalay A, Agarwal N, Baker SL, O’Neil JP, Janabi M, et al. Distinct clinical and metabolic deficits in PCA and AD are not related to amyloid distribution. Neurology. 2011;76(21):1789–1796. doi: 10.1212/WNL.0b013e31821cccad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe WB, Blalock EM, Chen KC, Kadish I, Wang D, Barrett JE, et al. Hippocampal expression analyses reveal selective association of immediate-early, neuroen-ergetic, and myelinogenic pathways with cognitive impairment in aged rats. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2007;27(12):3098–3110. doi: 10.1523/JNEUROSCI.4163-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schioth HB, Craft S, Brooks SJ, Frey WH, 2nd, Benedict C. Brain insulin signaling and Alzheimer’s disease: Current evidence and future directions. Molecular Neurobiology. 2011 doi: 10.1007/s12035-011-8229-6. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LS, Insel PS, Weiner MW. Treatment with cholinesterase inhibitors and memantine of patients in the Alzheimer’s disease neuroimaging initiative. Archives of Neurology. 2011;68(1):58–66. doi: 10.1001/archneurol.2010.343. [Clinical Trial Comparative Study Multicenter Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, et al. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA: The Journal of the American Medical Association. 1999;281(13):1197–1202. doi: 10.1001/jama.281.13.1197. [Clinical Trial Randomized Controlled Trial Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- Sherwin BB. The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature? Journal of Neuroendocrinology. 2007;19(2):77–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen therapy: is time of initiation critical for neuroprotection? Nature Reviews. Endocrinology. 2009;5(11):620–627. doi: 10.1038/nrendo.2009.193. [Research Support, Non-U.S. Gov’t Review] [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive functioning in women: Lessons we have learned. Behavioral Neuroscience. 2011;126(1):123–127. doi: 10.1037/a0025539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: A critical review. Frontiers in Neuroendocrinology. 2008;29(1):88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, McGill J. Oestrogen plus progestin doubles the risk of dementia in post-menopausal women. Evidence-Based Mental Health. 2003;6(4):111. doi: 10.1136/ebmh.6.4.111. [DOI] [PubMed] [Google Scholar]
- Shi J, Simpkins JW. 17 beta-Estradiol modulation of glucose transporter 1 expression in blood-brain barrier. The American Journal of Physiology. 1997;272(6 Pt 1):E1016–E1022. doi: 10.1152/ajpendo.1997.272.6.E1016. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thai L, Lane DS, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA: The Journal of the American Medical Association. 2004;291(24):2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thai L, Wallace RB, Ockene JK, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: A randomized controlled trial. JAMA: The Journal of the American Medical Association. 2003;289(20):2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Silva DF, Esteves AR, Arduino DM, Oliveira CR, Cardoso SM. Amyloid-beta-induced mitochondrial dysfunction impairs the autophagic lysosomal pathway in a tubulin dependent pathway. Journal of Alzheimer’s Disease: JAD. 2011;26(3):565–581. doi: 10.3233/JAD-2011-110423. [DOI] [PubMed] [Google Scholar]
- Simon AM, Frechilla D, del Rio J. [Perspectives on the amyloid cascade hypothesis of Alzheimer’s disease] Revista de Neu-rologia. 2010;50(11):667–675. [Research Support, Non-U.S. Gov’t Review] [PubMed] [Google Scholar]
- Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407(6803):538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins JW, Dykens JA. Mitochondrial mechanisms of estrogen neuroprotection. Brain Research Reviews. 2008;57(2):421–130. doi: 10.1016/j.brainresrev.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Wang J, Wang X, Perez E, Prokai L, Dykens JA. Mitochondria play a central role in estrogen-induced neuroprotection. Current Drug Targets. CNS and Neurological Disorders. 2005;4(1):69–83. doi: 10.2174/1568007053005073. [DOI] [PubMed] [Google Scholar]
- Singh M. Ovarian hormones elicit phosphorylation of Akt and extracellular-signal regulated kinase in explants of the cerebral cortex. Endocrine Journal-Uk. 2001;14(3):407–115. doi: 10.1385/ENDO:14:3:407. [DOI] [PubMed] [Google Scholar]
