Abstract
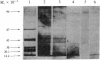
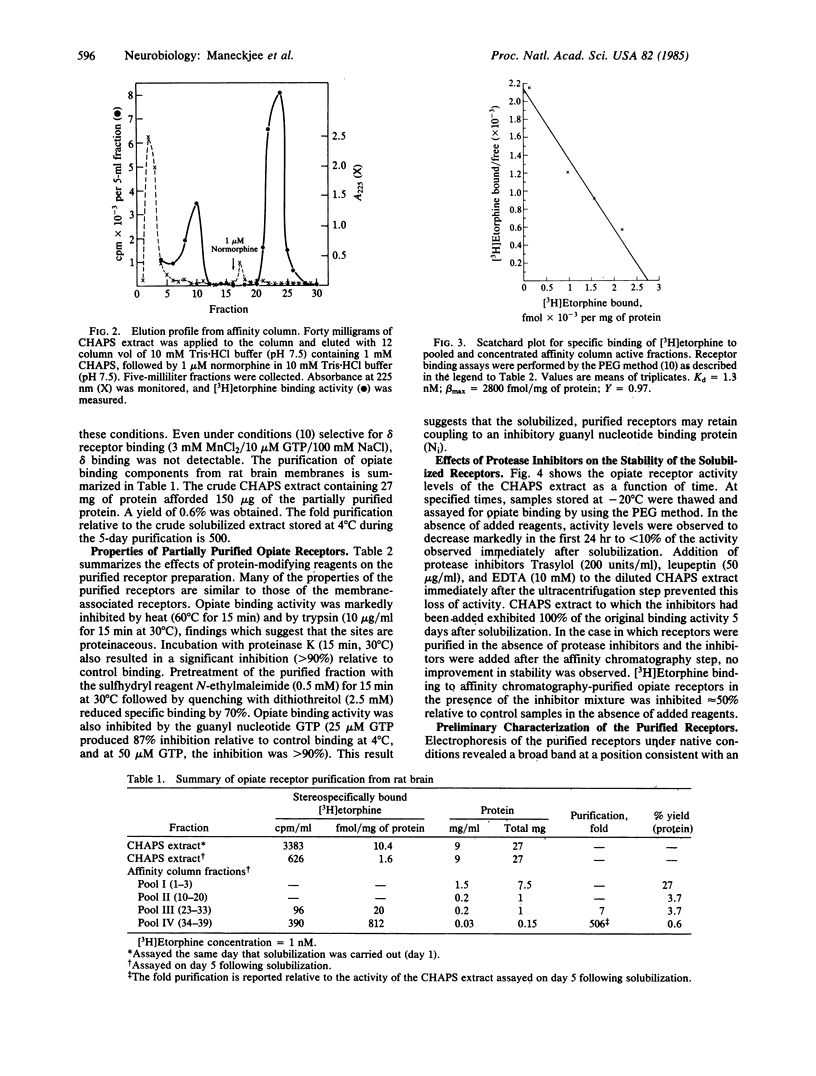
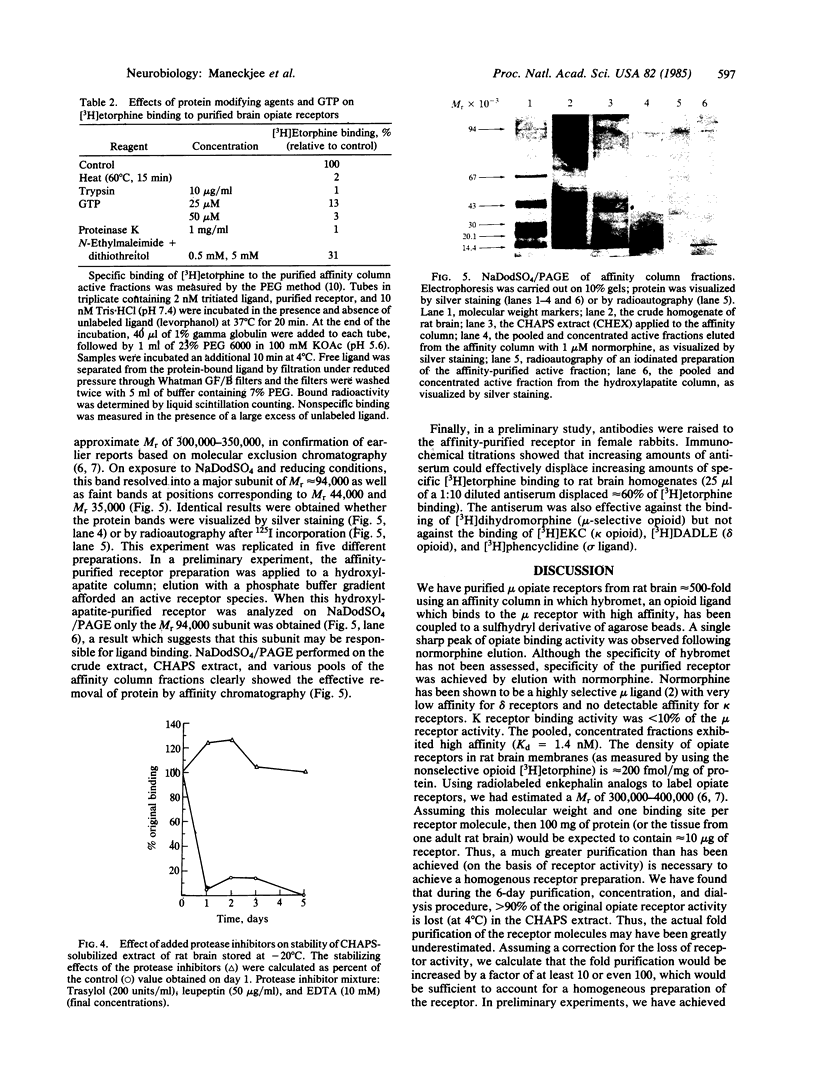
Opiate receptors have been solubilized from rat neural membranes and purified 500-fold (relative to the crude solubilized extract) by affinity chromatography. Active receptors were solubilized by using 3-[( 3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), a zwitterionic derivative of cholic acid. Affinity chromatography was carried out using Affi-Gel 401, a sulfhydryl derivative of agarose to which "hybromet," a newly synthesized opioid ligand with high affinity for the mu receptor, had been attached. Scatchard analysis of [3H]etorphine binding to the purified receptor revealed a single class of high-affinity sites (Kd = 1.4 nM; Bmax = 2800 fmol/mg of protein). Half-maximal binding was achieved at approximately equal to 1 nM. Activity was markedly inhibited by protein modifying reagents, findings which suggest that the sites are proteinaceous. Opiate binding activity was also inhibited by the guanyl nucleotide GTP. Electrophoresis of the purified material under denaturing conditions revealed three subunits of molecular weights 94,000, 44,000, and 35,000. The inhibitory guanyl nucleotide binding protein (Ni) implicated in opiate action has been shown to be comprised of two subunits of molecular weights 42,000 and 35,000. Thus, the opiate receptor may be an aggregate of multiple protein components that may include a guanyl nucleotide binding protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnew W. S., Raftery M. A. Solubilized tetrodotoxin binding component from the electroplax of Electrophorus electricus. Stability as a function of mixed lipid-detergent micelle composition. Biochemistry. 1979 May 15;18(10):1912–1919. doi: 10.1021/bi00577a010. [DOI] [PubMed] [Google Scholar]
