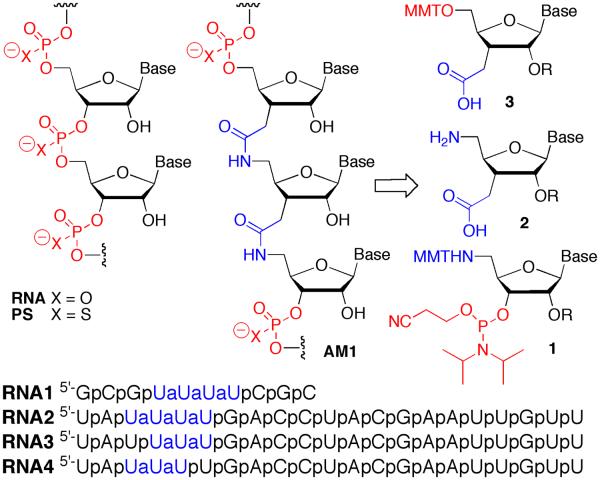
Abstract
RNA sequences having up to three consecutive internal amide linkages were synthesized and studied using UV and NMR spectroscopy. The amide modifications did not interfere with normal base-pairing and A-type RNA conformation. Three consecutive amides were well tolerated in the passenger strand of siRNA and caused little change in RNAi activity.
RNA interference (RNAi) has become a powerful and widely used technology for functional genomics and has intriguing potential for becoming a new therapeutic strategy.1,2 However, for in vivo applications in RNAi technologies the oligoribonucleotides need chemical modifications for optimization of properties such as enzymatic stability, targeted delivery, biodistribution and pharmacokinetics, and cellular uptake.1,2 While numerous good solutions for improving enzymatic stability have emerged, optimization of the latter properties remains challenging. Phosphorothioate (PS, Figure 1), where the non-bridging oxygen is replaced with sulfur, is one of a few modifications that enhances binding of antisense oligonucleotides to serum albumin, which improves pharmacokinetics and circulation time of the modified oligonucleotides.3 PS linkages are well tolerated in short interfering RNAs (siRNAs), especially at the ends of duplexes, and lead to more persistent gene silencing.4-6 While a fully modified siRNA (40 PS linkages) was not toxic in one study,4 others have observed toxicity of siRNAs with 12 and 20 PS modifications.5,6 Although these studies suggest that modification of the phosphodiester backbone has strong potential for optimization of siRNAs, such an approach has been little explored beyond the PS modification.
Fig. 1.
Structure and retrosynthetic analysis of amide-modified RNA.
Replacement of phosphodiesters with non-ionic amide linkages (such as AM1, Figure 1) has been explored as a means to increase enzymatic stability of antisense oligodeoxy-nucleotides.7 DNA having isolated AM1 modifications hybridized to complementary RNA with affinity similar to the unmodified DNA.8,9 NMR10 and molecular modeling9,11 studies suggested that AM1 linkages were well accommodated in an A-form DNA-RNA heteroduplex and favored an A-form conformation of the modified DNA. Von Matt et al. showed that DNA having several consecutive AM1 linkages had somewhat lower affinity (Δtm ca. −0.5 °C per modification) for the complementary RNA than the unmodified control.12
The relatively simple synthesis and favorable RNA binding of amide-modified DNA suggested that AM1 might also hold promise for optimization of siRNA properties. Robins, Peterson, and co-workers pioneered synthesis of AM1-linked RNA dimers13 and pentamers;14 however, the biophysical properties of these analogues were not reported. Later, we demonstrated that isolated AM1 linkages had little effect on structure, thermal stability and hydration of RNA double helices.15,16 Iwase and co-workers17 found that two consecutive amides at the 3′ overhangs (prepared using Robin’s methods) increased the enzymatic stability of siRNAs, but did not impair their RNAi activity. Taken together our preliminary results and Iwase’s encouraging studies prompted us to explore the structure and biological properties of RNA harboring consecutive amides. Herein, we report that three consecutive AM1 linkages somewhat decreased the thermal stability but maintained the A-form conformation of an RNA helix. When placed close to the 5′-end, the amide modifications were well tolerated in the passenger strand of siRNA. To our knowledge, this is the first time that the effect of three consecutive non-ionic linkages on structure and biological activity of RNA has been characterized. The results suggest that amides are excellent mimics of phosphate backbone in RNA, are well tolerated in biological systems, and may have potential to optimize properties of siRNAs for in vivo applications.
