Abstract
Vangl1 and Vangl2 are integral membrane proteins that play a critical role in establishing planar cell polarity (PCP) in epithelial cells and are required for convergent extension (CE) movements during embryogenesis. Their proper targeting to the plasma membrane (PM) is required for function. We created discrete deletions at the amino and carboxy termini of Vangl1 and monitored the effect of the mutations on PM targeting in Madin–Darby canine kidney cells. Our results show that the Vangl1 amino terminus lacks PM targeting determinants, and these are restricted to the carboxy terminus, including the predicted PDZBM motif at the C-terminus.
Keywords: planar cell polarity, Vangl1, Vangl2, plasma membrane, PDZ-binding motif
Introduction
Neural tube defects (NTDs) are a group of heterogeneous birth defects caused by failure of the neural tube (precursor of the central nervous system) to close properly during embryogenesis.1 These malformations are common (1/1000 births) and often cause infant mortality.2 Epidemiological studies indicate that NTDs are caused by complex genetic determinants in combination with environmental factors, both poorly understood.3 We have shown that the Vangl1 and Vangl2 genes are essential for the development of the neural tube, and that mutations in these genes cause NTDs in mice and humans.4
Vangl genes are vertebrate relatives of the fly Vang/Stbm, a gene required for polarization of epithelial cell layers and their associated cellular appendages, a process called “planar cell polarity” (PCP).4 Core PCP genes and proteins include Vangl/Stbm (van gogh-like, strabismus), Pk (prickle), Dgo (diego), Fz (frizzled), Dsh (dishevelled), and Fmi (flamingo).5 These assemble into membrane-bound multiprotein complexes that partition to the proximal (Vangl/Pk/Dgo) and distal side (Dsh/Fmi/Fz) of cells during PCP. The asymmetrical distribution of these complexes in adjacent cells propagates polarity signals to organize cells during PCP.5 The mechanisms underlying recruitment, assembly, and signaling by these membrane complexes remain unknown.
Mammalian Vangl1 and Vangl2 are integral membrane proteins that share high sequence similarity (∼75%), and that are expressed at the plasma membrane (PM) of different tissues during embryogenesis.6–8 Polarity mapping using Vangl1 recombinant proteins bearing exofacial eptitope tags expressed at the PM of transfected cells demonstrated that Vangl proteins contain four transmembrane domains, with intracellular amino and carboxyl termini. PM recruitment of Vangl proteins is essential for function during PCP.8,9 In the normal embryos in vivo, Vangl proteins are present at the baso-lateral side of epithelial cells of several tissues; this staining is lost in mutant embryos that harbor NTDs caused by defective Vangl proteins.7,8 PM recruitment of Vangl proteins is also required for formation of complexes with other PCP proteins.6,8,9
While sequence determinants required for Vangl membrane association are poorly characterized, several motifs responsible for targeting in other membrane proteins are found in Vangl proteins, including tyrosine based motifs YXXφ and NPXY/φ (where φ is hydrophobic) and di-leucine motifs known to act as internalization signals for clathrin-mediated endocytosis.10 Specifically, YSGY at the amino acid position (pst.) 7–10, YSYY (pst. 10–14), and a di-leucine motifs (pst. 56) are found in the amino terminus, while YKDF (pst. 284–287), NPNL (pst. 290–294) are found at the carboxyl terminus. Finally, Vangl proteins contain a PBM (PDZ binding motif), that is essential for interaction and recruitment of several PDZ containing partners including PCP proteins Scribble and Dvl1, Dvl2, and Dvl3.6,11 Here, we created linear deletions of the amino and carboxyl termini of Vangl1 and determine the effect of the deletions on PM targeting.