- Bentley K. W., Hardy D. G., Meek B. Novel analgesics and molecular rearrangements in the morphine-thebaine group. II. Alcohols derived from 6,14-endo-etheno- and 6,14-endo-ethanotetrahydrothebaine. J Am Chem Soc. 1967 Jun 21;89(13):3273–3280. doi: 10.1021/ja00989a031. [DOI] [PubMed] [Google Scholar]
- Bidlack J. M., Abood L. G., Osei-Gyimah P., Archer S. Purification of the opiate receptor from rat brain. Proc Natl Acad Sci U S A. 1981 Jan;78(1):636–639. doi: 10.1073/pnas.78.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlack J. M., Abood L. G. Solubilization of the opiate receptor. Life Sci. 1980 Jul 28;27(4):331–340. doi: 10.1016/0024-3205(80)90201-5. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Hewlett E. L., Gilman A. G. Identification of the predominant substrate for ADP-ribosylation by islet activating protein. J Biol Chem. 1983 Feb 25;258(4):2072–2075. [PubMed] [Google Scholar]
- Chang K. J., Cuatrecasas P. Heterogeneity and properties of opiate receptors. Fed Proc. 1981 Nov;40(13):2729–2734. [PubMed] [Google Scholar]
- Chow T., Zukin R. S. Solubilization and preliminary characterization of mu and kappa opiate receptor subtypes from rat brain. Mol Pharmacol. 1983 Sep;24(2):203–212. [PubMed] [Google Scholar]
- Codina J., Hildebrandt J., Iyengar R., Birnbaumer L., Sekura R. D., Manclark C. R. Pertussis toxin substrate, the putative Ni component of adenylyl cyclases, is an alpha beta heterodimer regulated by guanine nucleotide and magnesium. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4276–4280. doi: 10.1073/pnas.80.14.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Howells R. D., Gioannini T. L., Hiller J. M., Simon E. J. Solubilization and characterization of active opiate binding sites from mammalian brain. J Pharmacol Exp Ther. 1982 Sep;222(3):629–634. [PubMed] [Google Scholar]