Synthesis of RNA having consecutive internal amide linkages required three types of modified nucleosides, uridine derivatives in our case (Fig. 1): 1) 5′-aminouridine-3′-phosphoramidite 1 to switch from standard RNA to amide-linked RNA synthesis; 2) 5′-amino-3′-carboxymethyluridine 2 to extend the amide backbone; and 3) 3′-carboxymethyluridine 3 to switch back to standard RNA synthesis.
The approach for RNA1, for example, was to start with a standard RNA synthesis using commercially available phosphoramidites to assemble a stretch of phosphodiester backbone, 5′-CpGpC then, after introducing monomer 1, build a stretch of amide backbone using monomers 2, 5′-UaUaUpCpGpC, and finally, after adding 3, complete the 5′-GpCpGpUaUaUaUpCpGpCp sequence using standard phosphoramidite chemistry.
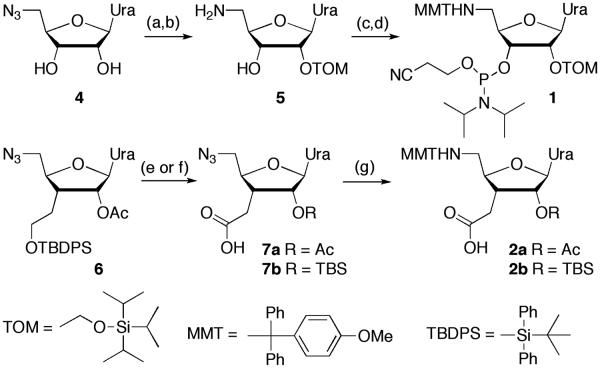
Compound 1 was prepared using standard nucleoside chemistry (Scheme 1). For construction of consecutive amide linkages, we explored two nucleoside amino acids, 2a and 2b. The 2′-O-Ac protected 2a was prepared from D-xylose following our recently developed procedure.18 The 2′-O-TBS protected 2b was prepared from the known 6.18 Protecting group exchange and oxidation gave the azido acid 7b,19 which was converted to 2b using the same procedures as for 2a.18 Modified uridines 3a (R= Ac) and 3b (R= TBS) required for switching from amide to phosphodiester synthesis were made as previously reported by us.15,16
Our initial experiments focused on preparing the amide-modified RNAs (Fig. 1) using monomers 1, 2a and 3a. A brief study of amide coupling conditions revealed that using 10 equivalents of 2a (0.125 M in DMF) activated by HATU in the presence of diisopropylethylamine gave ~90% yields after 8 hours. However, attempts to introduce amides at either 3′- or 5′-ends of siRNAs gave only phosphodiester-linked RNA fragments that were missing the amide-linked portions and terminated at the transition monomers 1 and 3. In other words, the amide linkages appeared to have degraded during the post-synthetic manipulation. In our earlier studies we noted decreased stability of amides in RNA (presumably due to the 1,5 relationship of carbonyl and 2′-OH) and optimized the deprotection conditions; we found that using ammonia in methanol (2 days at rt) did not cause amide cleavage.19 The apparent degradation of consecutive amides in RNA was surprising because we had successfully synthesized RNAs having isolated amides using the 2′-O-Ac protected 3a.16
Although the precise mechanism of the unexpected amide degradation remained unclear, using the 2′-O-TBS instead of 2′-O-Ac enabled synthesis of intact amide-modified RNAs. The synthesis started as a standard automated phosphoramidite protocol (1 μmol on Expedite 8909) concluding with monomer 1. The synthesis column was then removed from the synthesizer and the amide portion was synthesized manually using 2b as described in ESI. After manual addition of 3b, automated RNA synthesis finished preparation of RNA1-RNA4 (Fig. 1). The overall yields of amide-modified RNA were good for RNA1 (2 to 5 %) but relatively poor for the longer RNA2-RNA4 (0.2 to 1.2 %). MS analysis identified phosphodiester-linked RNA fragments terminated with acetylated monomer 1 (capped RNAs missing the amide-linked portions) as the major impurities (for details, see ESI). These results suggested that either inefficient amide couplings or residual degradation of the amide backbone might be responsible for decreased overall yields. Nevertheless, the modest efficiency (the synthesis was not thoroughly optimized) was adequate for the NMR and RNAi activity studies.
UV thermal melting studies showed that three consecutive amide linkages in RNA1 lowered the thermal stability of the decamer duplex by 3.5 °C per modification (Table 1). Nevertheless, CD (Fig. S8) and NMR spectroscopy studies showed that RNA1 formed a typical A-form duplex despite the consecutive amide modifications. As in our previous study,16 osmotic stress20 (Table 1, last three columns) showed that amide modifications caused little change in RNA hydration.