Results
Recombinant proteins were constructed using a human VANGL1 cDNA modified by addition of a GFP protein and a cMyc epitope tag at the amino terminus, and by the in-frame insertion of a hemagglutinin (HA) epitope tag at position 139 in the first extracellular loop delineated by trans-membrane domains 1 and 2 [Fig. 1(A)]. Six deletions overlapping different segments of the amino (N1, Δ1–13; N2, Δ1–95) and carboxyl termini (C1, Δ520–524; C2, Δ354–524; C3, Δ284–524; C4, Δ249–524) were created by PCR mutagenesis, cloned in an expression vector and transfected into Madin–Darby canine kidney (MDCK) kidney cells, in which transfected VANGL1 is expressed at the basal and lateral membranes.8 At the amino terminus, the putative membrane targeting function of sequence motifs YSGY (pst. 7–10), YSYY (pst. 10–14), and di-leucine LL (pst. 56–57) can be assessed using constructs N1 and N2, while at the carboxy terminus, a role of the PDZ binding motif ETSV (pst. 520–524) can be tested in construct C1. The functions of motifs YKDF (pst. 284–287) and NPNL (pst. 290–294) can be examined in constructs C3 and C4 (Fig. 1). The level of expression of the various recombinant Vangl proteins was measured by immunoblotting (anti-HA antibody) and shows low level of all variants, except for C4 in transfected MDCK cells [Fig. 1(B)].
Figure 1.
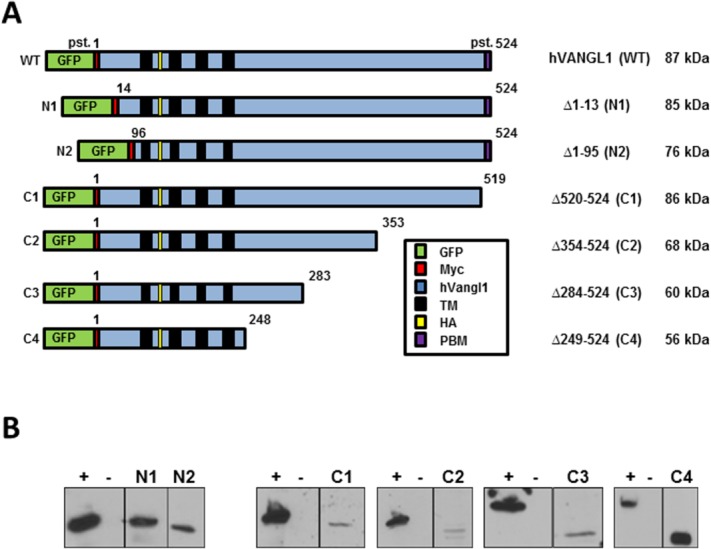
A: Schematic representation of the WT Vangl1 protein and of 6 deletion constructs, including position (pst.) corresponding to amino acid residue number, sequence features (GFP, Myc, and HA), and expected molecular mass of corresponding truncated proteins. B: Expression of the recombinant Vangl proteins was monitored by immunoblotting of total cell extracts from stably transfected MDCK cells, and using an anti-HA monoclonal antibody directed against an HA epitiope tag inserted in frame in all Vangl constructs. Positive control (+): WT protein and negative control (−): whole cellular extracts of untranscfected MDCK cells.
