Abstract
The vast majority of patients with plasma cell neoplasms die of progressive disease despite high response rates to novel agents. Malignant plasma cells are very radiosensitive, but the potential role of radioimmunotherapy (RIT) in the management of plasmacytomas and multiple myeloma (MM) has undergone only limited evaluation. Furthermore, CD38 has not been explored as a RIT target despite its uniform high expression on plasma cell malignancies. In this report, both conventional RIT (directly radiolabeled antibody) and streptavidin-biotin pretargeted RIT (PRIT) directed against the CD38 antigen, were assessed as approaches to deliver radiation doses sufficient for MM cell eradication. PRIT demonstrated biodistributions that were markedly superior to conventional RIT. Tumor-to-blood ratios as high as 638:1 were seen 24hr after PRIT, while ratios never exceeded 1:1 with conventional RIT. 90Yttrium absorbed dose estimates demonstrated excellent target-to-normal organ ratios (6:1 for the kidney, lung, liver; 10:1 for the whole body). Objective remissions were observed within 7 days in 100% of the mice treated with doses ranging from 800 µCi to 1200 µCi of anti-CD38 pretargeted 90Y-DOTA-biotin, including 100% complete remissions (no detectable tumor in treated mice compared to tumors that were 2982±2834% of initial tumor volume in control animals) by day 23. Furthermore, 100% of animals bearing NCI-H929 multiple myeloma tumor xenografts treated with 800 µCi of anti-CD38 pretargeted 90Y-DOTA-biotin achieved long-term myeloma-free survival (>70 days) compared to none (0%) of the control animals.
Keywords: Radioimmunotherapy, multiple myeloma, CD38, pretargeting, preclinical
INTRODUCTION
Bortezomib, lenalidomide and other novel agents have significantly improved the response rates, progression-free survival and overall survival for patients with multiple myeloma (MM) in recent years.(1, 2) Despite these advances, MM remains incurable. With currently available therapies the 77,000 MM patients living in the United States will almost universally relapse and die from progressive disease. Myeloma recurrence is presumably a function of malignant plasma cell clones, and possibly precursor stem cells, (3, 4) that evade or develop resistance to available therapies. The efficacy of radioimmunotherapy (RIT) in the treatment of hematologic malignancies is well established.(5–7) RIT selectively delivers radiation to target cells at multifocal disease sites and facilitates escalation to radiation doses not achievable through external beam therapy. The radiosensitivity of malignant plasma cells outside of the bone marrow has been well documented in clinical settings. Local recurrence of solitary extramedullary plasmacytomas occurs in less than 10% of cases after external beam radiation alone.(8) Radiation therapy is also effective as a palliative measure in patients experiencing pain or other sequelae resulting from MM-induced osteolysis. Steep dose response relationships have been demonstrated for most hematological malignancies, and the impact of radiation dose escalation may be of particular importance in the case of MM.(9)
A limited number of radionuclide based therapies have been explored in the treatment of MM.(10–14) While each of these radionuclide based approaches has theoretical promise, none have directly targeted radiation to the CD38 antigen on MM cells. The directed delivery of radionuclides to MM cells requires an antigen target that is specific, stable and uniformly expressed at high density. CD38 is a 45 kDa stable transmembrane glycoprotein receptor expressed at a high epitope density on 95–100% of malignant plasma cells.(15, 16) The CD38 antigen is expressed on activated T cells, monocytes and NK cells at relatively low levels when compared to plasma cells. Reports describing CD38 expression on cells that are not of hematopoietic origin have been inconsistent. Non-quantitative approaches have suggested that CD38 may be expressed in pancreatic, lung, brain and even skeletal muscle;(17) however, recent gene expression profiling of human tissues demonstrate that CD38 mRNA is largely restricted to cells of hematopoietic origin with minimal expression in non-hematopoietic derived tissue limited to the thymus and prostate. (18)
Unconjugated anti-CD38 monoclonal antibodies (mAbs) have induced myeloma cell killing both in culture and in xenograft mouse models.(19, 20) CD38 mAbs have reportedly been well tolerated in a small number of patients(21) and a phase I/II clinical trial with the anti-CD38 mAb daratumumab is currently accruing patients with relapsed or refractory MM. CD38 mAbs linked to cytotoxic agents have also demonstrated therapeutic activity in myeloma models.(22, 23)
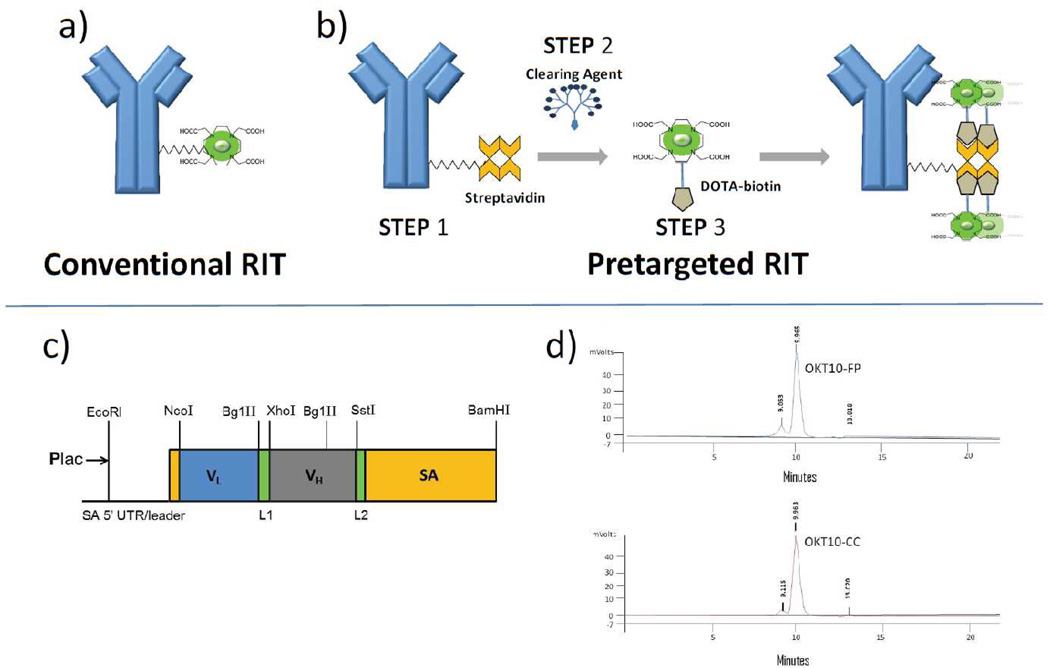
While conventional one-step RIT with directly conjugated radiolabeled antibodies (Figure 1a) is effective at achieving disease control, disease eradication is thought to be limited by relatively low tumor-to-normal organ ratios of absorbed radioactivity (e.g. 1.5:1 for tumor-to-lung using 131I-anti-CD20).(6) Multistep pretargeting methods can optimize delivery of the therapeutic radionuclide to tumor targets, while limiting normal organs from radiation exposure. Several approaches to pretargeting have been described.(24–26) One method employs an antibody-streptavidin (Ab-SA) construct, followed by administration of a small molecule, radio-DOTA-biotin. In the first step, the tumor-reactive Ab-SA localizes to tumor sites without subjecting the rest of the body to non-specific irradiation. After maximal accumulation of Ab-SA in the tumor, a small molecular weight radioactive moiety (radio-DOTA-biotin) possessing a high affinity for the Ab-SA bound to tumor sites is administered as a second-step. Due to its small size, the second-step reagent penetrates tumors rapidly where it binds tightly to the pretargeted Ab-SA (Figure 1b). Unbound radio-DOTA-biotin molecules are cleared from the blood and excreted in the urine within minutes. This rapid clearance of the second step reagent limits the time during which normal tissues are exposed to non-specific radiation. Dissociating the slow target antigen binding distribution phase from the radionuclide delivery phase generates more favorable target-to-normal organ ratios. As a further refinement, a clearing agent (CA) can be injected shortly before the radiolabeled small molecule to remove unbound Ab-SA from the bloodstream and prevent it from complexing with the radiolabeled small molecule in the circulation.(26, 27)
Figure 1.
Components for pretargeted radioimmunotherapy (PRIT). Schema depicting (a) one-step conventional radioimmunotherapy and (b) multistep PRIT. PRIT involves infusion of the antibody-SA construct [Step 1], followed by injection of a synthetic N-acetylgalactosamine-containing clearing agent [Step 2] designed to facilitate hepatic clearance of excess antibody-SA from the bloodstream, and then infusion of the radiolabeled small molecule DOTA-biotin [Step 3]. (c) Schematic diagram of OKT10 scFv-SA fusion gene. (d) Size exclusion HPLC performed on OKT10-FP and OKT10-CC anti-CD38 streptavidin constructs demonstrate a retention time of 9.965 and 9.963 minutes respectively. A small amount of aggregate is present.
This report describes a new approach to the treatment of MM. Anti-CD38 pretargeted radioimmunotherapy (PRIT) demonstrates superior biodistributions when compared with directly labeled conventional anti-CD38 antibody RIT and clear evidence of therapeutic efficacy which provides a compelling rationale for further study.