Table 1.
UV Melting and Osmotic Stress of Duplexes Formed by RNA1 and Unmodified Control.
| Sequencea | tm °C | - ΔH kcal/molb |
- ΔH kcal/molc |
- ΔH kcal/mold |
ΔS eud | - ΔG310
kcal/mold |
Ethylene glycol ΔnWe |
Glycerol ΔnWe |
Acetamide ΔnWe |
|---|---|---|---|---|---|---|---|---|---|
| 5′-GCGUaUaUaUCGC 3′-CGCApApApAGCG |
48.6 ± 0.2 | 74.0 ± 3.0 | 86.0 ± 7 | 76.1 ± 2.0 | 209 ± 6 | 11.5 ± 0.3 | 46 ± 3 | 59 ± 5 | 60 ± 5 |
| 5′-GCGUpUpUpUCGC 3′-CGCApApApAGCG |
59.1 ± 0.3 | 87.2 ± 2.7 | 116 ± 19 | 96.6 ± 4.4 | 263 ± 13 | 15.1 ± 0.3 | 52 ± 4 | 63 ± 7 | 66 ± 4 |
RNA strands, 2 μM each in 10 mM sodium cacodylate (pH 7.4), 0.1 mM EDTA, and 300 mM NaCl. Results ± standard deviations.
From dα/d(1/Tm) vs. Tm.
From concentration dependency of Tm.
From van’t Hoff analysis.
ΔnW = number of waters released upon melting. Results ± error estimates.
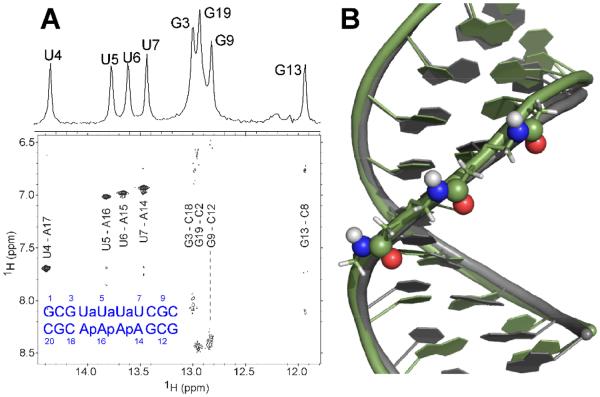
1D and 2D NMR spectra of the imino protons indicated that all expected Watson-Crick pairs were formed, including the four AU pairs flanking amide modifications (Fig. 2A). Consistent with the UV melting data, weaker NOE cross-peaks for the central AU pairs compared to the flanking AU pairs, especially at room temperature, suggested reduced stability towards the center of the duplex (Fig. S9). All riboses were in C3′-endo conformation typical of A-form RNA (Fig. S10). Amide protons show very weak or missing NOEs to H2′ and H3′ of the residue 5′ of the amide and medium NOEs to H4′ of the 3′-residue (Fig. S11). The NOE patterns were similar to those observed between the amide protons and sugar protons in a duplex with isolated amide linkages studied previously by us.16 Comparison of a representative model resulting from simulated-annealing with a relatively small number of restraints with the crystal structure of an all AU-pairs RNA duplex revealed that the three amide modifications caused notable, but relatively small deviations from the A-form conformation. The dominant conformation of the amide linkages oriented the C=O bond towards the major groove and NH towards the minor groove (Fig. 2B) and was consistent with previous molecular dynamics11 and our recent NMR16 studies.
Fig. 2.
NMR data and model of duplex formed by RNA1 and a complementary RNA strand: A 1D imino and 2D NOE spectrum of RNA1 indicate formation of four AU pairs. B model (green) overlaid with PDB 1H1K (grey, all AU pairs), the dominant orientation of the amide linkages is indicated.