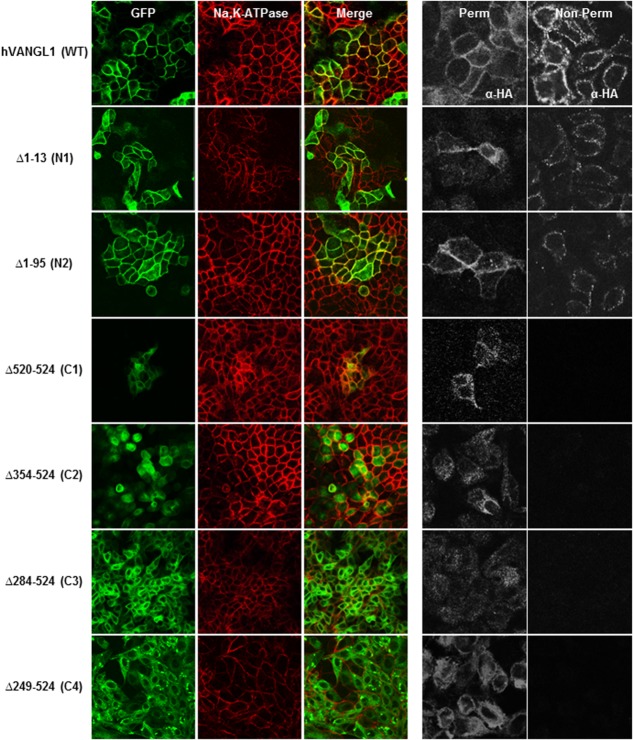
The effect of the deletions on PM targeting of VANGL1 was assessed by double immunofluorescence using the Na,K-ATPase as a PM marker.8 Recombinant VANGL1 proteins lacking N-terminal segments Δ1–13 (N1) and Δ1–95 (N2) are correctly targeted to the PM, and co-localize with Na,K-ATPase similarly to the WT VANGL1 (Fig. 2). Conversely, VANGL1 proteins lacking any portion of the C-terminal domain failed to reach the PM (Fig. 2). Indeed recombinant proteins C1 (Δ520–524), C2 (Δ354–524), C3 (Δ284–524), and C4 (Δ249–524) did not co-localize with Na,K-ATPase, but rather displayed a punctate intracellular staining. These results suggest that sequence elements in the carboxyl terminus of the protein are essential for PM association of VANGL1 (Fig. 2).
Figure 2.
MDCK cells stably transfected with either GFP-tagged hVANGL1 WT or GFP-tagged hVANGL1 amino or carboxy terminal truncated constructs (green) were grown on cover slips and either (A) stained with the PM marker Na,K-ATPase (red) or (B) incubated with an anti-HA antibody (each construct possessed an extracellular HA tag at position 139) under either non-permeabilized or permeabilized (0.5% TritonX-100) condition, followed by incubation with a Cy3-conjugated secondary antibody. Images were acquired by confocal microscopy.
To further validate the presence or absence of VANGL1 deletion variants at the PM, we monitored surface expression and accessibility of an exofacial HA epitope tag inserted in the first extracellular loop of VANGL1 (pst. 139, predicted TM1–TM2 interval). Intact cells producing a fluorescent signal with an anti-HA monoclonal antibody detect cell surface expression, while permeabilized conditions also reveal intracytoplasmic protein expression (Fig. 2). Under these conditions, wild type VANGL1 protein is inserted at the PM, exposing the HA tag to the extracellular milieu, resulting in immunofluorescence detection under both permeabilized and non-permeabilized conditions. While amino-terminal deletion constructs N1 and N2 produce staining similar to the WT protein, C1–C4 are only detected under permeabilized conditions, verifying that there is little if any expression of these proteins at the PM. Under these conditions, mutant variants C1–C4, but not WT or NI–N2 constructs, show overlapping staining with the ER marker calreticulin, suggesting inappropriate ER retention of these mutants (Fig. 3). Results reveal that (a) the N-terminal of VANGL1 is not essential for PM targeting, and (b) that the C-terminal domain is required for PM targeting of the protein in MDCK cells, including the short PDZ binding motif (ETSV; C1, Δ520–524), whose deletion abrogates PM targeting.
Figure 3.
