Abstract
To evaluate neurological effects of terbutaline, rats were injected with saline, terbutaline (Sigma or American Pharmaceutical Partners (APP™)) at 0.5mg/kg/day or 10mg/kg/day between postnatal days (PND)2–5 or 11–14. Brains collected 24hrs after last injection were used to determine corpus-callosum thickness, Purkinje cell and neuronal number in the cerebellum. Ambulation, distance traveled, resting time and time on rotarod were analyzed. Terbutaline (both doses/grades at PND11–14) decreased corpus-callosum thickness. Ambulation time was significantly decreased in the 10mg/kg/day (Sigma) and 0.5mg/kg/day of terbutaline (APP™) (PND2–5) juvenile-rats and 10mg/kg/day-Sigma adult-rats; 0.5mg/kg/day APP™ (PND11–14) adult-rats. Resting time was increased in both doses of APP™ (PND2–5) in juvenile-rats; 10mg/kg/day Sigma adult-rats. 10mg/kg/day-Sigma (PND2–5) decreased distance traveled in adult-rats. 0.5mg/kg/day-Sigma (PND2–5&PND11–14) decreased the time spent on rotarod (30RPM) in adult-rats. Sigma terbutaline Sigma had 2X as much free base compared to APP™. In conclusion, APP™ terbutaline did not have a deleterious effect on the developing rat brain.
Keywords: Terbutaline, Motor Impairment, Brain, Rodent, Sigma
1. Introduction
Preterm birth remains the single most common and costly obstetric complication in the United States, accounting for the majority of neonatal mortality and morbidity. In addition, preterm delivery is linked to the majority of childhood neurobehavioral problems [1]. According to recent statistics, preterm birth has risen from 8.2% in the 1980’s to just over 10% in the 1990’s and over 12% in 2003 [2]. As a large portion of preterm births are found in women who develop preterm labor, it is likely that such patients are treated with tocolytic drugs in an effort to prolong the gestation and permit corticosteroid administration [3].
In addition to the desired β2 effects of blocking uterine contractions and bronchiole relaxation, stimulation of the β1 adrenergic receptors accounts for maternal beta-agonist side effects. These include tachycardia, anxiety, nervousness, insomnia and, in some cases, angina and pulmonary edema. The vast majority of serious maternal adverse effects have been reported using intravenous beta-agonist dosing for acute preterm labor treatment. Infants delivering just after high dose intravenous beta-agonist tocolytic therapy for preterm labor have demonstrated short term renal, pulmonary, and cardiovascular side effects [4]. No consistent, adverse, long-term effects in children exposed to intravenous β-agonists have been noted when stratified for the effects of prematurity and long term neurologic studies following low dose beta agonists have not demonstrated any adverse consequences in children [5–7].
A series of studies by the Slotkin laboratory using terbutaline, obtained from Sigma Chemical Company, have found that perinatal administration of terbutaline causes profound changes in behavior, neurochemistry and morphology in the developing rat as well as changes in brain neurochemistry and activation of fetal β-adrenoreceptors on developing neurons and glia [8, 9]. Therefore the objective of this study was to determine if a pharmaceutical grade of terbutaline was also capable of changing basic neurobehavior among rats, we examined the effects of different preparations of terbutaline using the same administration schedule and doses of terbutaline as the Slotkin laboratory[8, 9]. We used the combination of histology and neurobehavioral testing to determine if this was also the case for American Pharmaceutical Partners™ terbutaline, a tocolytic agent in clinical practice.
2. Materials and Methods
The Institutional Animal Care and Use Committee approved the animal procedures in this study at the University of Mississippi Medical Center. Fifty-three timed pregnant, Sprague-Dawley rats were obtained (day 15 of gestation) from Harlan Laboratories. Pregnant rats were placed in separate cages under a 12/12-hour light/dark cycle and allowed to acclimate and deliver without interference. Water and food were available ad libitum.
2.1 Drug administration
Upon delivery, the number of pups for each litter was recorded and remained unadjusted, and the pups were maintained with dams until the beginning of the experimental period. The litters were assigned to one of five regimens: 1) saline 2) 0.5mg/kg/day terbutaline in the hemisulfate form (Sigma Chemical Company, St. Louis, MO) terbutaline 3) 10mg/kg/day of terbutaline obtained from Sigma [10] 4) 0.5mg/kg/day, pharmaceutical grade (American Pharmaceutical Partners, Inc. (APP™), Schaumburg, IL) terbutaline and 5) 10mg/kg/day pharmaceutical terbutaline. Terbutaline doses used were based on previous studies by the Slotkin laboratory indicating developmental neurotoxicity and β-adrenoreceptor stimulation [8, 9, 11]. Saline was used as a vehicle to obtain the different concentrations of terbutaline. Previous animal studies by Slotkin’s group also utilized saline as a vehicle[8, 9].
All animals were administered saline or terbutaline between PND 2–5 or PND 11–14 [9–10]. Single subcutaneous doses were administered daily to all groups during the appropriate dosing period using a 25-gauge needle inserted into the subcutaneous tissue of the lower hind legs. Equivalent volumes of saline were given at both time periods to control animals and all injections were given at the same time each day. All animals remained with their dams until PND30 at which time they were weaned and placed into new cages immediately following behavioral testing at PND30. As both neonatal handling and prenatal stress have been shown to increase locomotion in rats, especially Sprague Dawley rats, we elected to wean rats after their first exposure to the locomotor activity chamber [12, 13].