MATERIALS AND METHODS
Cell lines
The human MM cell lines NCI-H929 and L363 were a gift from David Maloney (obtained from American Type Culture Collection [ATCC, Bethesda, MD]). The human Ramos B-cell lymphoma and human RPMI 8226 MM cell lines were also obtained from ATCC. The MM1R cell line was a gift from Steven Rosen (Northwestern University, Chicago, IL) and was generated in his laboratory. Cells were grown in RPMI-1640 supplemented with 10% FBS, 50U/mL penicillin G and 50µg/mL streptomycin sulfate. Following two passages, cells were frozen and stored in liquid nitrogen for future use. For all studies a fresh vial of frozen cells was thawed and grown in culture for 7 to 21 days.
Antibodies, antibody conjugates, fusion proteins and pretargeted reagents
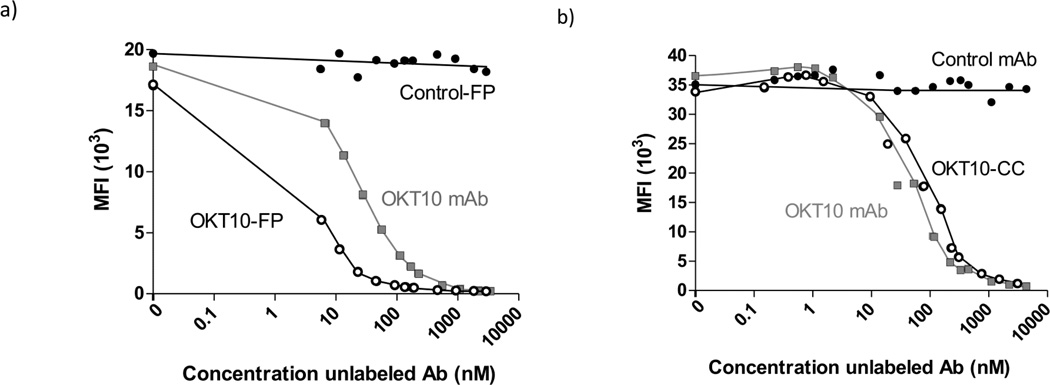
The OKT10 hybridoma expressing murine IgG1 anti-CD38 Ab and nonspecific IgG1 control Ab, BHV1 (specific for bovine herpes virus 1), were obtained from ATCC. The OKT10-Ab and BHV1-Ab were produced from ascites in pristine-primed mice purified by HiTrap Protein G HP 5 mL column chromatography. DOTA-Ab reagents were generated as described previously.(27) Each Ab was also conjugated to streptavidin (SA) to form covalent synthetic chemical conjugates using previously described methods.(27) The CC49 scFv4SA-FP, which recognizes the TAG-72 antigen on human adenocarcinomas, was a gift from NeoRx (NeoRx, Seattle, WA). Expression, purification and characterization of CC49-FP has been previously described. (28, 29) To produce OKT10-CC, intact OKT10 IgG1 anti-CD38 mAb was derivatized with either a DOTA chelate(27, 30) or SA using the heterobifunctional cross-linker SMCC using methods previously described.(27) Iminobiotin and cation exchange chromatography led to purities of >93%. The chemical conjugate (CC) harbors approximately one SA molecule conjugated to each OKT10 molecule as assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and size exclusion HPLC (Figure 1d). OKT10-FP was generated through cloning Fv regions from the OKT10 anti-CD38 hybridoma which were fused to the full-length genomic SA gene of Streptomyces avidinii, and the resultant fusion genes (Figure 1c) expressed as soluble tetramers (174 kDa) in the periplasmic space of E. coli(29) (FP construction detailed in supplemental methods). Lineweaver-Burke and Scatchard cell binding assays confirmed that both OKT10-CC and OKT10-FP retain the full immunoreactivity and avidity of the parent OKT10-Ab; binding specificity was confirmed by blocking with excess intact unconjugated mAb (Figure 2a,b). The biotin-binding capacity of the CC and FP were similar to recombinant SA by the HABA (4’-hydroxyazobenzene-2-carboxylic acid) assay (not shown).
Figure 2.
Binding of Anti-CD38 antibody constructs to CD38 on malignant plasma cells. Competitive binding assays demonstrate blocking of Alexa-647 conjugated OKT10 mAb binding to CD38-expressing L363 MM cells with escalating concentrations of unlabeled (a) OKT10-FP or (b) OKT10-CC. No blocking is observed with escalating concentrations of nonbinding control Ab constructs.
Radiolabeling
90Yttrium (90Y) and 111Indium (111In) [Perkin Elmer, Waltham, MA] radiolabeling of intact DOTA-Ab for conventional RIT, and DOTA-biotin for pretargeted RIT, were conducted as described previously.(27, 30) Radiochemical purity was generally greater than 95% as determined by iTLC and avidin bead assay. DOTA-biotin was synthesized as described.(27)
Trichloroacetic acid (TCA) precipitation
The amounts of intact and degraded radiolabeled antibody in culture supernatants were estimated by TCA precipitation as previously described (see Supplemental Methods Section for details).
Competitive cell binding assay
L363 cells (0.5x106 cells per sample) were plated in a 96-well plate. Serial concentrations of either OKT10-Ab, OKT10 synthetic chemical conjugate (OKT10-CC), OKT10scFv4 fusion protein (OKT10-FP), or isotype control, were added to each well followed by OKT10-APC (0.1µg, Alexa Fluor 647 Protein Labeling Kit from Molecular Probes [Invitrogen, Grand Island, NY]). The cells were incubated at 4°C for 45 minutes, washed and fluorescence intensity was measured on a flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
In vitro binding characterization
The CD38 binding ability of OKT10 was assessed using Ramos targeted cells. Cells (0.1x106 per sample) were incubated with 30µL of 10µg/mL 1F5-Ab (positive control), OKT10-Ab, and BHV1-Ab (non-binding Isotype control) for 30 minutes at 4°C. Cells were washed and incubated with 25µL of 1:64 goat anti-mouse IgG (Fab specific) fluorescein isothiocyanate conjugate (Sigma-Aldrich, St. Louis, MO) in PBS. Cells were washed and fluorescence intensity was measured on a FACS Canto I flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Binding ability of OKT10 to L363 targeted cells was assessed using OKT10-APC.
Cell binding assay
Anti-CD38 (OKT10-FP/OKT10-CC) [20µg/mL] or nonbinding control (CC49-FP/BHV1-CC) [20µg/mL] were added to 1x106 cells pelleted in 96 well round bottomed plates on ice. Pellets were resuspended in the antibody solution, incubated at 4°C for 1hr, washed and resuspended in 200µL DMEM for 24hr and stored at 4°C or 37°C. Cells were pelleted and then resuspended in 25µL of 111In-DOTA-biotin (100 ng/mL), incubated at 4°C for 1hr, washed twice, and activity was measured on a gamma counter.
Mouse RIT and PRIT studies
Female athymic nude-Foxn1nu mice, aged 5 to 6 weeks, were purchased from Harlan Sprague-Dawley (Indianapolis, IN). NCI-H929 and L363 cells (1 x107) were injected subcutaneously into the right flank 9 to 11 days prior to study start dates. Mice bearing palpable plasmacytoma xenografts measuring 100 mm3±10% were selected for the studies and randomly assigned to experimental groups. MM tumor-bearing mice were placed on biotin-free diet for 5 days and injected with either 1.4nmol anti-CD38 OKT10-DOTA Ab or control BHV1-DOTA Ab each directly labeled with 111In, or 1.4nmol of anti-CD38 OKT10 Ab-SA (OKT10-CC or OKT10-FP) or control Ab-SA (BHV1-CC or CC49 [recognizes the irrelevant TAG-72 antigen on human adenocarcinomas]- scFv4SA-FP) followed 22hr later by 5.8nmol (50µg) CA and 2hr later by 1.2nmol (1µg) 111In-DOTA-biotin for biodistributions or 90Y-DOTA-biotin labeled with 400µCi (14.9 MBq), 800µCi (29.6 MBq), or 1200µCi (44.4 MBq) 90Y for therapy studies. Mice were monitored thrice weekly for general appearance, tumor volume measurements, and body weight. Mice were injected with anti-asialoGM1 antiserum (200uL, WAKO, Richmond, VA) 9 days and 5 days prior to the injection of Ab-SA to abrogate natural killer cell activity and prevent spontaneous tumor regressions. Mice were euthanized when tumors reached a maximum bi-directional measurement of ≥20mm×20mm, when tumor ulceration occurred, or when mice lost >30% of baseline body weight, as required by institutional animal care guidelines.