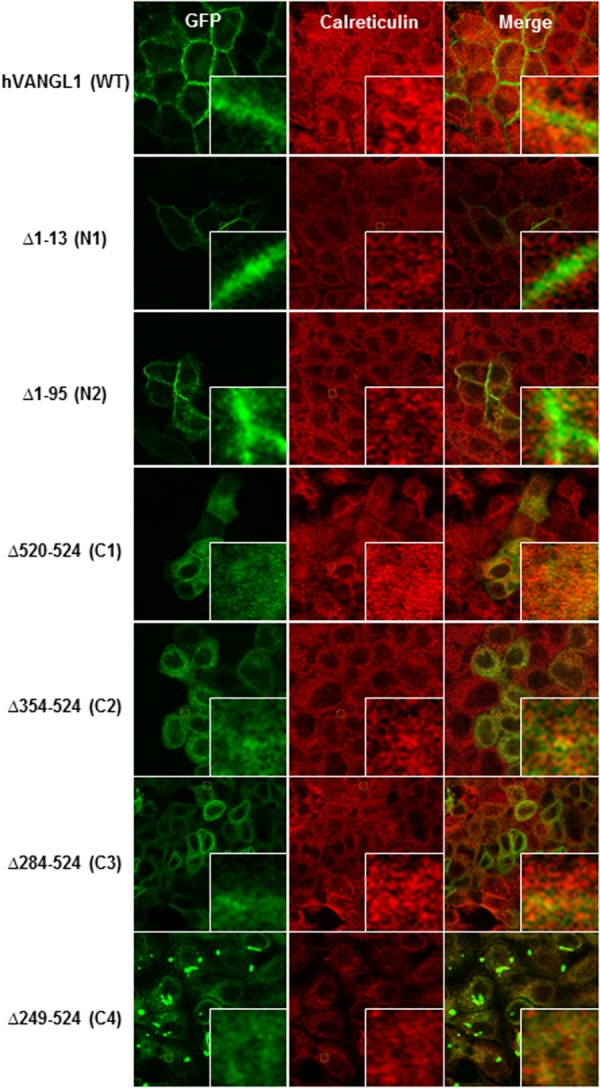
MDCK cells stably transfected with either GFP-tagged hVANGL1 WT or GFP-tagged hVANGL1 amino or carboxy terminal truncated constructs (green) were immunostained with the ER marker calreticulin (red) to determine subcellular localization. The magnification views point to overlapping staining of the ER marker with mis-targeted Vangl constructs. Images were acquired by confocal microscopy.
Discussion
Previous work in vivo and in transfected mammalian cell lines has demonstrated that PM targeting of Vangl proteins and asymmetric partitioning of Vangl-containing PCP complexes to polar opposites of epithelial cells are critical for biological function.4 For example, Vangl mutations in Lp mice (D255E, S464N) abrogate this partitioning to PM domains and cause PCP defects, including NTDs.6,8,9 Additionally, Lp-associated Vangl2 variants (D255E, S464N) fail to interact with Sec24b, a cargo-selecting component of COPII vesicles responsible for ER–Golgi transport, resulting in ER retention of Vangl proteins. This, combined with the observation that Sec24b mutant mice exhibit craniorachischisis, further emphasized the critical importance of Vangl proteins trafficking in regulating PCP and ultimately neural tube closure in mammals.12,13 Here, we used deletion analysis to locate sequence determinants responsible for Vangl membrane targeting.
Both amino terminal deletions, including Δ1–13 (N1) which eliminates both tyrosine-based motifs YSGY(7–10) and YSYY(10–14) and Δ1–95 (N2) which additionally removes the di-leucine motif LL(56) did not affect PM targeting. Indeed, both truncated constructs showed baso-lateral staining in MDCK cells, co-localization with Na,K-ATPase membrane marker, and showed staining with an HA antibody under non-permabilized conditions, suggesting that the amino terminus of Vangl proteins does not contain major determinants for PM membrane targeting. Interestingly, previous study of the Vangl zebrafish relative trilobite showed that a 13 amino-acid addition at pst. 21 in the N-terminus of this protein causes a loss of function in vivo.14 In addition, the N-terminal segment of VANGL1/VANGL2 proteins harbors pathological variants (S83L and S84F) associated with sporadic cases of NTD15,16; in particular, S84F maps to a Ser/Thr cluster that is phosphorylated in response to a Wnt5 signaling gradient required for PCP.17 Hence, the amino terminal segment of Vangl proteins does not harbor critical determinants for PM targeting, but is nevertheless required for other functional aspects.