2.2 Histological Examination
Histological examination of brains was performed in accordance (brain regions, and parameters studied) to previous studies by the Slotkin lab[8]. Twenty-four hours after the last terbutaline injection, PND 6 or PND 15, 2–3 pups per litter were sacrificed and their brains were harvested. There was equal sex distribution between the treatment groups at the time of sacrifice. After decapitation brains were extracted from the skull, immediately weighed followed by immediate placement into 10% formalin buffered solution. Paraffin embedded sections were prepared, cut to 8 μm-thickness, mounted and stained with hematoxylin and eosin (H&E). For each animal, two slides containing 3 adjacent brain sections were examined. H&E sections were submitted for pathological review and examined microscopically for alterations in histopathology including thickness of the corpus callosum, number of Purkinje cells in the cerebellum and the thickness of the external granular cerebellar layer. The pathologist (JWA) was blind to the treatment group.
2.3 Motor and balance assessment
Perinatal terbutaline treatment in rats was reported to increase hyperactivity in the open field and decreases the number of Purkinje cells in the cerebellum [8, 9]. Animals with decreases in Purkinje cell density or function often exhibit deficits in motor coordination and balance [14, 15]. In order to determine if either terbutaline obtained from Sigma or pharmaceutical grade terbutaline could lead to impairment in these behaviors the remaining pups were subjected to basic neurobehavioral testing (motor coordination, balance, locomotion and exploratory behavior) in order to determine long-lasting or delayed effects of perinatal drug injections on motor or neurobehavioral development [16, 17]. On the day of behavioral testing, rats were brought to a sound-attenuated testing room and allowed to acclimate for 1 hour. Animals from each experimental group underwent behavioral testing at two different ages, PND 30–35 (Juvenile) and PND 50–55 (Adulthood). All testing was done at the same time of day for each time period.
To measure locomotor activity, rats were placed individually into a monitoring system (Opto-Varimex-Minor System, Columbus Instruments, Columbus, Ohio) under low lights for 30 minutes to test locomotion. A computer system recorded horizontal and vertical activity as determined by the breaking of infrared detectors and sensors. Data was analyzed for distance traveled, resting time and ambulatory time.
To measure motor coordination and balance, rats were placed on a rotating cylinder (Economy Rotamex, Columbus Instruments, Columbus, Ohio) at a slow rotational speed (15-RPM) for 3 minutes. At the end of 3 minutes the speed was increased to 30-RPM for an additional 3 minutes. The first 30 seconds in each time period was used as an acclimation period, and any falls were not scored. The time spent on the rota-rod was the main outcome.
2.4 Terbutaline Composition by Mass Spectrometry
Mass spectrometry was used to ascertain if a difference exists in either of the molecular ion spectra of the two terbutaline preparations (Sigma vs APP™) or their fragmentation patterns. Terbutaline obtained from Sigma was dissolved in water at a concentration of 1 μg/mL and medicinal grade terbutaline diluted to 1 μg/mL were extracted with 1 mL of deionized water: methanol: methylene chloride (1:1:1) by mixing for 10 min at room temperature. After centrifugation at 1400g for 5 min, the organic phase was transferred to recovery vials and evaporated to dryness under a stream of nitrogen gas. The residue was re-dissolved in 1 mL of methanol: deionized water (1:1) with 1% acetic acid. This step was undertaken to purify the free base from the salt to improve ionization efficiency. All solvents used were analytical grade and obtained from Fisher Scientific.
Samples were analyzed in an Electrospray Ionization – Triple Quadrupole mass spectrometer (API 365 – Applied Biosystems). Analytes were introduced by direct infusion into the mass spectrometer at a flow rate of 10 μL/min. Nitrogen was used as the nebulizer gas at 40-psi pressure. Data were collected by positive ionization in MS mode and the origin of the fragment ions confirmed by MS/MS analysis. The source was optimized and the orifice and ring voltages were set at 51 and 210 V.
2.5 Statistical Analysis
One-way Analysis of Variance (ANOVA) or Students T-test with Bonferroni correction was used to determine statistical significance for the thickness of the corpus callosum and external cerebellar granular cell layers, as well as the number of Purkinje cells within each treatment group. Locomotor and rota-rod data were analyzed using a mixed model which allowed us to incorporate within-rat correlations and also to adjust for dams per treatment group and litters using variance components models. An ANOVA incorporating random effects for dams and litters with drug treatment (per injection regimen) serving as the repeated measure was used to analyze behavioral data. Tukey-Kramer multiple comparisons tests were used for post-hoc analysis. Data are represented as mean ± standard error mean. p values < 0.05 were considered significant.
3. Results
In agreement with previous reports [8–9] neither the 0.5mg/kg/day or 10mg/kg/day terbutaline (Sigma and APP™) regimen had a significant effect on brain (p = 0. 853) or pup weight (p = 0.712). In addition, none of the pups died following injections and body weights were similar among groups regardless of treatment type (data not shown).
3.1 Terbutaline alters corpus callosum thickness based on age at exposure
In the PND 2–5 injection group, terbutaline (neither Sigma or APP™ at either dose) did not significantly increase corpus callosum thickness compared to animals in the saline group (p = 0.626, Table 1) when analyzed at PND6. Terbutaline (neither Sigma nor APP™ at either dose) injection between PND 2–5 did not significantly decrease Purkinje cell density (p = 0.386, Table 1) or significantly decrease neurons in the cerebellar external granular cell layer (p=0.133, Table 1) when analyzed at PND6.
Table 1.