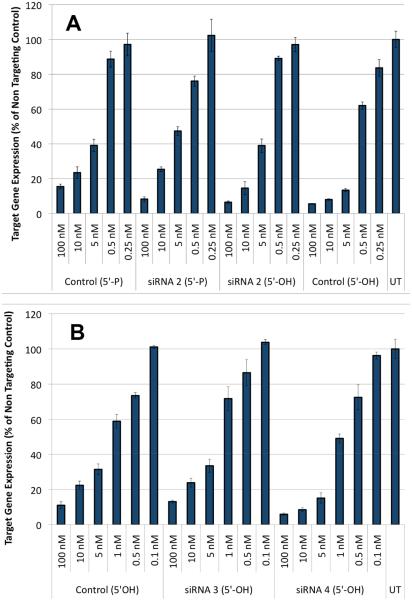
Finally, the effect of consecutive amide modifications on RNAi activity was tested using RNA2-RNA4, which is a passenger strand of siRNA targeting the Cyclophilin B (PPIB) mRNA. Introduction of three consecutive amides near the 5′-end caused very little change in the silencing activity of RNA2 (Fig. 3A). This result was both encouraging and perhaps unexpected because Gong and Desaulniers have recently reported that an amide linkage derived from insertion of a PNA monomer was not well tolerated near the 5′-end of siRNA’s passenger strand.21 The somewhat higher activity of RNA4 compared to RNA3 (Fig. 3B, siRNAs having two consecutive amide linkages) suggested that an amide modification at the third position may help loading of the guide strand, but further testing is needed to confirm this. Overall, the data indicate that consecutive amides are well tolerated in biological systems.
Fig. 3.
RNAi activity ofsiRNAs formed with amide-modified passenger strand in HeLa cells:ARNA2 and BRNA3 and RNA4. Control and UT represents unmodified siRNA and lipid-only treated cells, respectively.
For RNA2 we synthesized and studied both 5′-phosphorylated (5′-P) and non-phosphorylated (5′-OH) variants (Figure 3A). In RNAi the guide strand must be 5′-phosphorylated before loading in Argonaute 2, the key protein of the RNA Induced Silencing Complex (RISC). Consequently, phosphorylation of the passenger strand may decrease the activity by enhancing loading of the passenger strand instead of the guide strand. Consistent with this notion, the non-phosphorylated Control and RNA2 were more active than the respective phosphorylated siRNAs (Figure 3A). Interestingly, phosphorylation had smaller effect on activity of amide-modified siRNAs than on the unmodified controls, indicating that the amide modifications may participate in RISC loading.
Most of the studies on backbone-modified DNA and RNA have been done on isolated modifications imbedded in unmodified nucleic acids.7 However, optimization of the biological properties of siRNAs is likely to require multiple and consecutive modifications that are more difficult to accommodate because even small conformational changes may multiply and significantly distort an RNA duplex. Herein, we show for the first time that three consecutive amide linkages are well accommodated in the middle of a short RNA duplex. Although the amides cause some loss of thermal stability, the overall RNA structure is only minimally altered. Consistent with small structural changes, three consecutive amide modifications in the passenger strand cause little change in siRNA activity. Our results suggest that amides are excellent mimics of phosphate backbone in RNA and may have potential to optimize biological properties of siRNAs.
Supplementary Material
Scheme 1.
Synthesis of protected uridine 5′-amino 3′-C-carboxylic acids. Steps: (a) Bu2SnCl2, diisopropylethylamine, dichloroethane, rt, 1 h, then TOM-Cl, 80 °C, 0.5 h, 36%; (b) H2S gas, pyridine/water, 4:1, 1h, then stirred overnight, 88%; (c) methoxytrityl chloride, pyridine, rt, overnight, 77%; (d) 2-cyanoethyl N,N-diisopropylchlorophosphoramidite, diisopropylethylamine, CH2Cl2, rt, 14 h, 61%; (e) ref. 18; (f) ref. 19; (g) ref. 18.
Acknowledgwments
We thank NIH (R01 GM071461) for financial support of this research. The Regional NMR Facility (600 MHz instrument) at Binghamton University is supported by NSF (CHE-0922815). We thank Daniel Mutisya for synthesis of 3b.