Blood clearance studies
Blood clearance studies were conducted according to the double-label method of Pressman. (31, 32) 131Iodine (131I)-OKT10-CC (1.4nmol) and 125Iodine (125I)-OKT10-FP (1.4nmol) were co-injected into mice via the tail vein (i.v.). NAGB (N-acetyl-galactosamine-biotin) CA (5.8nmol) was injected 24hr later. Venous sampling was conducted via the retro-orbital plexus at serial time points. 125I and 131I were counted on a gamma counter, and the %IDs/g of blood were calculated. Counts were corrected for 131I crossover into the 125I channel. Counts were also corrected for radioactive decay using an aliquot of the injectate.
Dosimetry
Absorbed radiation doses to organs were calculated for 90Y using beta kernel methods for localized beta dosimetry expressly developed for accurately calculating the radiation doses to small organs and tissues of the mouse. (33, 34) These methods account for energy losses by source and take into account the organ self-dose specific absorbed fractions and the beta-particle cross-organ dose contributions. Femoral bone marrow (BM) doses were determined using a model which incorporates Monte Carlo calculations of the energy absorbed fractions in the marrow shafts. (35) This model also accounts for the contributions of 90Y on bone surfaces, if any, that may contribute to BM dose.
Statistical Considerations
Differences in MM tumor xenograft volumes were compared by computing the means and standard deviations of each treatment group and employing Student’s t-test to determine statistical significance. For relatively large differences in tumor volume, 8–10 mice per group were projected to provide adequate power to detect statistically significant differences. Only the detection of large differences between treatment groups was considered to be of clinical interest.
RESULTS
OKT10 anti-CD38 reagents are cell surface stable and enable excellent pretargeting
Experiments assessing binding and internalization of 90Y labeled OKT10-Ab and FP were performed using 4 MM cell lines (L363, NCI-H929, RPMI-8226, MM1R), a CD38-expressing non-Hodgkin’s lymphoma (NHL) line (Ramos), and 4 MM patient biopsy samples. These studies evaluated the degree of internalization, cell surface retention, passive dissociation (“shedding”), intracellular metabolism, and exocytosis of 90Y-labeled metabolites, using flow cytometric, cell binding, and “acid-wash” methods as previously published for other targets.(36, 37) Only a minority (9±3%; Supplemental Figure 1) of CD38-targeted Ab or FP was internalized by L363 MM tumor cells after 24hr (the time interval between the 2 reagents in PRIT). After 24hr, ~60% of initially bound OKT10-Ab remained on the cell surface, while 30–40% dissociated passively from the cell surface into the culture medium. A TCA precipitation assay was used to demonstrate that the radioactivity released into the culture medium reflected passive “shedding” of intact Ab rather than exocytosis of 90Y-labeled small molecular weight fragments from cells (detailed in supplemental materials). To demonstrate effective PRIT targeting of 90Y-DOTA-biotin, MM cells were labeled with Ab-SA, washed and incubated either at 4°C, (a temperature which completely inhibits endocytosis), or at 37°C. After 24hr of incubation, 90Y-DOTA-biotin was added and the amount of radioactivity targeted to MM cells was measured. The difference in cell associated radioactivity was minor, indicating that the magnitude of endocytosis of the 1st step reagent in 24hr was not sufficient to prevent effective targeting of the 2nd step reagent (90Y-DOTA-biotin). Concordant results were seen for 3 other cell lines and the patient samples tested. These conclusions also pertain in vivo since favorable in vivo targeting of 111In-DOTA-biotin to xenografts is observed [Figure 3b–e].
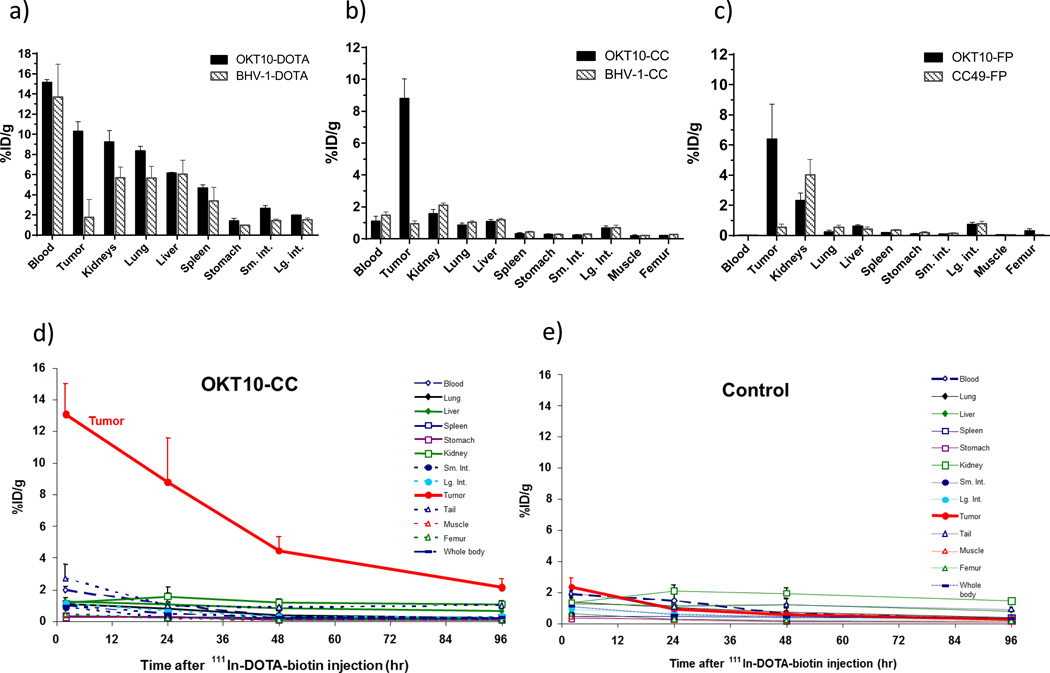
Figure 3.
Biodistributions of radioactivity in athymic nude- Foxnlnu mice (n=5/group) bearing CD38+ MM xenograft tumors (1×107 cells injected into left flank). Blood, tumor and normal organ specimens were obtained 24hr after (a) 111In-OKT10-DOTA or 111In-BHV1-DOTA (control) (b) 111In-DOTA-biotin pretargeted by OKT10-CC or BHV1-CC (control CC) (c) 111In-DOTA-biotin pretargeted by OKT10-FP or CC49-FP (control FP). In PRIT animal studies (b,c) NAGB-clearing agent (5.8 nmol) was administered 2hr before the 111In-DOTA-biotin (1.2 nmol). Comprehensive tissue biodistributions of radioactivity obtained at sequential timepoints 2hr, 24hr, 48hr and 96hr following 111In-DOTA-biotin are shown for the mice from the OKT10-CC (d) and BHV1-CC (e) PRIT groups.
Comparative biodistributions of radioactivity demonstrate OKT10 PRIT improves the therapeutic index
Blood, tumor and nonspecific organ uptake of 1-step directly radiolabeled (conventional) OKT10 (OKT10-DOTA) was compared with multistep PRIT in 6 biodistribution experiments involving athymic nude mice bearing s.c. CD38+ human myeloma tumor xenografts (NCI-H929 and L363). Studies were designed to evaluate the relative merits of two distinct pretargeting constructs, OKT10-CC and OKT10-FP (administered at equimolar concentrations). Sixteen groups of 5 mice each were injected with 1.4nmol of OKT10-DOTA, OKT10-FP or OKT10-CC (including matched antibody isotype or FP matched controls). The PRIT reagents (OKT10-FP or OKT10-CC) were followed, 18–22hr later, by 5.8nmol CA and 2hr thereafter by 1.2nmol 111In-DOTA-biotin (1µg). Groups of animals were euthanized at 2, 24, 48, and 96hr after injection of the radioactivity. Tumors excised from mice pretargeted with OKT10-CC contained 8.8±2.8% of the injected dose of 111In-DOTA-biotin per gram(%ID/g) after 24hr compared to 0.9 ±0.4%ID/g after 24hr in tumors excised from control mice pretargeted with a control Ab-SA chemical conjugate (BHV1-CC; all values are mean ±S.D.) [Figure 3b]. The 24hr tumor-to-normal organ ratios of absorbed radioactivity were 10:1; 8:1; and 6:1 respectively for lung, liver and kidney in mice pretargeted with OKT10-CC; compared to < 1:1 for the lung, liver and kidney in control mice pretargeted with BHV1-CC.
Tumors excised from mice pretargeted with OKT10-FP demonstrated uptakes of radioactivity in tumor sites (6.4%ID/g±2.3% after 24hr) that were similar to those observed with the chemical conjugate while minimal tumor uptakes were seen in the controls treated with CC49-FP (0.55%ID/g±0.21% after 24hr; all values are mean ±S.D.) [Figure 3c]. Tumor-to-organ ratios of absorbed radioactivity with OKT10-FP PRIT were 27:1; 10:1 and 3:1, respectively, for lung, liver and kidney at the 24hr time-point compared to ≤ 1:1 for the same organs in control mice pretargeted with the negative control CC49-FP. The minimal tumor uptake of radiobiotin detected in both the CC49-FP and BHV1-CC control groups demonstrates the specificity of targeting with OKT10-FP and OKT10-CC (Figure 3b,c).