- Singh M, Setalo G, Jr, Guan X, Frail DE, Toran-Allerand CD. Estrogen-induced activation of the mitogen-activated protein kinase cascade in the cerebral cortex of estrogen receptor-alpha knock-out mice. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20(5):1694–1700. doi: 10.1523/JNEUROSCI.20-05-01694.2000. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Sumien N, Kyser C, Simpkins JW. Estrogens and progesterone as neuroprotectants: What animal models teach us. Frontiers in Bioscience. 2008;13:1083–1089. doi: 10.2741/2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith YR, Bowen L, Love TM, Berent-Spillson A, Frey KA, Persad CC, et al. Early initiation of hormone therapy in menopausal women is associated with increased hippocampal and posterior cingulate cholinergic activity. The Journal of Clinical Endocrinology and Metabolism. 2011;96(11):E1761–E1770. doi: 10.1210/jc.2011-0351. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F. Estrogen: A neuroprotective or proinflammatory hormone? Emerging evidence from reproductive aging models. Annals of the New York Academy of Sciences. 2005;1052:75–90. doi: 10.1196/annals.1347.006. [DOI] [PubMed] [Google Scholar]
- Sommer B. Alzheimer’s disease and the amyloid cascade hypothesis: Ten years on. Current Opinion in Pharmacology. 2002;2(1):87–92. doi: 10.1016/s1471-4892(01)00126-6. [Review] [DOI] [PubMed] [Google Scholar]
- Sorbi S, Bird ED, Blass JP. Decreased pyruvate dehydrogenase complex activity in Huntington and Alzheimer brain. Annals of Neurology. 1983;13(1):72–78. doi: 10.1002/ana.410130116. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- Spencer-Segal JL, Tsuda MC, Mattei L, Waters EM, Romeo RD, Milner TA, et al. Estradiol acts via estrogen receptors alpha and beta on pathways important for synaptic plasticity in the mouse hippocampal formation. Neuroscience. 2011;202:131–146. doi: 10.1016/j.neuroscience.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spulber G, Niskanen E, Macdonald S, Smilovici O, Chen K, Reiman EM, et al. Whole brain atrophy rate predicts progression from MCI to Alzheimer’s disease. Neurobiology of Aging. 2008;31(9):1601–1605. doi: 10.1016/j.neurobiolaging.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, et al. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004;24(36):7779–7788. doi: 10.1523/JNEUROSCI.1899-04.2004. [Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirone C, Boroujerdi A, Duckies SP, Krause DN. Estrogen receptor activation of phosphoinositide-3 kinase, akt, and nitric oxide signaling in cerebral blood vessels: rapid and long-term effects. Molecular Pharmacology. 2005;67(1):105–113. doi: 10.1124/mol.104.004465. [DOI] [PubMed] [Google Scholar]
- Stirone C, Duckies SP, Krause DN, Procaccio V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Molecular Pharmacology. 2005;68(4):959–965. doi: 10.1124/mol.105.014662. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. Mitochondria in cybrids containing mtDNA from persons with mito-chondriopathies. Journal of Neuroscience Research. 2007;85(15):3416–3428. doi: 10.1002/jnr.21167. [Research Support, N.I.H., Extramural Review] [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis. Journal of Alzheimer’s disease: JAD. 2010;2(Suppl. 20):S265–S279. doi: 10.3233/JAD-2010-100339. [Research Support, N.I.H., Extramural Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: an update. Experimental Neurology. 2009;218(2):308–315. doi: 10.1016/j.expneurol.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma K, Yao J, Huang J, Xu H, Chen X, Luddy J, et al. ABAD enhances Abeta-induced cell stress via mitochondrial dysfunction. The FASEB journal: Official Publication of the Federation of American Societies for Experimental Biology. 2005;19(6):597–598. doi: 10.1096/fj.04-2582fje. [DOI] [PubMed] [Google Scholar]
- Tang M, Subbiah MT. Estrogens protect against hydrogen peroxide and arachi-donic acid induced DNA damage. Biochimica et Biophysica Acta. 1996;1299(2):155–159. doi: 10.1016/0005-2760(95)00227-8. [Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- Toescu EC, Verkhratsky A, Landfield PW. Ca2+ regulation and gene expression in normal brain aging. Trends in Neurosciences. 2004;27(10):614–620. doi: 10.1016/j.tins.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Vandeerstichele H, Korecka M, Clark CM, Aisen PS, Petersen RC, et al. Update on the biomarker core of the Alzheimer’s disease neuroimaging initiative subjects. Alzbeimers & Dementia. 2010;6(3):230–238. doi: 10.1016/j.jalz.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trushina E, McMurray CT. Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience. 2007;145(4):1233–1248. doi: 10.1016/j.neuroscience.2006.10.056. [DOI] [PubMed] [Google Scholar]
- Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME. Regional aerobic glycolysis in the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(41):17757–17762. doi: 10.1073/pnas.1010459107. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valla J, Schneider L, Niedzielko T, Coon KD, Caselli R, Sabbagh MN, et al. Impaired platelet mitochondrial activity in Alzheimer’s disease and mild cognitive impairment. Mitochondrion. 2006;6(6):323–330. doi: 10.1016/j.mito.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrivel KS, Thinakaran G. Membrane rafts in Alzheimer’s disease beta-amyloid production. Biochimica et Biophysica Acta. 2010;1801(8):860–867. doi: 10.1016/j.bbalip.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villain N, Fouquet M, Baron JC, Mezenge F, Landeau B, de La Sayette V, et al. Sequential relationships between grey matter and white matter atrophy and brain metabolic abnormalities in early Alzheimer’s disease. Brain. 2010;133(11):3301–3314. doi: 10.1093/brain/awq203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vina J, Borras C, Gambini J, Sastre J, Pallardo FV. Why females live longer than males: Control of longevity by sex hormones. Science of Aging Knowledge Environment. 2005;2005(23):pel7. doi: 10.1126/sageke.2005.23.pe17. [DOI] [PubMed] [Google Scholar]
- Vina J, Sastre J, Pallardo FV, Gambini J, Borras C. Role of mitochondrial oxidative stress to explain the different longevity between genders: protective effect of estrogens. Free Radical Research. 2006;40(12):1359–1365. doi: 10.1080/10715760600952851. [DOI] [PubMed] [Google Scholar]
- Vingtdeux V, Dreses-Werringloer U, Zhao H, Davies P, Marambaud P. Therapeutic potential of resveratrol in Alzheimer’s disease. BMC Neuroscience. 2008;2(Suppl. 9):S6. doi: 10.1186/1471-2202-9-S2-S6. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassenko AG, Vaishnavi SN, Couture L, Sacco D, Shannon BJ, Mach RH, et al. Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta) deposition. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(41):17763–17767. doi: 10.1073/pnas.1010461107. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner BK, Kitami T, Gilbert TJ, Peck D, Ramanathan A, Schreiber SL, et al. Large-scale chemical dissection of mitochondrial function. Nature Biotechnology. 2008;26(3):343–351. doi: 10.1038/nbt1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annual Review of Genetics. 2005;39:359–07. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xiong S, Xie C, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer’s disease. Journal of Neuro-chemistry. 2005;93(4):953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Quesenberry CP, Zhou J, Yaffe K. Timing of hormone therapy and dementia: The critical window theory revisited. Annals of Neurology. 2011;69(1):163–169. doi: 10.1002/ana.22239. [Comparative Study Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, et al. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer’s disease. Brain. 2007;130(Pt 7):1777–1786. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. Biomarkers: Warning signs. Nature. 2011;475(7355):S5–S7. doi: 10.1038/475S5a. [DOI] [PubMed] [Google Scholar]
- Wise PM. Estrogen therapy: Does it help or hurt the adult and aging brain? Insights derived from animal models. Neuroscience. 2006;138(3):831–835. doi: 10.1016/j.neuroscience.2005.08.046. [DOI] [PubMed] [Google Scholar]
- Wollen KA. Alzheimer’s disease: The pros and cons of pharmaceutical, nutritional, botanical, and stimulatory therapies, with a discussion of treatment strategies from the perspective of patients and practitioners. Alternative Nedicine Review: A Journal of Clinical Therapeutic. 2010;15(3):223–244. [Review] [PubMed] [Google Scholar]
- Wong DF, Rosenberg PB, Zhou Y, Kumar A, Raymont V, Ravert HT, et al. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F–AV-45 (florbetapir [corrected] F 18) Journal of Nuclear Medicine: Official Publication, Society of Nuclear Medicine. 2010;51(6):913–920. doi: 10.2967/jnumed.109.069088. [Clinical Trial Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annual Review of Pharmacology and Toxicology. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Wu TW, Brinton RD. Estrogen membrane receptor imaging coupled with estradiol activation of intracellular calcium rise and ERK activation in single neurons: Paper presented at the Society for Neuroscience Abstracts. 2004 [Google Scholar]