Brain Pathological evaluation.
| PND 2–5 | PND 11–14 | |||||||
|---|---|---|---|---|---|---|---|---|
| Corpus Callosum (microns) | Purkinje Cells (#/350 microns) | ECGL (# of cells) | N | Corpus Callosum (microns) | Purkinje Cells (#/350 microns) | ECGL (# of cells) | N | |
| Saline | 201 ± 18.8 | 8.0 ± .71 | 5.3 ± .14 | 12 | 251 ± 12.2 | 7.6 ± .63 | 2.9 ± .23 | 10 |
| 0.5mg/kg/day Sigma | 214 ± 17.2 | 8.9 ± .58 | 6.0 ± .27 | 12 | 195 ± 16.7* | 7.8 ± .52 | 3.8 ± .35 | 10 |
| 0.5mg/kg/day APP™ | 209 ± 5.3 | 8.1 ± .59 | 6.4 ± .26 | 12 | 185 ± 7.5* | 9.1 ± .73 | 3.4 ± .27 | 10 |
| 10mg/kg/day Sigma | 216 ± 7.8 | 8.4 ± .35 | 5.8 ± .15 | 12 | 196 ± 8.9* | 7.8 ± .59 | 2.8 ± .43 | 10 |
| 10 mg/kg/day APP™ | 213 ± 7.2 | 8.6 ± .45 | 5.7 ± .14 | 12 | 192 ± 7.2* | 7.7 ± .6 | 3.7 ± .15 | 10 |
| P Value | .626 | .386 | .133 | .003 | .445 | .161 | ||
Data is displayed as Mean ± SD. ECGL = external cerebellar granule cell layer.
P<0.05 rats injected between PND 11–14 with terbutaline from APP™ or Sigma at 0.5mg/kg/day or 10mg/kg/day had statistically significant thinner corpus callosums compared to rats injected with saline at the same time period.
In the PND 11–14 injected group, terbutaline injection, both Sigma and APP™ at 0.5mg/kg/day and 10mg/kg/day, significantly decreased corpus callosum thickness compared to animals in the saline group (p = 0.003, Table 1, Fig. 1) when analyzed at PND15. Terbutaline injection (neither Sigma nor APP™ at either dose) between PND 11–14 did not significantly decrease Purkinje cell density (p = 0.445, Table 1) or significantly decrease neurons in the cerebellar external granular cell layer (p=0.161, Table 1) when analyzed at PND15.
Figure 1. Effects of terbutaline treatment on corpus callosum thickness.
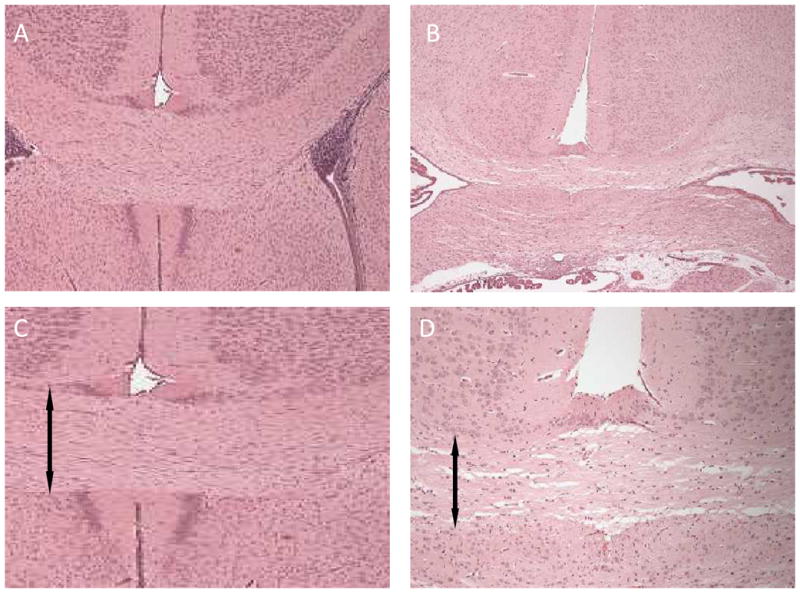
(A, and C) corpus callosum from a representative control animal on PND 15 which was administered saline between PND 11–14. (B, and D) the same area on PND 15 from an animal given 10mg/kg/day doses of terbutaline between PND 11–14. Arrows indicate where the width of the corpus callosum was measured for each section. Terbutaline treatment administered at this time point resulted in thinning of the corpus callosum.
3.2 The effect of terbutaline on motor skills
Ambulation time and behavior
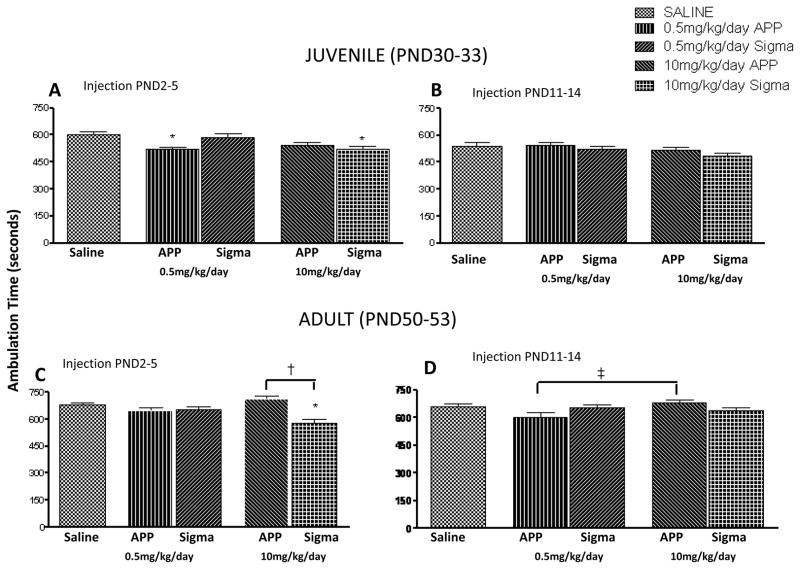
As there were no statistical differences in ambulation time between male and female rodents at either the juvenile (PND 2–5 regimen, p = 0.097; PND 11–14 regimen, p = 0.315) or adult time (PND 2–5 regimen, p = 0.751; PND 11–14 regimen, p = 0.917) period among either the 0.5mg/kg/day or 10mg/kg/day dose, males and females are reported together (data not shown). Juvenile animals in the PND 2–5 regimen that received 0.5mg/kg/day of terbutaline from APP™ or the 10mg/kg/day dose of terbutaline obtained from Sigma had significantly decreased ambulation times (p < 0.05), in comparison to the saline group (Fig. 2A). There were no differences in ambulation time seen in juvenile animals in the PND 11–14 regimen regardless of terbutaline treatment (p = 0.089, Fig 2B).