While levels of radioactivity in tumor xenografts measured 24hr after administration of the radioactive species were similar in the OKT10-FP, OKT10-CC and OKT10-DOTA treated mice (6.4±2.3%, 8.8±2.8%ID/g, and 9.4%±2.2% respectively); PRIT (OKT10-CC and OKT10-FP) led to biodistributions of radioactivity that were far superior to the conventional (OKT10-DOTA) approach (Figures 3a–e). At 24hr following injection of the radiolabeled species, the tumor-to-blood ratio for the conventional RIT was <1:1, while for the OKT10-FP and OKT10-CC ratios were 638:1 and 9:1 respectively, confirming the capacity for PRIT to circumvent the tumor-to-normal organ distribution limitations seen with conventional 1-step RIT.(38, 39)
Excellent target specificity was demonstrated for both PRIT constructs. While tumor-to-blood ratios were most dramatic with the FP, this finding appears to be a consequence of very rapid circulatory clearance of the FP (see blood clearance studies below) and did not lead to superior overall biodistributions. For both constructs the kidney represented the normal organ with the largest non-specific radiation uptake. Tumor-to-kidney ratios were 6:1 and 3:1 for the CC and FP respectively 24hr after the 111In-DOTA-biotin infusion. Thus, the kidney was identified as the organ likely to define dose limiting toxicity in high dose therapy settings, and the findings supported an overall advantage for OKT10-CC pretargeting.
Blood clearance studies favor OKT10-CC
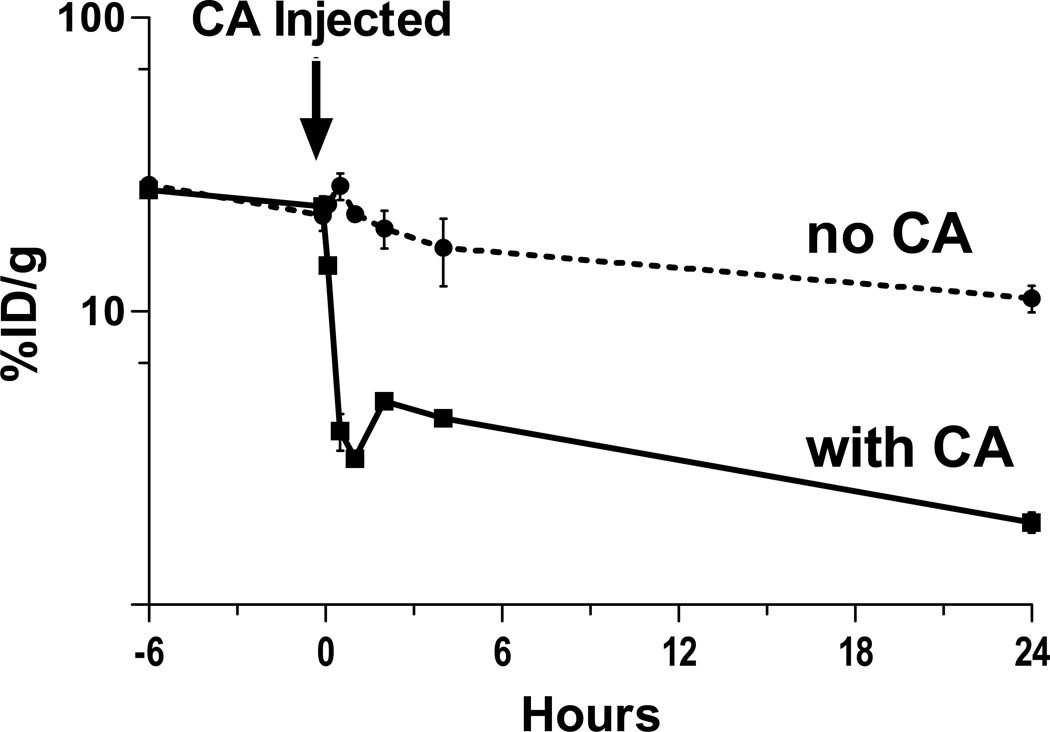
Optimal tumor target accretion in PRIT models requires a sustained concentration of the first step SA reagent in the bloodstream during the biodistribution phase of the treatment algorithm, followed by rapid blood clearance after administration of biotinylated polymeric N-acetyl-galactosamine CA. The rapid clearance of OKT10-CC or FP complexed with CA is mediated by asialoglycoprotein receptors in the liver.(26) Detailed blood clearance studies conducted in athymic mice both when administered separately and co-injected(32) with both 131Iodine (131I )-OKT10-CC and 125Iodine (125I)-OKT10-FP at equimolar concentrations, revealed that 16% of the OKT10-CC remained in the circulation 24hr after injection, compared with only 1.4% of the OKT10-FP (not shown).
Synthetic CA reproducibly removed >85% of circulating OKT10-CC or OKT10-FP from the bloodstream within 60 minutes of administration in 5 blood clearance studies. In a representative experiment CA [5.8nmol] was injected 24hr after 131I-OKT10-CC (300µg) into 5 athymic nude mice (i.v.). Retro-orbital venous sampling was conducted at serial time points up to 24hr and 131I was counted on a γ counter. After CA administration the concentration of 131I-OKT10-CC dropped from 22.8%±3.1%ID/g of blood to 3.2%±0.3%ID/g 60 minutes later [all values are mean ±S.D.] (Figure 4). Consistent with prior studies(27), re-equilibration with conjugate in the extravascular space caused a slight rebound rise of the 131I-OKT10-CC during the subsequent 2hrs (Figure 4).
Figure 4.
Effects of biotinylated N-acetyl galactosamine clearing agent (CA) on circulating OKT10-CC. CA [5.8 nmol] was injected 24hr after 131I-OKT10-CC (1.4 nmol) into 5 athymic nude mice (i.v.). Five control mice received 131I-OKT10-CC (1.4 nmol) and no CA. Retro-orbital venous sampling was conducted at serial time points up to 24 hr.
Organ dosimetry
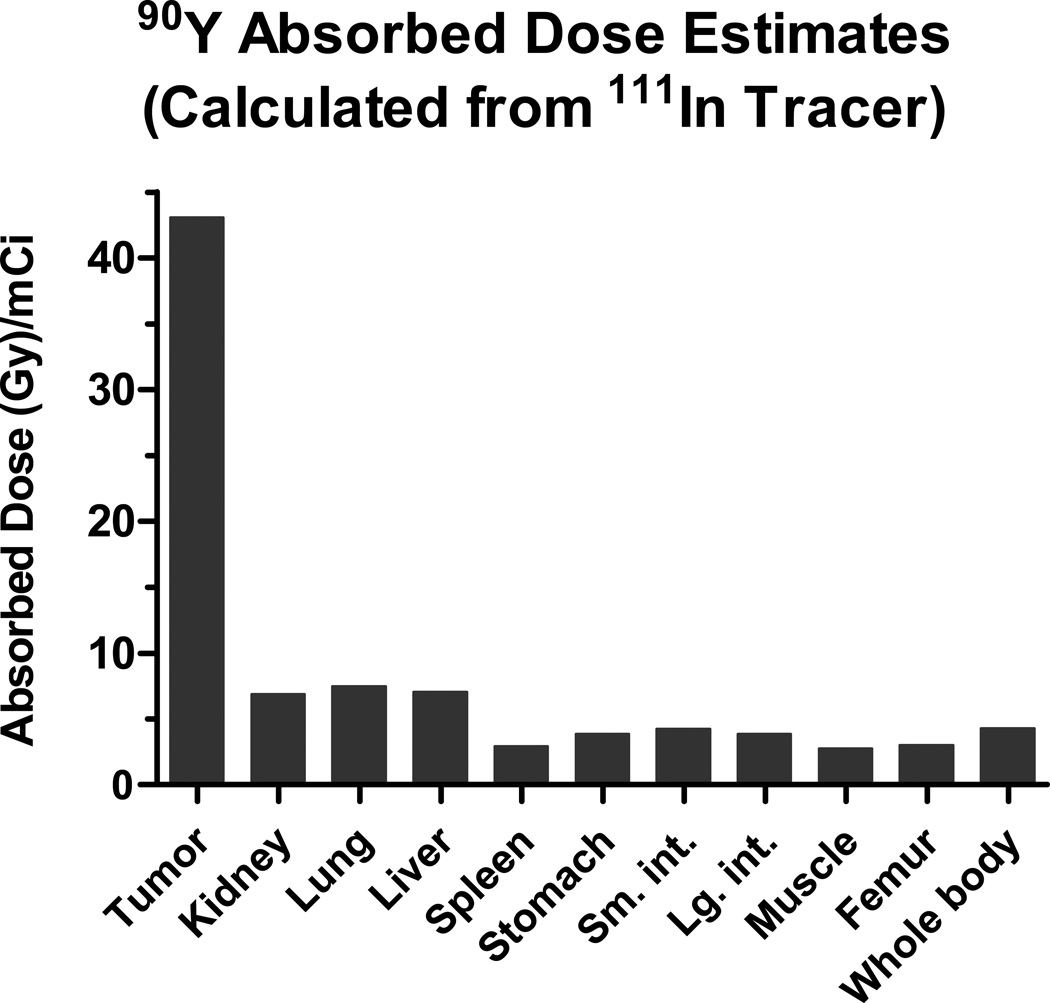
A detailed organ dosimetry analysis was performed to calculate 90Y absorbed dose estimates after OKT10-CC pretargeting (Figure 5). Radiation absorbed doses to tumors, whole body and nine normal tissues were determined based on integrating the areas under time-activity curves constructed by plotting the concentration of 111In-DOTA-biotin in tissues after various intervals of time.(33) The dosimetry method used accounted for organ self-dose absorbed fractions as well as beta-particle cross-organ dose contributions.(33) The 90Y absorbed dose estimates (Gy) per millicurie administered calculated from the 111In tracer generated tumor-to-normal organ ratios of 6:1 for the kidney, lung and liver; 15:1 for the spleen and 10:1 for the whole body.