Footnotes
Electronic Supplementary Information (ESI) available: Experimental procedures and results, and copies of 1H and 13C NMR data. See DOI: 10.1039/c000000x/
References
- 1.Watts JK, Corey DR. J. Pathol. 2012;226:365–379. doi: 10.1002/path.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deleavey GF, Damha MJ. Chem. Biol. 2012;19:937–954. doi: 10.1016/j.chembiol.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Bennett CF, Swayze EE. Annu. Rev. Pharmacol. Toxicol. 2010;50:259–293. doi: 10.1146/annurev.pharmtox.010909.105654. [DOI] [PubMed] [Google Scholar]
- 4.Braasch DA, Jensen S, Liu Y, Kaur K, Arar K, White MA, Corey DR. Biochemistry. 2003;42:7967–7975. doi: 10.1021/bi0343774. [DOI] [PubMed] [Google Scholar]
- 5.Harborth J, Elbashir SM, Vandenburgh K, Manninga H, Scaringe SA, Weber K, Tuschl T. Antisense Nucl. Acid Drug Dev. 2003;13:83–105. doi: 10.1089/108729003321629638. [DOI] [PubMed] [Google Scholar]
- 6.Amarzguioui M, Holen T, Babaie E, Prydz H. Nucleic Acids Res. 2003;31:589–595. doi: 10.1093/nar/gkg147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freier SM, Altmann KH. Nucleic Acids Res. 1997;25:4429–4443. doi: 10.1093/nar/25.22.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mesmaeker A. De, Waldner A, Lebreton J, Hoffmann P, Fritsch V, Wolf RM, Freier SM. Angew. Chem., Int. Ed. Engl. 1994;33:226–229. [Google Scholar]
- 9.Mesmaeker A. De, Lesueur C, Bevierre MO, Waldner A, Fritsch V, Wolf RM. Angew. Chem., Int. Ed. 1996;35:2790–2794. [Google Scholar]
- 10.Blommers MJJ, Pieles U, Mesmaeker A. de. Nucleic Acids Res. 1994;22:4187–4194. doi: 10.1093/nar/22.20.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nina M, Fonne-Pfister R, Beaudegnies R, Chekatt H, Jung PMJ, Murphy-Kessabi F, Mesmaeker A. De, Wendeborn S. J. Am. Chem. Soc. 2005;127:6027–6038. doi: 10.1021/ja0486566. [DOI] [PubMed] [Google Scholar]
- 12.Matt P. Von, Mesmaeker A. De, Pieles U, Zurcher W, Altmann KH. Tetrahedron Lett. 1999;40:2899–2902. [Google Scholar]
- 13.(a) Robins MJ, Sarker S, Xie M, Zhang W, Peterson MA. Tetrahedron Lett. 1996;37:3921–3924. [Google Scholar]; (b) Robins MJ, Zou R, Guo Z, Wnuk SF. J. Org. Chem. 1996;61:9207–9212. [Google Scholar]; (c) Peterson MA, Nilsson BL, Sarker S, Doboszewski B, Zhang W, Robins MJ. J. Org. Chem. 1999;64:8183–8192. doi: 10.1021/jo9908647. [DOI] [PubMed] [Google Scholar]
- 14.Robins MJ, Doboszewski B, Nilsson BL, Peterson MA. Nucleosides, Nucleotides Nucleic Acids. 2000;19:69–86. doi: 10.1080/15257770008032997. [DOI] [PubMed] [Google Scholar]
- 15.(a) Rozners E, Strömberg R. Nucleosides Nucleotides. 1997;16:967–970. [Google Scholar]; (b) Rozners E, Katkevica D, Bizdena E, Strömberg R. J. Am. Chem. Soc. 2003;125:12125–12136. doi: 10.1021/ja0360900. [DOI] [PubMed] [Google Scholar]
- 16.Selvam, Thomas S, Abbott J, Kennedy SD, Rozners E. Angew. Chem., Int. Ed. 2011;50:2068–2070. doi: 10.1002/anie.201007012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Iwase R, Miyao H, Toyama T, Nishimori K. Nucleic Acids Symposium Series. 2006:175–176. doi: 10.1093/nass/nrl087. [DOI] [PubMed] [Google Scholar]; (b) Iwase R, Toyama T, Nishimori K. Nucleosides, Nucleotides & Nucleic Acids. 2007;26:1451–1454. doi: 10.1080/15257770701542389. [DOI] [PubMed] [Google Scholar]
- 18.Tanui P, Kullberg M, Song N, Chivate Y, Rozners E. Tetrahedron. 2010;66:4961–4964. doi: 10.1016/j.tet.2010.04.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.(a) Rozners E, Liu Y. Org. Lett. 2003;5:181–184. doi: 10.1021/ol027229z. [DOI] [PubMed] [Google Scholar]; (b) Rozners E, Liu Y. J. Org. Chem. 2005;70:9841–9848. doi: 10.1021/jo0515879. [DOI] [PubMed] [Google Scholar]
- 20.(a) Rozners E, Moulder J. Nucleic Acids Res. 2004;32:248–254. doi: 10.1093/nar/gkh175. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rozners E. Curr. Protoc. Nucleic Acid Chem. 2010;43:7.14.11–17.14.13. doi: 10.1002/0471142700.nc0714s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong W, Desaulniers J-P. Bioorg. Med. Chem. Lett. 2012;22:6934–6937. doi: 10.1016/j.bmcl.2012.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.