Figure 2. Terbutaline had differential effects on ambulation time.
Mean (±S.E.M) total ambulation time (sec) of juvenile and young adult rats treated at either PND 2–5 (A&C) or PND 11–14 (B&D) with 0.5mg/kg/day or 10mg/kg/day doses of Terbutaline. Animals numbers for these studies are as follows: A&C; Saline (n=42), 0.5mg/kg/day APP™ and Sigma (n=55,55), 10mg/kg/day APP™ and Sigma (n=50,48), B&D; Saline (n=34),0.5mg/kg/day APP™andSigma (n=45,50), 10mg/kg/day APP™ and Sigma (n=48,47). Statistical differences are indicated by the following: * p<0.05 vs saline group, † p<001 10mg/kg/day Sigma vs 10mg/kg/day APP™, ‡ p< 0.05 0.5mg/kg/day APP™ vs 10mg/kg/day APP™.
Adult animals in the PND 2–5 regimen injected with 10mg/kg/day of terbutaline obtained from Sigma had significantly decreased ambulation times (p < 0.05) when compared to the saline and 10mg/kg/day APP™ terbutaline (Fig. 2C). Adult rats injected with 0.5mg/kg/day of APP™ terbutaline between PND 11–14 had decreased (p < 0.05) ambulation time compared to the 10mg/kg/day APP™ terbutaline group (Fig. 2D).
Distance traveled
As there were no statistical differences in distance traveled between male and female rodents at either the juvenile (PND 2–5 regimen, p = 0.076; PND 11–14 regimen, p = 0.257) or adult time (PND 2–5 regimen, p = 0.102; PND 11–14 regimen, p = 0.508) period among either the 0.5mg/kg/day or 10mg/kg/day dose, males and females are reported together (data not shown). Terbutaline did not significantly change distance traveled in juvenile rats receiving terbutaline (from either Sigma or APP™ at either dose) compared to the saline group when administered between PND 2–5 (p = 0.448) or PND 11–14 (p = 0.524).
The 10mg/kg/day dose of terbutaline obtained from Sigma when injected between PND2–5 significantly decreased distance traveled in adult animals compared to animals injected with the same dose of pharmaceutical grade terbutaline (p < 0.001, data not shown). Terbutaline (from either Sigma or APP™ from either dose) injection at PND 11–14 did not significantly change distance traveled among adult rats (p = 0.125).
Resting time
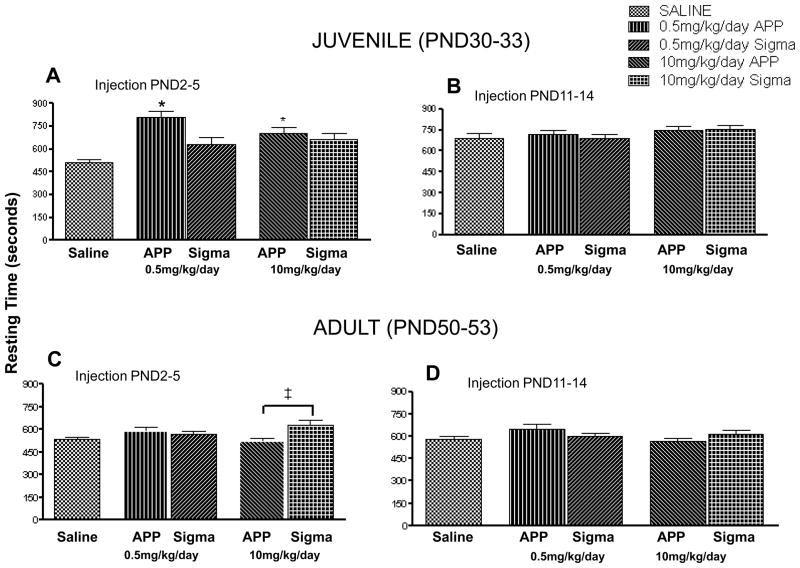
As there were no statistical differences in resting time between male and female rodents at either the juvenile (PND 2–5 regimen, p = 0.211; PND 11–14 regimen, p = 0.729) or adult time (PND 2–5 regimen, p = 0.086; PND 11–14 regimen, p = 0.219) period among either the 0.5mg/kg/day or 10mg/kg/day doses, males and females are reported together (data not shown). To determine the amount of time the animals were inactive during the 30min. testing period, resting time in the locomotor activity chamber was analyzed. Both doses of pharmaceutical grade terbutaline administered at PND 2–5 significantly increased (p < 0.05) resting times among juvenile animals (Fig. 3A). There were no statistically significant changes in resting time between groups in the PND11–14 regimen in juvenile animals (p = 0.302).
Figure 3. Terbutaline administered between PND 2–5 increases resting time.
Mean (±S.E.M) total resting time (sec) of juvenile and young adult rats treated at either PN2–5 (A&C) or PND 11–14 (B&D) with 0.5mg/kg/dayand 10mg/kg/day doses of APP™ or Sigma terbutaline. There were no significant differences among the groups in animals injected between PND 11–14. Animal numbers for these studies were as follows: A&C; Saline (n=42), 0.5mg/kg/dayAPP™ and Sigma (n=55,55), 10mg/kg/dayAPP™ and Sigma (n=50,48), B&D; Saline (n=34), 0.5mg/kg/dayAPP™ and Sigma (n=45,50), 10mg/kg/dayAPP™ and Sigma (n=48,47). Statistical differences are indicated by the following: * = p<.05vs saline group, and ‡ = p<0.05 10mg/kg/day Sigma vs 10mg/kg/dayAPP™.