Figure 5.
90Y absorbed dose estimates (Gy/mCi) from an 111In-DOTA-biotin tracer. Radiation absorbed doses to tumor, whole body and normal tissues are estimated by integrating the areas under time-activity curves constructed by plotting the concentration of 111In-DOTA-biotin (after OKT10-CC pretargeting) measured in tissues by γ-counting at 2, 24, 48 and 96hr.(33)
Therapy studies
90Y-DOTA-biotin pretargeted to CD38 eradicates plasmacytomas in vivo
Therapy studies were performed in athymic mice (n=8–10/group) bearing s.c. xenografts of either L363 or NCI-H929 malignant plasma cells, producing tumors similar to human plasmacytomas. Reagent concentrations and time-points for administration of OKT10-CC, BHV1-CC and CA were identical to those reported for the biodistribution studies.
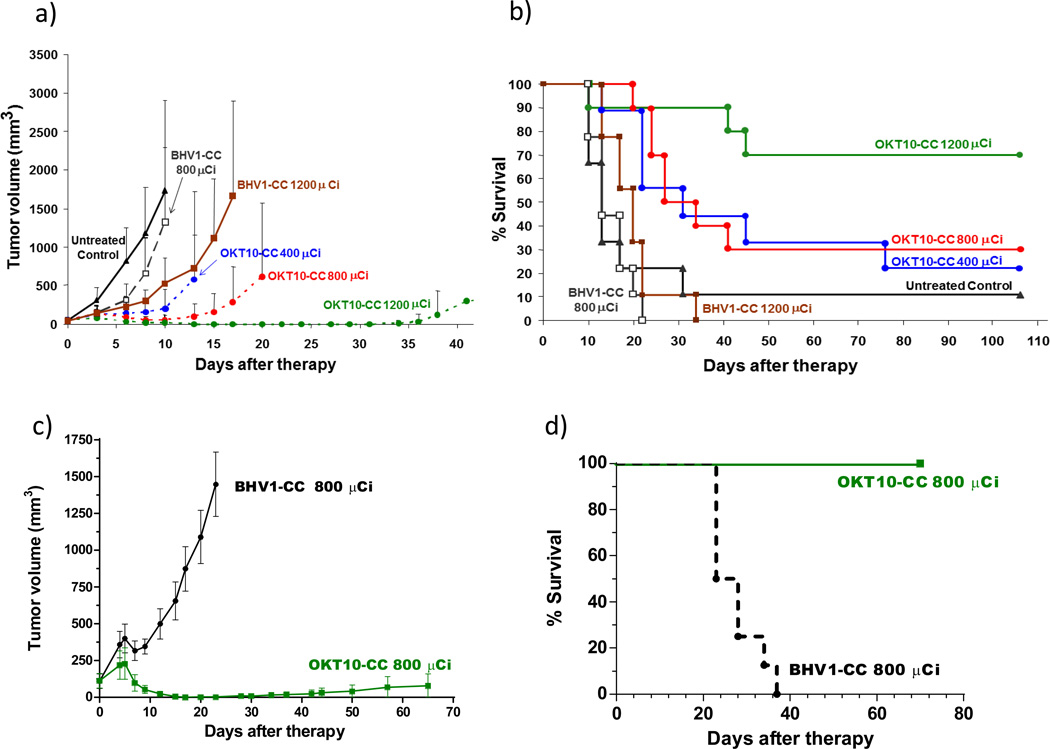
90Y-DOTA-biotin (2µg) was labeled with 400, 800, or 1200µCi per mouse in 3 OKT10-CC groups and 3 control groups (untreated control; 800 or 1200µCi 90Y-DOTA-biotin after BHV1-CC). All L363 tumor xenograft mice in the untreated control and BHV1-CC control groups experienced exponential MM tumor growth and 78% of the untreated control animals required euthanasia within 17 days (Figure 6a). After 22 days, 100% of the 800µCi and 89% of 1200µCi BHV1-CC control groups had required euthanasia due to progressive tumor growth. All mice pretargeted with OKT10-CC prior to 90Y-DOTA-biotin demonstrated tumor shrinkage by day 6 at all dose levels (Figure 6a). After 20 days, 90% of the OKT10-CC treated animals in the 400, 800 and 1200µCi groups remained alive. One animal treated with 1200 µCi was euthanized on day 10 due to weight loss (as required by institutional animal care guidelines); however the other 9 animals from that group were healthy, weighing 106±9%(S.D.) of initial body weight on day 17 (Supplemental Figure 2). Objective remissions were observed within 6 days in 100% of the mice treated with OKT10-CC followed by 1200µCi of 90Y-DOTA-biotin, including 100% complete remissions (no detectable tumor in OKT10-CC treated mice compared to tumors that were 5240±2495% of initial tumor volume in untreated control animals) by day 17 (p< 0.0001, Student’s t-test) [Figure 6a]. After 100 days, 70% of the OKT10-CC treated animals in the 1200µCi group, 30% in the 800µCi group and 20% in the 400µCi group remained alive and tumor-free (Figure 6b). In mice bearing NCI-H929 MM xenograft tumors, 800µCi pretargeted to OKT10-CC was sufficient to eradicate 100% of tumor xenografts within 23 days. At the identical time point, tumors progressed in all BHV1-CC treated controls receiving 800µCi. Control tumors were 2982±2834% of the initial tumor volume (p<0.0001, Student’s t-test) [Figure 6c]. Seventy days after treatment with 800µCi of anti-CD38 pretargeted 90Y-DOTA-biotin, 100% of animals initially bearing NCI-H929 MM tumor xenografts were alive compared with no mice surviving in the control group [Figure 6d]. Hepatic and renal function was assessed through measurement of serum transaminases, blood urea nitrogen and creatinine levels 160 days after mice received 90Y-DOTA-biotin (800µCi, n=5). No significant toxicity was observed. The measured serum creatinine was <0.4mg/dl in all animals, the average BUN, ALT and AST values were 48±33mg/dl, 46.8±40 and 120.4±116.7U/L respectively.
Figure 6.
Tumor responses in athymic nude-mice (n=8–10/group) bearing L363 (a, b) or NCI-H929 (c, d) right flank MM xenografts. Mice received 1.4 nmol (300 µg) of OKT10-CC or BHV1-CC (control) and CA [5.8 nmol (50 µg)]. Treatment groups received 90Y-DOTA-biotin 24hr after the OKT10-CC at doses of 400 µCi (14.9 MBq), 800 µCi (29.6 MBq) & 1200 µCi (44.4MBq); and at doses of 800 µCi and 1200 µCi for the BHV1-CC control groups. (a) Mice were monitored thrice weekly for tumor volume measurements and were euthanized when tumors were ≥10% of body weight or when ulceration occurred as mandated by the institutional animal care committee. (Curves are truncated at the time the first animal was euthanized in each group). A dose dependent regression of MM tumor xenografts is demonstrated. (b) Kaplan-Meier analysis of cumulative survival of mice bearing L363 MM xenografts. (c) NCI-H929 treatment groups received 800 µCi (29.6 MBq) of 90Y-DOTA-biotin 24hr after the OKT10-CC or BHV1-CC (control group). Complete regression of MM tumor xenografts in the OKT10-CC group is demonstrated. (d) Kaplan-Meier survival analysis of mice bearing NCI-H929 MM xenografts.
Tumor-to-normal organ ratios from the PRIT biodistribution studies described above were markedly superior to conventional RIT and provided the rationale for dose escalation up to 1200µCi in PRIT therapy studies. In contrast to the efficacy and tolerability demonstrated with anti-CD38 PRIT, conventionally radiolabeled 90Y-OKT10-DOTA administered as a single step to athymic nude mice (n=10/group) bearing s.c. NCI-H929 tumor xenografts was not well tolerated. Within 15 days of infusion, 100% of animals receiving 400µCi of 90Y-OKT10-DOTA died from complications of radiation toxicity (weight loss, thrombocytopenia [petechiae], and failure to thrive). On day 8, these animals weighed 79%±9% of their initial body weight. When mice were treated with 200µCi of 90Y-OKT10-DOTA, all tumor xenografts transiently decreased in size, but 6 of 10 animals exhibited tumor progression by day 40 and one died of radiation toxicity on day 19. All animals in the untreated and 90Y-BHV1-DOTA control groups (200 and 400µCi) had either died of tumor progression or radiation toxicity (60%) or demonstrated tumor growth by day 40 (not shown).