Conversely, none of the C-terminal deletions Δ520–524 (C1), Δ354–524 (C2), Δ284–524 (C3), Δ249–524 (C4) were recruited to the PM, and they displayed intracellular staining (Fig. 2), reflecting retention in the ER, as demonstrated by overlapping staining with calreticulin (Fig. 3). The ∼280 amino acid residues carboxy terminal intracellular domain is highly conserved across species (> 80% sequence similarity), and all three Lp mutant alleles (D255E, R259L, and S464N) and the majority of human variants associated with NTDs (VANGL1: R274Q, M328T, and A404S and VANGL2: L242V, T247M, R270H, R353C, F437S, and R482H) map to this domain. Furthermore, a recent study deleting the Vangl segment spanning amino acid positions 280–291 (Vangl2 numbering) impaired membrane targeting. More specifically, the Phe in the YXXF(280–283) motif was found to be a critical amino acid responsible for the binding to the µ subunit of the clathrin adaptor complex AP1, thus mediating the export of Vangl proteins from the trans-Golgi network (TGN) to the PM.18 Finally, the recent description of a new Lp-allele, Vangl2m1Yzcm (displaying craniorachischisis) showing a stop codon at position 449, thus lacking the PDZ binding motif, additionally highlights the importance of this domain for PCP signaling and neural tube closure.19
Our data suggests that deleting the PDZ binding motif in the Vangl1 protein is sufficient for intracellular retention and impaired PM targeting. Our findings do not indicate that the PDZ binding motif functions directly as a membrane targeting motif, but that its action could be indirect, for example, by permitting interactions with other proteins which are relevant for membrane targeting. This motif is generally thought to mediate interactions with other PDZ containing proteins. These include interaction with the PDZ and DIX domains of Dvl1, Dvl2, and Dvl3, which is abrogated by Lp mutations (D255E, S464N) and by NTD specific human variants (R353C, F437S).6,15 Additionally, the Vangl2 PDZ binding motif recruits the PDZ domain of Scribble, and is disrupted by the Lp variant S464N and by absence of the PBM itself.11 More recently, interaction with a member of the nexin family, the PDZ containing protein SNX-27, which promotes the recycling of transmembrane proteins from endosomes to the PM has also been demonstrated.20 Additionally, this motif was also shown to affect the localization of Vangl2 at post-synaptic sites in the rat brain, by directly mediating interaction between Vangl2 and the third PDZ motif of PSD-95.20 These findings together with the results reported here strongly suggest that the PDZ binding motif of Vangl proteins is required for interactions with a number of PCP proteins, which is essential for targeting PCP complexes to the PM.
Materials and Methods
Material and antibodies
Geneticin (G418) and penicillin/streptomycin were purchased from Invitrogen (Carlsbad, CA). Restriction endonucleases were from New England Biolabs (Ipswich, MA), and Taq DNA polymerase was from Invitrogen. The mouse monoclonal antibodies directed against the influenza hemagglutinin epitope (HA.11) were purchased from Covance (Berkeley, CA). The mouse monoclonal antibody recognizing Na,K-ATPase (α) was from Santa Cruz Biotechnology (Santa Cruz, CA). Cy3-conjugated goat anti-mouse antibody and peroxidase-coupled goat anti-mouse antiserum were from Jackson ImmunoResearch Laboratories (West Grove, PA). The rabbit polyclonal antibody against calreticulin was obtained from Affinity BioReagents (Golden, CO).