In adult rats the 10mg/kg/day dose of terbutaline obtained from Sigma administered between PND 2–5 significantly increased (p < 0.05) resting time compared to the 10mg/kg/day APP™ terbutaline group (Fig. 3C). There were no significant differences in resting times in adult rats that were in the PND11–14 regimen (p = 0.204). These results suggest that when terbutaline is administered in the early postnatal period, it increases the amount of time spent resting as tested through the locomotor activity chamber.
Coordination and balance
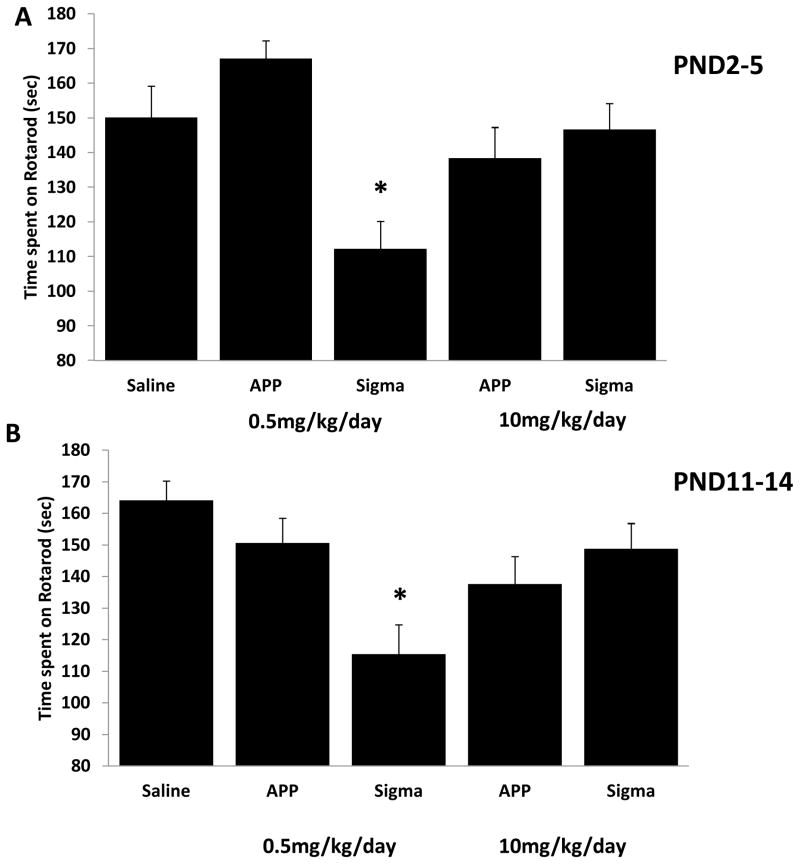
As there were no statistical differences in the time spent on the rotarod between male and female rodents at either the juvenile (PND 2–5 regimen, p = 0.373; PND 11–14 regimen, p = 0.601) or adult time (PND 2–5 regimen, p = 0.726; PND 11–14 regimen, p = 0.581) period among either the 0.5mg/kg/day or 10mg/kg/day doses, males and females are reported together (data not shown). Coordination and balance were tested over a series of increasing speeds on a rotating rotarod. Juvenile rats injected with 10mg/kg/day of APP™ terbutaline at PND 2–5 spent significantly less time (P<.05) on the rotarod at 15RPM compared to rats injected with 0.5mg/kg/day of APP™ terbutaline (Supplementary data table 1). There were no statistically significant differences at 30-RPM (p = 0.323) when compared to rats in the saline group (Supplementary data table 1). There were no significant differences among juvenile rats injected at PND 11–14 at either 15-RPM (p = 0.719) or 30-RPM (p = 0.267) (Supplementary data table 1).
There were no statistically significant differences in the time spent on the rotarod among adult rats at 15RPM (p=.222) when they were injected with terbutaline (neither Sigma nor APP™ at either dose) or saline between PND2–5 (Supplementary data table 2). There was a significant difference in time spent on the rotarod at 15RPM between adult animals injected at the PND11–14 regimen (p=.036), however post-hoc analysis with Tukey-Kramer Multiple comparison failed to detect a significant difference between the groups (Supplementary data table 2). Adult animals injected between PND2–5 and between PND11–14 with 0.5mg/kg/day of terbutaline obtained from Sigma spent significantly less time on the rotarod at 30RPM compared to rats in the saline, 0.5mg/kg/day dose of APP™ terbutaline and 10mg/kg/day of terbutaline from Sigma groups (Figure 4).
Figure 4. Sigma terbutaline decreases time spent on rotarod.
Latency to fall (sec) at 30-RPM for adult rats treated at PND 2–5 (A) or at PND11–14 (B) with 10mg/kg/day of terbutaline from Sigma. Adult rats injected with terbutaline from Sigma at either PND2–5 or PND11–14 had impaired motor coordination signified by significantly less time spent on the rotarod. Animal numbers for these studies were as follows: Animal numbers for these studies were as follows: PND2–5; Saline (n=42), 0.5mg/kg/day APP™ and Sigma (n=55,55), 10mg/kg/day APP™ and Sigma (n=50,48), PND11–14; Saline (n=34), 0.5mg/kg/day APP™ and Sigma (n=45,50), 10mg/kg/day APP™ and Sigma (n=48,47). Statistical differences are reported as *0.5mg/kg/day of terbutaline from Sigma was p< 0.05 compared to animals in the saline, 0.5mg/kg/day terbutaline from APP™, and 10mg/kg/day from Sigma terbutaline groups.