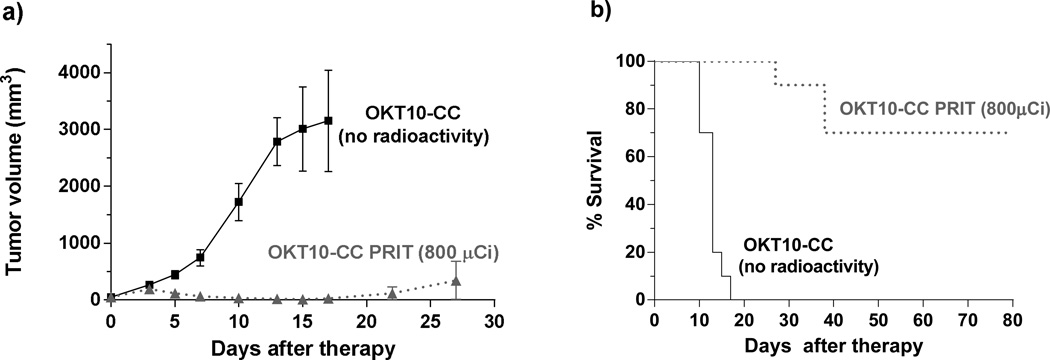
To assess if any of the observed anti-tumor activity is attributable to the CD38 mAb in the absence of radiolabeled DOTA-biotin, mice bearing L363 MM xenografts (n=10) were treated with OKT10-CC administered as a single agent without subsequent 90Y-DOTA-biotin infusion. These animals treated with OKT10-CC alone demonstrated no tumor response and 100% required euthanasia by day 17 for tumors that were 7837±3492% of their initial volume. In contrast, a matched cohort of mice (n=10) treated concurrently with identical doses of OKT10-CC followed by CA and 90Y-DOTA-biotin (800µCi) demonstrated complete remissions by day 17 in 100% of animals and 70% of these animals were alive and tumor free at day 80 [Figure 7a,b].
Figure 7.
Tumor responses in athymic nude-mice (n=10/group) bearing L363 right flank MM xenografts. All mice received 1.4 nmol (300 µg) of OKT10-CC as a single agent (no radioactivity) or followed by CA [5.8 nmol (50 µg)] and 90Y-DOTA-biotin (800 µCi [29.6 MBq]) 24hr after the OKT10-CC. (a) Mice were monitored thrice weekly for tumor volume measurements and were euthanized when tumors were ≥10% of body weight or when ulceration occurred as mandated by the institutional animal care committee. (Curves are truncated at the time the first animal was euthanized in each group). Complete regression of MM tumor xenografts in the group receiving OKT10-CC followed by 800 µCi (29.6 MBq) is demonstrated. Animals receiving OKT10-CC as a single agent (no radioactivity) demonstrated rapid tumor progression. (b) Kaplan-Meier analysis of cumulative survival of mice bearing L363 MM xenografts.
DISCUSSION
Plasma cell malignancies, including MM, are rarely cured. Despite the higher response rates and longer survival afforded by novel agents (2) the vast majority of patients with plasma cell malignancies die of progressive disease emphasizing the importance of continuing to investigate new treatment strategies. RIT is a therapeutic modality that is not cross-resistant with chemotherapy or “novel agents” and has been under-explored in this disease.
External beam radiation can cure isolated malignant plasmacytomas and radiation therapy is frequently used to palliate painful MM bone lesions. Sustained local disease control and durable symptom relief has been reported for 98% of lesions receiving >10Gy. (40) Further, the poor prognostic implications associated with high risk bone marrow cytogenetics in active MM are not predictive of a decrement in the very high rates of local control and cure after external beam radiation therapy is used to treat solitary extramedullary plasmacytomas with the same cytogenetic derangements. (41) This suggests that the unique attributes associated with the targeted delivery of radiation may augur clinical efficacy even among patients classified as “high risk”. When ionizing radiation has been combined with bortezomib, synergistic inhibition of MM cell proliferation has been reported. (12) It has also been suggested that the efficacy of bortezomib can be improved when combined with skeletally targeted 153samarium lexidronam. (42) These findings raise the possibility that anti-CD38 RIT may be enhanced when combined with novel therapies.
Although several plasma-cell associated antigens have been identified, we elected to target CD38 because it has a high density and consistency of expression on clonal plasma cells. (15, 16) We demonstrate that the receptor has minimal internalization and is not shed from the plasma cell surface after binding to OKT10. The unmodified CD38 mAb daratumumab, which binds a unique epitope on the antigen receptor, has demonstrated anti-MM tumor cell activity both in vitro and in mouse xenografts; (19) however, perhaps as a consequence of binding a different epitope, OKT10-CC administered as a single agent without radioactivity demonstrates no anti-tumor effects. The promising results achieved with daratumomab however, complement our approach targeting CD38 for radionuclide delivery and validate the antigen as a desirable target. Despite the salient favorable attributes enumerated for CD38, no target antigen is perfect and it must be acknowledged that CD38 expression is not limited exclusively to malignant plasma cells. Nevertheless the expression of CD38 on normal plasma cells and some activated lymphoid cells must be viewed in the context of the successful selection strategies which have resulted in clinically approved RIT therapeutics for other hematologic malignancies, such as 131Iodine-tositumomab (Bexxar) and 90Yttrium-ibritumomab tiuxetan (Zevalin). The efficacy and favorable toxicity profiles of anti-CD20 RIT in B cell lymphoma have been clearly established despite expression of this antigen on normal B cells. (5–7) The CD38 antigen has many attributes parallel to CD20 to support its selection as viable target for RIT.
Several therapeutic radionuclides might be considered for RIT of MM. We selected 90Y as the radioisotope for these studies because it is a pure, high-energy β-emitter that is readily complexed by the DOTA chelate and is commercially available in high specific activity and purity. The long path length of 90Y’s β particles permits the delivery of lethal radiation across several cell diameters, resulting in a “crossfire effect” that can eradicate antigen-negative MM cells (or MM cells that are in the center of a tumor cluster) by lethal radiation emitted from surrounding antibody-binding CD38+ MM cells. Furthermore, the current biotin reagents employed for PRIT can only accommodate radiometals, not 131I, and attempts to produce effective 131I-labeled biotin have generally been disappointing, re-enforcing the selection of 90Y.
In this report, we demonstrate that the 90Y-based anti-CD38 PRIT approach chosen, rapidly and completely eradicates plasmacytoma xenografts with minimal toxicity. This therapeutic efficacy was predicted by anti-CD38 PRIT biodistributions that were markedly superior to those achievable by conventional RIT, as confirmed by radiation dosimetry estimates demonstrating excellent tumor-to-normal organ ratios. While both anti-CD38-SA constructs were effective, the rapid circulatory clearance of OKT10-FP resulted in a lower AUC during the distribution phase; and, despite excellent blood-to-tumor ratios of activity, the FP had lower tumor-to-kidney ratios of absorbed activity (3:1) when compared with OKT10-CC PRIT (6:1) after 24hr. Consistent with prior published reports, biodistribution studies predicted the kidney to be the dose limiting normal organ.(43) The renal biodistribution profile for the chemical conjugate therefore provided a rationale for selection of OKT10-CC as the best pretargeting reagent for subsequent therapy studies.
PRIT enables the administration of 90Y doses not attainable using conventional one-step RIT and appears to improve the therapeutic index, enhance efficacy and diminish toxicity. Removal of excess OKT10-CC from the vascular compartment by CA, combined with rapid urinary excretion of excess 90Y-DOTA-biotin, limited nonspecific radiation exposure to normal organs even at doses of 1200µCi (three times the previously defined lethal doses in conventional 90Y labeled RIT).(44) Ninety percent of animals in the highest dose group demonstrated only mild transient weight loss, and regained their normal weight within 15 days (Supplemental Figure 2), while animals receiving either 400 or 800µCi demonstrated no significant drop in weight. Among the OKT10-CC animals alive and disease free >70 days after therapy, no significant toxicity was detectable.
We elected to initiate preclinical studies of CD38-targeted RIT and PRIT using a plasmacytoma model because traditional radiation dosimetry and biodistribution methods can be most accurately performed using solid tumor nodules, and are much more difficult to apply in a quantitative fashion using marrow-based, disseminated disease. Tumor xenografts are therefore the standard for initial RIT validation studies (e.g. CD20 in NHL, CD45 in AML). (27) (45–48) In addition, xenograft models have played a vital role in validating the impressive efficacy of proteasome inhibitor therapy in MM and we have emulated this initial approach. (49)
Despite serving as a well validated model for the assessment of new RIT targets and MM therapeutics in large cohorts of animals, MM tumor xenografts do not fully recapitulate the clinical spectrum of the disease. (49, 50) The absence of human CD38 antigen expression in murine tissues is an inherent limitation of most xenograft models, and the xenograft model applies most directly to soft tissue plasmacytomas rather than disseminated MM. Furthermore xenografts do not provide a bone based platform that recapitulates the human marrow microenvironment. To address this concern we are conducting studies in a SCID-hu MM mouse model and have seen preliminary results that are encouraging, but beyond the scope of the current manuscript.