- Klee W. A., Simonds W. F., Sweat F. W., Burke T. R., Jr, Jacobson A. E., Rice K. C. Identification of a Mr 58 000 glycoprotein subunit of the opiate receptor. FEBS Lett. 1982 Dec 13;150(1):125–128. doi: 10.1016/0014-5793(82)81318-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin H. K., Simon E. J. Phospholipase A inhibition of opiate receptor binding can be reversed by albumin. Nature. 1978 Jan 26;271(5643):383–384. doi: 10.1038/271383a0. [DOI] [PubMed] [Google Scholar]
- Maneckjee R., Baylin S. B. Use of radiolabeled monofluoromethyl-Dopa to define the subunit structure of human L-Dopa decarboxylase. Biochemistry. 1983 Dec 20;22(26):6058–6063. doi: 10.1021/bi00295a003. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. Loss of the inhibitory function of the guanine nucleotide regulatory component of adenylate cyclase due to its ADP ribosylation by islet-activating protein, pertussis toxin, in adipocyte membranes. J Biol Chem. 1983 Mar 10;258(5):3319–3326. [PubMed] [Google Scholar]
- Rüegg U. T., Hiller J. M., Simon E. J. Solubilization of an active opiate receptor from Bufo marinus. Eur J Pharmacol. 1980 Jun 27;64(4):367–368. doi: 10.1016/0014-2999(80)90247-2. [DOI] [PubMed] [Google Scholar]
- Simon E. J., Hiller J. M., Edelman I. Stereospecific binding of the potent narcotic analgesic (3H) Etorphine to rat-brain homogenate. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1947–1949. doi: 10.1073/pnas.70.7.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds W. F., Koski G., Streaty R. A., Hjelmeland L. M., Klee W. A. Solubilization of active opiate receptors. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4623–4627. doi: 10.1073/pnas.77.8.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukin R. S., Kream R. M. Chemical crosslinking of a solubilized enkephalin macromolecular complex. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1593–1597. doi: 10.1073/pnas.76.4.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukin R. S., Zukin S. R. Multiple opiate receptors: emerging concepts. Life Sci. 1981 Dec 28;29(26):2681–2690. doi: 10.1016/0024-3205(81)90527-0. [DOI] [PubMed] [Google Scholar]