3.3 Mass Spectrum Analysis is different for pharmaceutical and terbutaline from Sigma
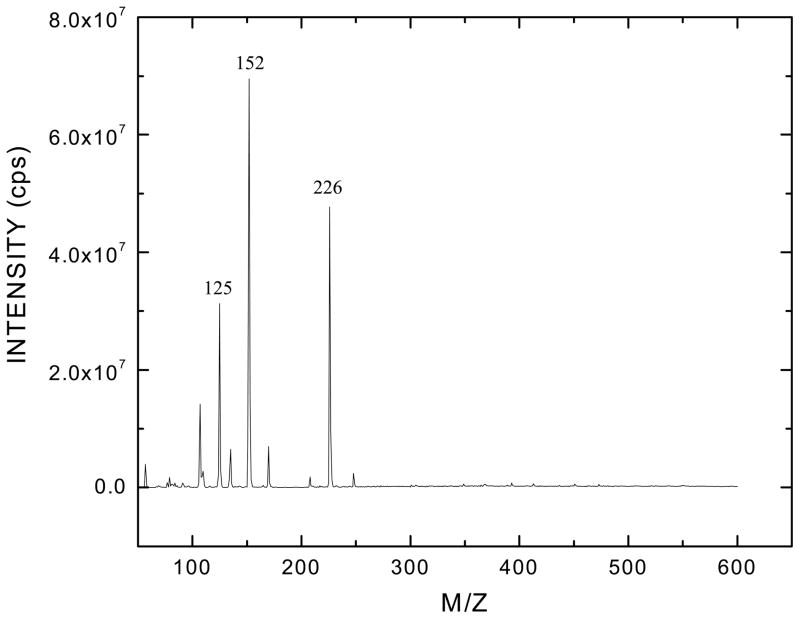
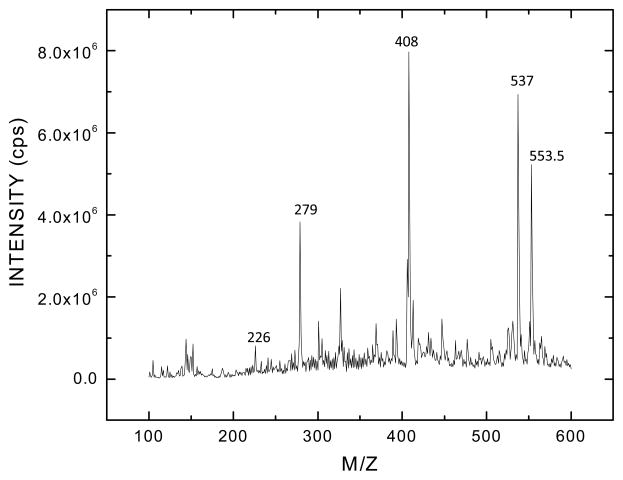
Mass spectrum analysis of terbutaline revealed differences in fragmentation between the two preparations. Electro-spray ionization spectra were scanned from a mass to charge ratio (m/z) of 50 – 650 in the single-stage MS mode using positive ionization. In Fig. 5, the molecular ion peak at m/z of 226, with an ion intensity of 5×107, corresponds to the molecular weight of the free base of terbutaline in the Sigma preparation. The peak at m/z 226 in Fig. 6 is the molecular ion arising from the free base of the medicinal grade terbutaline. It is noteworthy that the intensity of the molecular ion arising from the APP™ terbutaline is 1×106, approximately 50 fold lower than the free base generated from the terbutaline obtained from Sigma. Other molecular ions are seen at m/z of 279, 408, 537 and 553.5 are consistent with the presence of stabilizing agents in the pharmaceutical formulation (Fig. 6).
Figure 5. Mass spectrum analysis of terbutaline obtained from Sigma.
The molecular ion is seen at a mass to charge ratio (m/z) 226. The peaks at 125 and 152 denote fragment ions generated from the molecular ion. Data shown was collected in the MS mode.
Figure 6. Mass spectrum analysis of APP™ terbutaline.
The molecular ion is seen as a dimer with a sulfate adduct at 537 m/z. The peak seen at m/z 553.5 is suggestive of a water adduct of the molecular ion. The peaks at 408 and 279 are fragments arising from the molecular ion with the loss of a single aromatic ring and both aromatic rings from the parent molecule respectively. The peak at m/z 226 represents the monomelic form of the analyte. The remaining peaks were not identified. Only partial extraction of the inorganic component of the analyte was achieved as evidenced by the presence of the sulfate moiety as an adduct on the molecular ion. Data shown was collected in the MS mode.
The sample of terbutaline obtained from Sigma was prepared based on a molecular weight of 274.32g for terbutaline hemisulfate, as listed in the material safety data sheet and the molecular weight of the APP™ terbutaline was taken to be 548.65g during sample preparation. The molecular weight of the free base used in both formulations is 225 which gave a peak at 226 in our mass spectrometer (Figs. 5 & 6). When samples are prepared as weight by volume assuming different molecular weights, an error occurs in our estimation of the concentration of the free base. Therefore, assuming equivalent extraction efficiencies for both formulations, terbutaline obtained from Sigma prepared at 0.5mg/mL, the low dose, has a free base concentration of twice that for APP™ terbutaline at 0.5mg/mL when prepared according to the molecular formula of the two preparations.
4. Discussion
Previous studies by the Slotkin laboratory have suggested that perinatal administration of terbutaline from Sigma Chemical Company into rats alters brain neurochemistry, morphology and behavior [8, 18]. Based on these rodent experiments the Slotkin laboratory has suggested that terbutaline administration in human women can be harmful to the developing fetus. It was our goal to determine if 1) we could replicate these findings and 2) if a pharmaceutical grade of terbutaline would produce similar results.