In conclusion, this is the first report of anti-CD38 RIT in MM and the first report documenting the efficacy of PRIT in this disease. The complete eradication of MM tumor xenografts provides a scientifically compelling rationale for further exploring high dose anti-CD38 PRIT as conditioning therapy prior to autologous stem cell transplant for MM. While xenograft tumors are not ideal, they have been used to accurately predict the value of CD20 and CD45 directed RIT or PRIT in lymphoma and leukemia mouse models, and have been used to establish the value of “novel agents” to treat MM despite the same limitations. Ultimately, clinical trials will be necessary to demonstrate safety and evaluate the potential for anti-CD38 PRIT to improve progression-free and long term survival among patients with MM.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by grants from the US National Institutes of Health NCI K08 CA151682 (D.J.G.); NCI R01 CA154897 (O.W.P.), NCI R01 CA076287 (O.W.P); Multiple Myeloma Research Foundation Research Fellow Award (D.J.G.); The Orin Edson Foundation (D.J.G. and O.W.P.); A.K.G. is a Clinical Research Scholar of the Leukemia and Lymphoma Society.
Footnotes
Disclosure of COI: The authors have no conflicts of interest to disclose.
Author Contributions:
D.J.G. designed experiments, performed experiments, wrote and revised the manuscript, analyzed results and designed the figures; N.N.O. performed experiments, analyzed results and contributed to figures; J.C.J. performed experiments and analyzed results; M.D.H. performed experiments and analyzed results; J.M.P. analyzed results; D.K.H. and D.S.W. produced essential reagents and analyzed results; Y.L. performed experiments, edited the manuscript and produced essential reagents; D.R.F. performed dosimetry analysis; A.L.K. and S.L.F. performed experiments; A.K.G. analyzed results and edited the manuscript; J.J.O. performed experiments and edited the manuscript; T.A.G. provided statistical support; B.L.W. performed experiments; W.I.B. analyzed results; O.W.P. designed experiments, performed experiments, analyzed results, revised the manuscript and edited figures.
References
- 1.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson KC. The 39th David A. Karnofsky Lecture: bench-to-bedside translation of targeted therapies in multiple myeloma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:445–452. doi: 10.1200/JCO.2011.37.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pilarski LM, Hipperson G, Seeberger K, Pruski E, Coupland RW, Belch AR. Myeloma progenitors in the blood of patients with aggressive or minimal disease: engraftment and self-renewal of primary human myeloma in the bone marrow of NOD SCID mice. Blood. 2000;95:1056–1065. [PubMed] [Google Scholar]
- 4.Matsui W, Wang Q, Barber JP, Brennan S, Smith BD, Borrello I, et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer research. 2008;68:190–197. doi: 10.1158/0008-5472.CAN-07-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nademanee A, Forman S, Molina A, Fung H, Smith D, Dagis A, et al. A phase 1/2 trial of high-dose yttrium-90-ibritumomab tiuxetan in combination with high-dose etoposide and cyclophosphamide followed by autologous stem cell transplantation in patients with poor-risk or relapsed non-Hodgkin lymphoma. Blood. 2005;106:2896–2902. doi: 10.1182/blood-2005-03-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Press OW, Eary JF, Appelbaum FR, Martin PJ, Badger CC, Nelp WB, et al. Radiolabeled-antibody therapy of B-cell lymphoma with autologous bone marrow support. The New England journal of medicine. 1993;329:1219–1224. doi: 10.1056/NEJM199310213291702. [DOI] [PubMed] [Google Scholar]
- 7.Kaminski MS, Tuck M, Estes J, Kolstad A, Ross CW, Zasadny K, et al. 131I-tositumomab therapy as initial treatment for follicular lymphoma. The New England journal of medicine. 2005;352:441–449. doi: 10.1056/NEJMoa041511. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S. Solitary plasmacytoma: is radiation therapy sufficient? Am J Hematol. 2008;83:695–696. doi: 10.1002/ajh.21248. [DOI] [PubMed] [Google Scholar]
- 9.Gluck S, Van Dyk J, Messner HA. Radiosensitivity of human clonogenic myeloma cells and normal bone marrow precursors: effect of different dose rates and fractionation. International journal of radiation oncology, biology, physics. 1994;28:877–882. doi: 10.1016/0360-3016(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 10.Giralt S, Bensinger W, Goodman M, Podoloff D, Eary J, Wendt R, et al. 166Ho-DOTMP plus melphalan followed by peripheral blood stem cell transplantation in patients with multiple myeloma: results of two phase 1/2 trials. Blood. 2003;102:2684–2691. doi: 10.1182/blood-2002-10-3250. [DOI] [PubMed] [Google Scholar]
- 11.Rousseau C, Ferrer L, Supiot S, Bardies M, Davodeau F, Faivre-Chauvet A, et al. Dosimetry results suggest feasibility of radioimmunotherapy using anti-CD138 (B-B4) antibody in multiple myeloma patients. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2012;33:679–688. doi: 10.1007/s13277-012-0362-y. [DOI] [PubMed] [Google Scholar]
- 12.Goel A, Dispenzieri A, Geyer SM, Greiner S, Peng KW, Russell SJ. Synergistic activity of the proteasome inhibitor PS-341 with non-myeloablative 153-Sm-EDTMP skeletally targeted radiotherapy in an orthotopic model of multiple myeloma. Blood. 2006;107:4063–4070. doi: 10.1182/blood-2005-09-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prashant Kapoor PTG, Morice William G, Rajkumar S Vincent, Witzig Thomas E, Greipp Philip R. Anti-CD20 monoclonal antibody therapy in multiple myeloma. British journal of haematology. 2008;141:135–148. doi: 10.1111/j.1365-2141.2008.07024.x. [DOI] [PubMed] [Google Scholar]
- 14.Supiot S, Faivre-Chauvet A, Couturier O, Heymann MF, Robillard N, Kraeber-Bodere F, et al. Comparison of the biologic effects of MA5 and B-B4 monoclonal antibody labeled with iodine-131 and bismuth-213 on multiple myeloma. Cancer. 2002;94:1202–1209. doi: 10.1002/cncr.10286. [DOI] [PubMed] [Google Scholar]
- 15.Deaglio S, Aydin S, Vaisitti T, Bergui L, Malavasi F. CD38 at the junction between prognostic marker and therapeutic target. Trends Mol Med. 2008;14:210–218. doi: 10.1016/j.molmed.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Bataille R, Jego G, Robillard N, Barille-Nion S, Harousseau JL, Moreau P, et al. The phenotype of normal, reactive and malignant plasma cells. Identification of "many and multiple myelomas" and of new targets for myeloma therapy. Haematologica. 2006;91:1234–1240. [PubMed] [Google Scholar]
- 17.Fernandez JE, Deaglio S, Donati D, Beusan IS, Corno F, Aranega A, et al. Analysis of the distribution of human CD38 and of its ligand CD31 in normal tissues. Journal of biological regulators and homeostatic agents. 1998;12:81–91. [PubMed] [Google Scholar]
- 18.Su AWC, MacLeod M. BioGPS. 2012 May 9; http://biogps.org/#goto=genereport&id=952.