Plasmids and site-directed mutagenesis
The human VANGL1 cDNA was used for constructing all deletions. The cDNA was amplified from total human RNA by reverse transcriptase polymerase chain reaction (RT-PCR) and cloned into the pCS2+ plasmid vector. PCR-mediated mutagenesis with overlapping oligonucleotides was used to insert a short antigenic peptide epitope (EQKLISEEDL) from the human c-Myc protein at the N-terminus of VANGL1 and a small hemagglutinin (HA) epitopes (YPYDVPDYA) in the extra-cellular loop between TM1–2 at position 139, followed by cloning into the pCB6 mammalian expression vector, as we have previously described.8 To facilitate identification of transfected cells expressing recombinant hVANGL1 proteins, c-Myc/HA-tagged hVANGL1 cDNAs were fused in-frame to the green fluorescent protein (GFP) by cloning into the peGFP-C1 vector (Clontech, Mountain View, CA). The deletions were introduced in hVANGL1 cDNA by PCR overlap extension mutagenesis, using the following primers; N1 (Δ 1–15) (Forward: ATCGAATTCCATGGAGCAGAAGCTAA; Reverse: CTTTGTAGTAATTTTCTAGGACCACC), N2 (Δ 1–95) (F: ATCGAATTCATGGAGCAGAAGCTAAT; R: CTTTGTAGTAATTTTCTAGGACCACC), C1 (Δ 520–524) (F: TACTACAAAGATTTCACCATCTATAAC; R: ATCTCTAGACTGTAAGCGAAGGACAA), C2 (Δ 354–524) (F: CTATTGGCTTTTTTACGGGGTCCGCAT; R: GTCATCTCTAGATACTCGCCGTTCAT), C3 (Δ 284–524) (F: TTTACCTCCGATCCTGTGGAGGTACCC; R: GTCATCTCTAGAGTAATTTTCTAGGA), C4 (Δ 249–524) (F: TTTACCTCCGATCCTGTGGAGGTACC; R: GTCATCTCTAGAGGGCTGCAGCTGCC). The integrity of all VANGL1 cDNA constructs was verified by nucleotide sequencing.
Cell culture, transfection, and Western blotting
MDCK epithelial cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin (37°C, 5% CO2). To generate stable transfectants, MDCK cells were transfected with c-Myc/HA-tagged VANGL1 WT or linear deletion constructs (subcloned in peGFP-C1) using Lipofectamine Plus Reagent as described in the manufacturer's instructions (Invitrogen). Total cell lysates, Western blot analysis, immunofluorescence (HA, Na,K-ATPase, calreticulin), and cell surface expression were performed as we have previously described.9
Acknowledgments
Image acquisition, data analysis, and image processing were conducted on equipment and with the assistance of the McGill Life Science Complex Imaging Facility, which was founded by the Canadian Foundation for Innovation.
References
- 1.Wallingford JB, Niswander LA, Shaw GM, Finnell RH. The continuing challenge of understanding, preventing, and treating neural tube defects. Science. 2013;339:1222002. doi: 10.1126/science.1222002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Copp AJ, Stanier P, Greene ND. Neural tube defects: recent advances, unsolved questions, and controversies. Lancet Neurol. 2013;12:799–810. doi: 10.1016/S1474-4422(13)70110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juriloff DM, Harris MJ. A consideration of the evidence that genetic defects in planar cell polarity contribute to the etiology of human neural tube defects. Birth Defects Res A Clin Mol Teratol. 2012;94:824–840. doi: 10.1002/bdra.23079. [DOI] [PubMed] [Google Scholar]
- 4.Torban E, Iliescu A, Gros P. An expanding role of Vangl proteins in embryonic development. Curr Top Dev Biol. 2012;101:237–261. doi: 10.1016/B978-0-12-394592-1.00005-3. [DOI] [PubMed] [Google Scholar]