Based on studies by the Slotkin laboratory, we used the same drug regimen and terbutaline concentrations in our studies. It should be pointed out that in order to successfully increase the circulating levels of terbutaline a more effective method would be, to continuously infuse terbutaline through a miniosmotic pump. Follow-up studies will include a treatment arm which employs an Alzet miniosmotic pump to ensure a continuous infusion of terbutaline. Although terbutaline obtained from Sigma is not used clinically since it is non-medicinal grade and may contain impurities harmful to humans, it was employed to replicate the previously reported studies of the rat model of terbutaline neurotoxicity [8, 9, 18]. Alternatively, APP™ pharmaceutical grade terbutaline, which is a preparation used clinically to treat preterm labor in humans was also represented in the treatment arms. Clinical doses of terbutaline for management of preterm labor rarely exceed 0.03mg/kg/day [19], which is much lower than any of the doses used in this study or the studies performed by Slotkin’s group.
In the first part of our study we examined the pathology of brains from pups injected with 0.5mg/kg/day or 10mg/kg/day dose of terbutaline obtained from Sigma or APP™. When compared to control animals, we found decreased thickness of the corpus callosum (Figure 1) when either preparation (dose and brand) of terbutaline was administered between PND 11–14. Based on these findings it appears as if terbutaline targets the developing corpus callosum when injected in the later postnatal period. A study from the Slotkin laboratory reported similar white matter damage after terbutaline was injected between PND 2–5 and the brains were examined at PND 30, suggesting that terbutaline exposure can have a delayed effect on gross brain pathology [9]. We did not see a decrease in the number of Purkinje cells due to terbutaline (Sigma) treatment as reported by the Slotkin laboratory [8], which might be due to the developmental time at which we conducted our pathologic evaluations. A decrease in Purkinje cell number has been reported when terbutaline from Sigma was administered at the PND 2–5 regimen and the brains are evaluated at PND 30 [8]. Since we performed pathology 24hrs after the last injection we were unable to confirm the findings of the Slotkin laboratory or to determine if APP™ leads to delayed white matter damage after injection between PND2–5.
In the second arm of this study the four treatment groups receiving terbutaline were assessed for locomotor activity. A decrease in ambulation time was seen in both juvenile and adult animals in the PND 2–5 regimen that received 0.5mg/kg/day of APP™ terbutaline or 10mg/kg/day of terbutaline from Sigma, respectively. Injection of terbutaline from Sigma between PND2–5 decreased ambulation in a dose-dependent manner, but terbutaline from APP™ failed to do so. The 0.5mg/kg/day APP™ had a greater impact on resting time in rats compared to the 10mg/kg/day APP™ group (Figure 3), which could have a direct effect on the ambulation time.
Both concentrations of pharmaceutical grade terbutaline and the 10mg/kg/day dose of terbutaline (Sigma) administered at PND2–5 increased resting times of juvenile and adult animals respectively. Since there were no changes or decreases in locomotor activity by these groups despite the increases in resting time, since only adult rats injected with 10mg/kg/day of terbutaline from Sigma between PND2–5 had a significant decrease in distance travel (data not shown), we are unsure of the meaning, if any, of this increase in resting time, and cannot confirm the Slotkin report on hyperactivity [9].
When terbutaline from Sigma was injected at either PND 2–5 or PND 11–14 an impairment in motor coordination and balance in adult rats resulted. A previous study from the Slotkin laboratory has reported decreases in Purkinje cells as a result of terbutaline injection when rats are examined at PND 30 [9]. While this study did not examine brain pathology at PND 30, a decrease in Purkinje cells as previously indicated supports the findings in which animals receiving terbutaline from Sigma spent less time on the rotarod, since rotarod performance is directly linked to Purkinje cell output [14, 20]. Therefore the impairment in motor coordination seen in the 0.5mg/kg/day dose of terbutaline in Sigma does support what was seen in Slotkin’s studies. However, there was no impairment in the group receiving the pharmaceutical grade of terbutaline.
Differences in the fragmentation between both Sigma and pharmaceutical grade terbutaline were found after mass spectrum analysis. Since pharmaceutical grade terbutaline dimerizes it is possible that the two different preparations can have a different bioavailability, thus possibly explaining some of the behavioral differences between the two grades that were seen in the present study. It may be helpful to evaluate specific brain regions to see if there is a difference in terbutaline absorption between the preparations. It is important to recognize that even though the empirical formula of these preparations may be similar, they are indeed acting differently. The neurotoxicity that has been reported with the Sigma preparation may merely be due to the use of a higher effective dose of the compound. In the current study the pharmacokinetics of these drugs was not studied, as such we cannot conclude that the active ingredient(s) from any particular preparation (Sigma vs APP™) was responsible for the neurotoxicity and changes in behavior seen in this study. In addition, while there were some changes in ambulation, resting time and corpus callosum thickness, the changes did not appear to have a dose-response effect. Future studies will need to be performed to determine the fate of the active ingredient(s) after drug administration and to see if a dose-response effect can be achieved.
5. Conclusion
In conclusion, our study findings indicate that animals receiving pharmaceutical grade terbutaline do not exhibit any basic neurobehavioral problems, despite the decrease in corpus callosum size. We were able to reproduce some of the results from the Slotkin laboratory in that terbutaline from Sigma does alter neurobehavior and causes gross pathological abnormalities, in animals injected with terbutaline between PND11–14. However it is important to point out that these changes were not seen in animals injected with APP™ terbutaline which could indicate a functional and possible chemical difference between these preparations. The results from this study, which incorporates pathologic findings, mass spectrum analysis, and neurobehavioral testing in this model, indicate that APP™ terbutaline does not have a clinically significant deleterious effect on the developing rat brain.
Supplementary Material
Acknowledgments
We would like to thank Dr. Ian Paul for the use of his neurobehavioral facilities and Dr. Babbette LaMarca for her critical analysis of this work. Supported in part by The Perinatal Research Fund and The Center for Psychiatric Neuroscience (NIH RR17701).