- 19.de Weers M, Tai Y-T, van der Veer MS, Bakker JM, Vink T, Jacobs DCH, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. The Journal of Immunology. 2011;186:1840–1848. doi: 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- 20.Tesar M. Fully human antibody MOR202 against CD38 for the treatment of multiple myeloma and other blood-bourne malignancies. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:8106. [Google Scholar]
- 21.Vooijs WC, Schuurman HJ, Bast EJ, de Gast GC. Evaluation of CD38 as target for immunotherapy in multiple myeloma. Blood. 1995;85:2282–2284. [PubMed] [Google Scholar]
- 22.Goldmacher VS, Bourret LA, Levine BA, Rasmussen RA, Pourshadi M, Lambert JM, et al. Anti-CD38-blocked ricin: an immunotoxin for the treatment of multiple myeloma. Blood. 1994;84:3017–3025. [PubMed] [Google Scholar]
- 23.Bolognesi A, Polito L, Farini V, Bortolotti M, Tazzari PL, Ratta M, et al. CD38 as a target of IB4 mAb carrying saporin-S6: design of an immunotoxin for ex vivo depletion of hematological CD38+ neoplasia. J Biol Regul Homeost Agents. 2005;19:145–152. [PubMed] [Google Scholar]
- 24.Goldenberg DM, Chang CH, Sharkey RM, Rossi EA, Karacay H, McBride W, et al. Radioimmunotherapy: is avidin-biotin pretargeting the preferred choice among pretargeting methods? Eur J Nucl Med Mol Imaging. 2003;30:777–780. doi: 10.1007/s00259-002-1089-6. [DOI] [PubMed] [Google Scholar]
- 25.Forero A, Weiden PL, Vose JM, Knox SJ, LoBuglio AF, Hankins J, et al. A Phase I trial of a novel anti-CD20 fusion protein in pretargeting radioimmunotherapy for B cell non-Hodgkin's lymphoma. Blood. 2004;104:227–236. doi: 10.1182/blood-2003-09-3284. [DOI] [PubMed] [Google Scholar]
- 26.Axworthy DB, Fritzberg AR, Hylarides MD, Mallett RW, Theodore LJ, Gustavson LM, et al. Preclinical evaluation of an anti-tumor monoclonal antibody/streptavidin conjugate for pretargeted Y-90 radioimmunotherapy in a mouse xenograft model. J Immunother. 1994;16 [Google Scholar]
- 27.Press OW, Corcoran M, Subbiah K, Hamlin DK, Wilbur DS, Johnson T, et al. A Comparative Evaluation of Conventional and Pretargeted Radioimmunotherapy of CD20-expressing Lymphoma Xenografts. Blood. 2001;98:2535–2543. doi: 10.1182/blood.v98.8.2535. [DOI] [PubMed] [Google Scholar]
- 28.Graves SS, Dearstyne E, Lin Y, Zuo Y, Sanderson J, Schultz J, et al. Combination Therapy with Pretarget CC49 Radioimmunotherapy and Gemcitabine Prolongs Tumor Doubling Time in a Murine Xenograft Model of Colon Cancer More Effectively Than Either Monotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:3712–3721. [PubMed] [Google Scholar]
- 29.Schultz J, Lin Y, Sanderson J, Zuo Y, Stone D, Mallett R, et al. A tetravalent single-chain antibody-streptavidin fusion protein for pretargeted lymphoma therapy. Cancer research. 2000;60:6663–6669. [PubMed] [Google Scholar]
- 30.Mirzadeh S, Brechbiel MW, Atcher RW, Gansow OA. Radiometal labeling of immunoproteins: covalent linkage of 2-(4- isothiocyanatobenzyl)diethylenetriaminepentaacetic acid ligands to immunoglobulin. Bioconjugate chemistry. 1990;1:59–65. doi: 10.1021/bc00001a007. [DOI] [PubMed] [Google Scholar]
- 31.Park SI, Shenoi J, Frayo SM, Hamlin DK, Lin Y, Wilbur DS, et al. Pretargeted radioimmunotherapy using genetically engineered antibody-streptavidin fusion proteins for treatment of non-hodgkin lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:7373–7382. doi: 10.1158/1078-0432.CCR-11-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pressman D, Day ED, Blau M. The Use of Paired Labeling in the Determination of Tumor-localizing Antibodies. Cancer research. 1957;17:845–850. [PubMed] [Google Scholar]
- 33.Fisher DR, Shen S, Meredith RF. MIRD Dose Estimate Report No. 20: Radiation Absorbed-Dose Estimates for 111In- and 90Y-Ibritumomab Tiuxetan. J Nucl Med. 2009 doi: 10.2967/jnumed.108.057331. [DOI] [PubMed] [Google Scholar]
- 34.Hui TE, Fisher DR, Kuhn JA, Williams LE, Nourigat C, Badger CC, et al. A mouse model for calculating cross-organ beta doses from yttrium-90- labeled immunoconjugates. Cancer. 1994;73:951–957. doi: 10.1002/1097-0142(19940201)73:3+<951::aid-cncr2820731330>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 35.Beatty BG, Kuhn JA, Hui TE, Fisher DR, Williams LE, Beatty JD. Application of the cross-organ beta dose method for tissue dosimetry in tumor-bearing mice treated with a 90Y-labeled immunoconjugate. Cancer. 1994;73:958–965. doi: 10.1002/1097-0142(19940201)73:3+<958::aid-cncr2820731331>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 36.Press OW, Shan D, Howell-Clark J, Eary J, Appelbaum FR, Matthews D, et al. Comparative metabolism and retention of iodine-125, yttrium-90, and indium-111 radioimmunoconjugates by cancer cells. Cancer research. 1996;56:2123–2129. [PubMed] [Google Scholar]
- 37.Press OW, Howell-Clark J, Anderson S, Bernstein I. Retention of B-cell-specific monoclonal antibodies by human lymphoma cells. Blood. 1994;83:1390–1397. [PubMed] [Google Scholar]
- 38.Paganelli G, Grana C, Chinol M, Cremonesi M, De Cicco C, De Braud F, et al. Antibody-guided three-step therapy for high grade glioma with yttrium- 90 biotin. Eur J Nucl Med. 1999;26:348–357. doi: 10.1007/s002590050397. [DOI] [PubMed] [Google Scholar]
- 39.DeNardo DG, Xiong CY, Shi XB, DeNardo GL, DeNardo SJ. Anti-HLA-DR/anti-DOTA diabody construction in a modular gene design platform: bispecific antibodies for pretargeted radioimmunotherapy. Cancer biotherapy & radiopharmaceuticals. 2001;16:525–535. doi: 10.1089/10849780152752128. [DOI] [PubMed] [Google Scholar]
- 40.Leigh BR, Kurtts TA, Mack CF, Matzner MB, Shimm DS. Radiation therapy for the palliation of multiple myeloma. International Journal of Radiation Oncology*Biology*Physics. 1993;25:801–804. doi: 10.1016/0360-3016(93)90308-i. [DOI] [PubMed] [Google Scholar]
- 41.Bink K, Haralambieva E, Kremer M, Ott G, Beham-Schmid C, de Leval L, et al. Primary extramedullary plasmacytoma: similarities with and differences from multiple myeloma revealed by interphase cytogenetics. Haematologica. 2008;93:623–626. doi: 10.3324/haematol.12005. [DOI] [PubMed] [Google Scholar]
- 42.Berenson JR, Yellin O, Patel R, Duvivier H, Nassir Y, Mapes R, et al. A phase I study of samarium lexidronam/bortezomib combination therapy for the treatment of relapsed or refractory multiple myeloma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:1069–1075. doi: 10.1158/1078-0432.CCR-08-1261. [DOI] [PubMed] [Google Scholar]
- 43.Green DJ, Pagel JM, Nemecek ER, Lin Y, Kenoyer A, Pantelias A, et al. Pretargeting CD45 enhances the selective delivery of radiation to hematolymphoid tissues in nonhuman primates. Blood. 2009;114:1226–1235. doi: 10.1182/blood-2009-03-210344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subbiah K, Hamlin DK, Pagel J, Wilbur DS, Meyer DL, Axworthy DB, et al. Comparative immunoscintigraphy, toxicity, and efficacy of conventional and pretargeted radioimmunotherapy in a CD20-expressing human lymphoma xenograft model. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2003;44:437–445. [PubMed] [Google Scholar]
- 45.Pagel JM, Orgun N, Hamlin DK, Wilbur DS, Gooley TA, Gopal AK, et al. A comparative analysis of conventional and pretargeted radioimmunotherapy of B-cell lymphomas by targeting CD20, CD22, and HLA-DR singly and in combinations. Blood. 2009;113:4903–4913. doi: 10.1182/blood-2008-11-187401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma D, McDevitt MR, Barendswaard E, Lai L, Curcio MJ, Pellegrini V, et al. Radioimmunotherapy for model B cell malignancies using 90Y-labeled anti-CD19 and anti-CD20 monoclonal antibodies. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2002;16:60–66. doi: 10.1038/sj.leu.2402320. [DOI] [PubMed] [Google Scholar]
- 47.Tuscano JM, O'Donnell RT, Miers LA, Kroger LA, Kukis DL, Lamborn KR, et al. Anti-CD22 ligand-blocking antibody HB22.7 has independent lymphomacidal properties and augments the efficacy of 90Y-DOTA-peptide-Lym-1 in lymphoma xenografts. Blood. 2003;101:3641–3647. doi: 10.1182/blood-2002-08-2629. [DOI] [PubMed] [Google Scholar]
- 48.Sharkey RM, Karacay H, Litwin S, Rossi EA, McBride WJ, Chang CH, et al. Improved therapeutic results by pretargeted radioimmunotherapy of non-Hodgkin's lymphoma with a new recombinant, trivalent, anti-CD20, bispecific antibody. Cancer research. 2008;68:5282–5290. doi: 10.1158/0008-5472.CAN-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LeBlanc R, Catley LP, Hideshima T, Lentzsch S, Mitsiades CS, Mitsiades N, et al. Proteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model. Cancer research. 2002;62:4996–5000. [PubMed] [Google Scholar]
- 50.Lentzsch S, LeBlanc R, Podar K, Davies F, Lin B, Hideshima T, et al. Immunomodulatory analogs of thalidomide inhibit growth of Hs Sultan cells and angiogenesis in vivo. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2003;17:41–44. doi: 10.1038/sj.leu.2402745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.