- 5.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torban E, Wang HJ, Groulx N, Gros P. Independent mutations in mouse Vangl2 that cause neural tube defects in looptail mice impair interaction with members of the Dishevelled family. J Biol Chem. 2004;279:52703–52713. doi: 10.1074/jbc.M408675200. [DOI] [PubMed] [Google Scholar]
- 7.Torban E, Wang HJ, Patenaude AM, Riccomagno M, Daniels E, Epstein D, Gros P. Tissue, cellular and sub-cellular localization of the Vangl2 protein during embryonic development: effect of the Lp mutation. Gene Expr Patterns. 2007;7:346–354. doi: 10.1016/j.modgep.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Gravel M, Iliescu A, Horth C, Apuzzo S, Gros P. Molecular and cellular mechanisms underlying neural tube defects in the loop-tail mutant mouse. Biochemistry. 2010;49:3445–3455. doi: 10.1021/bi902180m. [DOI] [PubMed] [Google Scholar]
- 9.Iliescu A, Gravel M, Horth C, Kibar Z, Gros P. Loss of membrane targeting of Vangl proteins causes neural tube defects. Biochemistry. 2011;50:795–804. doi: 10.1021/bi101286d. [DOI] [PubMed] [Google Scholar]
- 10.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 11.Kallay LM, McNickle A, Brennwald PJ, Hubbard AL, Braiterman LT. Scribble associates with two polarity proteins, Lgl2 and Vangl2, via distinct molecular domains. J Cell Biochem. 2006;99:647–664. doi: 10.1002/jcb.20992. [DOI] [PubMed] [Google Scholar]
- 12.Wansleeben C, Feitsma H, Montcouquiol M, Kroon C, Cuppen E, Meijlink F. Planar cell polarity defects and defective Vangl2 trafficking in mutants for the COPII gene Sec24b. Development. 2010;137:1067–1073. doi: 10.1242/dev.041434. [DOI] [PubMed] [Google Scholar]
- 13.Merte J, Jensen D, Wright K, Sarsfield S, Wang Y, Schekman R, Ginty DD. Sec24b selectively sorts Vangl2 to regulate planar cell polarity during neural tube closure. Nat Cell Biol. 2010;12:41–46. doi: 10.1038/ncb2002. sup pp 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, Solnica-Krezel L. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei YP, Zhang T, Li H, Wu BL, Jin L, Wang HY. VANGL2 mutations in human cranial neural-tube defects. N Engl J Med. 2010;362:2232–2235. doi: 10.1056/NEJMc0910820. [DOI] [PubMed] [Google Scholar]
- 16.Kibar Z, Bosoi CM, Kooistra M, Salem S, Finnell RH, Marco De P, Merello E, Bassuk AG, Capra V, Gros P. Novel mutations in VANGL1 in neural tube defects. Hum Mutat. 2009;30:E706–E715. doi: 10.1002/humu.21026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, Andre P, Robinson J, Sood R, Minami Y, Economides AN, Yang Y. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell. 2011;20:163–176. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo Y, Zanetti G, Schekman R. A novel GTP-binding protein-adaptor protein complex responsible for export of Vangl2 from the trans Golgi network. Elife. 2013;2:e00160. doi: 10.7554/eLife.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen B, Mao HH, Chen L, Zhang FL, Li K, Xue ZF. Loop-tail phenotype in heterozygous mice and neural tube defects in homozygous mice result from a nonsense mutation in the Vangl2 gene. Genet Mol Res. 2013;12:3157–3165. doi: 10.4238/2013.January.22.2. [DOI] [PubMed] [Google Scholar]
- 20.Belotti E, Polanowska J, Daulat AM, Audebert S, Thome V, Lissitzky JC, Lembo F, Blibek K, Omi S, Lenfant N, Gangar A, Montcouquiol M, Santoni MJ, Sebbagh M, Aurrand-Lions M, Angers S, Kodjabachian L, Reboul J, Borg JP. The human PDZome: a gateway to PSD95-Disc large-zonula occludens (PDZ)-mediated functions. Mol Cell Proteom. 2013;12:2587–2603. doi: 10.1074/mcp.O112.021022. [DOI] [PMC free article] [PubMed] [Google Scholar]