- Wu TW, Wang JM, Chen S, Brinton RD. 17Beta-estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: A potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience. 2005;135(1):59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Xing C, Dong M, Li X, Zou Y, Fan L, Wang X, et al. Neuroprotective effects of puerarin against beta-amyloid-induced neurotoxicity in PC12 cells via a PI3K–dependent signaling pathway. Brain Research Bulletin. 2011;85(3–4):212–218. doi: 10.1016/j.brainresbull.2011.03.024. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- Yaffe K. Hormone therapy and the brain: Deja vu all over again? JAMA: the Journal of the American Medical Association. 2003;289(20):2717–2719. doi: 10.1001/jama.289.20.2717. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA: the Journal of the American Medical Association. 1998;279(9):688–695. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- Yager JD, Chen JQ. Mitochondrial estrogen receptors-new insights into specific functions. Trends in Endocrinology and Metabolism: TEM. 2007;18(3):89–91. doi: 10.1016/j.tem.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM, Jr, Valencia T, et al. Mitochondrial localization of estrogen receptor beta. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(12):4130–135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Chen S, Cadenas E, Brinton RD. Estrogen protection against mitochondrial toxin-induced cell death in hippocampal neurons: Antagonism by progesterone. Brain research. 2011;1379:2–10. doi: 10.1016/j.brainres.2010.11.090. [Comparative Study Research Support, N.I.H., Extramural Research Support, 1379, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Hamilton RT, Cadenas E, Brinton RD. Decline in mitochondrial bio-energetics and shift to ketogenic profile in brain during reproductive senescence. Biochi-mica etBiophysica Acta. 2010;1800(10):1121–1126. doi: 10.1016/j.bbagen.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Irwin R, Chen S, Hamilton R, Cadenas E, Brinton RD. Ovarian hormone loss induces bioenergetic deficits and mitochondrial beta-amyloid. Neurobiology of Aging. 2011;33:1507–1521. doi: 10.1016/j.neurobiolaging.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(34):14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Petanceska SS, Montine TJ, Holtzman DM, Schmidt SD, Parker CA, et al. Aging, gender and APOE isotype modulate metabolism of Alzheimer’s Abeta peptides and F-isoprostanes in the absence of detectable amyloid deposits. Journal of Neurochemistry. 2004;90(4):1011–1018. doi: 10.1111/j.1471-4159.2004.02532.x. [DOI] [PubMed] [Google Scholar]
- Yao J, Rettberg JR, Klosinski LP, Cadenas E, Brinton RD. Shift in brain metabolism in late onset Alzheimer’s disease: Implications for biomarkers and therapeutic interventions. Molecular Aspects of Medicine. 2011;32(4–6):247–257. doi: 10.1016/j.mam.2011.10.005. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KJ, Bennett JP. The mitochondrial secret(ase) of Alzheimer’s disease. Journal of Alzheimer’s disease: J AD. 2010;2(Suppl. 20):S381–S400. doi: 10.3233/JAD-2010-100360. [Research Support, N.I.H., Extramural Review] [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: The Cache County Study. JAMA : the Journal of the American Medical Association. 2002;288(17):2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Jahng GH, Bayne W, Mori S, Schad L, et al. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68(1):13–19. doi: 10.1212/01.wnl.0000250326.77323.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Brinton RD. WHI and WHIMS follow-up and human studies of soy isoflavones on cognition. Expert Review of Neurotherapeutics. 2007;7(11):1549–1564. doi: 10.1586/14737175.7.11.1549. [Comparative Study Research Support, Non-U.S. Gov’t Review] [DOI] [PubMed] [Google Scholar]
- Zhao L, Mao Z, Brinton RD. A select combination of clinically relevant phytoestrogens enhances estrogen receptor beta-binding selectivity and neuroprotective activities in vitro and in vivo. Endocrinology. 2009;150(2):770–783. doi: 10.1210/en.2008-0715. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- Zhao WQ, De Felice FG, Fernandez S, Chen H, Lambert MP, Quon MJ, et al. Amyloid beta oligomers induce impairment of neuronal insulin receptors. The FASEB journal: Official Publication of the Federation of American Societies for Experimental Biology. 2008;22(1):246–260. doi: 10.1096/fj.06-7703com. [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- Zhao X, MacBride MM, Peterson BR, Pfaff DW, Vasudevan N. Calcium flux in neuroblastoma cells is a coupling mechanism between non-genomic and genomic modes of estrogens. Neuroendocrinology. 2005;81(3):174–182. doi: 10.1159/000087000. [DOI] [PubMed] [Google Scholar]
- Znamensky V, Akama KT, McEwen BS, Milner TA. Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal CA1 dendrites. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2003;23(6):2340–2347. doi: 10.1523/JNEUROSCI.23-06-02340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]