References
- 1.Ananth CV, Joseph KS, Oyelese Y, KD, AMV Trends in preterm birth and perinatal mortality among singletons: United States, 1989 through 2000. Obstetrics and Gynecology. 2005;105:1084–91. doi: 10.1097/01.AOG.0000158124.96300.c7. [DOI] [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, MLM Births: Final data for 2003. National vital statistics reports. 2005 [PubMed] [Google Scholar]
- 3.Assessment of risk factors for preterm birth. ACOG Educational and Practice Bulletin. 2001 [Google Scholar]
- 4.Katz VL, Seeds JW. Fetal and neonatal cardiovascular complications from beta-sympathomimetic therapy for tocolysis. American Journal of Obstetrics and Gynecology. 1989;161:1. doi: 10.1016/0002-9378(89)90219-6. [DOI] [PubMed] [Google Scholar]
- 5.Polowxzyk D, Tejani N, Lauersen N. Evaluation of Seven-to-nine year-old children exposed to ritodrine in utero. Obstetrics and Gynecology. 1984;64:485. [PubMed] [Google Scholar]
- 6.Hadders-Algra M, Touwen BCL, Huisjes JH. Long-term follow-up of children prenatally exposed to ritodrine. British Journal of Obstetrics and Gynecology. 1986;1:156. doi: 10.1111/j.1471-0528.1986.tb07880.x. [DOI] [PubMed] [Google Scholar]
- 7.Pitzer M, Schmidt MH, Esser G, et al. Child development after maternal tocolysis with B-sympathomimetic drugs. Child Psychiatry and Human Development. 2001;31:165–82. doi: 10.1023/a:1026419720410. [DOI] [PubMed] [Google Scholar]
- 8.Rhodes MC, Seidler FJ, Abdel-Rahman A, Tate C, Nyska A, HLR, et al. Terbutaline is a developmental neurotoxicant: Effects of neuroproteins and morphology in the cerebellum, hippocampus, and somatosensory cortex. Journal of Pharmacology and Experimental Therapeutics. 2004;308:529–37. doi: 10.1124/jpet.103.060095. [DOI] [PubMed] [Google Scholar]
- 9.Zerrate M, Pletnikov M, Connors S, Vargas D, Seidler F, Zimmerman A, et al. Neuroinflammation and Behavioral Abnormalities after Neonatal Terbutaline Treatment in Rats: Implications for Autism. The Journal of Pharmacology and Experimental Therapeutics. 2007;322:16–22. doi: 10.1124/jpet.107.121483. [DOI] [PubMed] [Google Scholar]
- 10.Rodier PM. Structural-functional relationships in experimentally induced brain damage. Progress in Brain Research. 1988;73:335–48. doi: 10.1016/S0079-6123(08)60514-2. [DOI] [PubMed] [Google Scholar]
- 11.Rhodes MC, Seidler FJ, Qiao D, Tate C, MMC, Slotkin T. Does pharmacotherapy for preterm labor sensitize the developing brain to environmental neurotoxicants? Cellular and synaptic effects of sequential exposure to terbutaline and chlorpyrifos in neonatal rats. Toxicology and Applied Pharmacology. 2004;195:203–17. doi: 10.1016/j.taap.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Schmitt U, Hiemke C. Strain Differences in Open-field and Elevated Plus-maze behavior of rats without and with pretest handling. Pharmacology Biochemistry and Behavior. 1998;59:807–11. doi: 10.1016/s0091-3057(97)00502-9. [DOI] [PubMed] [Google Scholar]
- 13.Wakshlak A, Martan W. Neonatal handling reverses behavioral abnormalities induced in rats by prenatal stress. Physiology & Behavior. 1990;48:289–92. doi: 10.1016/0031-9384(90)90315-u. [DOI] [PubMed] [Google Scholar]
- 14.Sajdel-Sulkowska EM, Nguon K, Sulkowski ZL, Rosen GD, MGB Purkinje cell loss accompanies motor impairment in rats developing at altered gravity. NeuroReport. 2005;16:2037–40. doi: 10.1097/00001756-200512190-00014. [DOI] [PubMed] [Google Scholar]
- 15.Wallace K, Veerisetty S, Paul I, May W, Miguel-Hidalgo J, Bennett W. Prenatal infection decreases calbindin, decreases purkinje cell volume and density and produces long-term motor deficits in Sprague-Dawley rats. Developmental Neuroscience. 2010 doi: 10.1159/000319506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Assaiante C, BA An ontogenetic model for the sensorimotor organization of balance control in humans. Human Movement Science. 1995;14:13–43. [Google Scholar]
- 17.Gramsbergen A. Posture and locomotion in the rat, inter or independent development. Neuroscience and BioBehavioral Reviews. 1998;22:547–54. [PubMed] [Google Scholar]
- 18.Slotkin T, Seidler F. Developmental exposure to terbutaline and chlorpyrifos, separately or sequentially, elicits presynaptic serotonergic hyperactivity in juvenile and adolescent rats. Brain Research Bulletin. 2007;73:301–9. doi: 10.1016/j.brainresbull.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam F, Elliott J, Jones JS, Katz M, Knuppel RA, Morrison J, et al. Clinical issues surrounding the use of terbutaline sulfate for preterm labor. Obstetrics Gynecological Survey. 1998;53:585–95. doi: 10.1097/00006254-199811002-00001. [DOI] [PubMed] [Google Scholar]
- 20.Barski J, Hartmann J, Rose C, Hoebeek F, Morl K, Noll-Hussong D, et al. Calbindin in cerebellar purkinje cells is a critical determinant of the precision of motor coordination. The Journal of Neuroscience. 2003;23:3469–77. doi: 10.1523/JNEUROSCI.23-08-03469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.