Abstract
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS) characterized by encephalitogenic leukocyte infiltration and multifocal plaques of demyelination. Patients present with debilitating clinical sequelae including motor, sensory, and cognitive deficits. For the past 30 years, immune modulating treatments have entered the marketplace and continue to improve in limiting the frequency and severity of relapses, but no cure has been found and no drug has successfully stopped chronic progressive disease. Recent work focusing on the oligodendrocyte, the myelin-producing cell, has provided needed insight into the process of demyelination, the spontaneous ability of the CNS to regenerate, and the inevitable failure of remyelination. From this a number of promising molecular targets have been identified to protect oligodendrocytes and promote remyelination. Combining immunomodulatory therapy with strategies to protect oligodendrocytes from further degeneration and enhance remyelination presents a very real means to improve clinical outcome for chronic progressive patients in the near future. Here we lay out a combination therapy approach to treating MS and survey the current literature on promising drug candidates potentially capable of mediating oligodendrocyte protection and enhancing remyelination.
Myelin Is Necessary for Normal Central Nervous System Function
Electrical impulses conducted through the axonal segment of the neuron are essential for proper functioning of the central nervous system (CNS). Axonal conduction is integrally supported by sheaths of insulating membrane called myelin that are produced by glial cells termed oligodendrocytes. A single axon is wrapped with many segments of myelin often from multiple oligodendrocytes distributed along the length of the axon, and one oligodendrocyte can generate up to 40 myelin segments. Unlike the largely static morphology of a neuron, the oligodendrocyte is constantly generating new myelin and replacing segments in a form of ongoing myelin maintenance throughout adulthood (Lajtha et al., 1977). In addition to supporting axonal conduction, oligodendrocytes have more recently been shown to promote the health of neurons by other mechanisms, specifically providing growth factor and structural support. Indeed there is compelling evidence that axonal survival is dependent on intact oligodendrocytes (Pohl et al., 2011). It comes as no surprise then that oligodendrocyte deficiency and coincident demyelination can have devastating effects on a multitude of CNS functions. The prototypical demyelinating disease multiple sclerosis (MS) is characterized by multifocal lesions of demyelination in the brain and spinal cord ultimately presenting as progressive axonal loss and substantial neurological decline. Although the etiological origins of MS remain to be definitively explained – and as evidence suggests may not necessarily be restricted to any single mechanism – there is in most cases a definitive immunopathological component usually manifested as aberrant immune responses to host CNS cells and proteins.
Combining Therapeutic Strategies
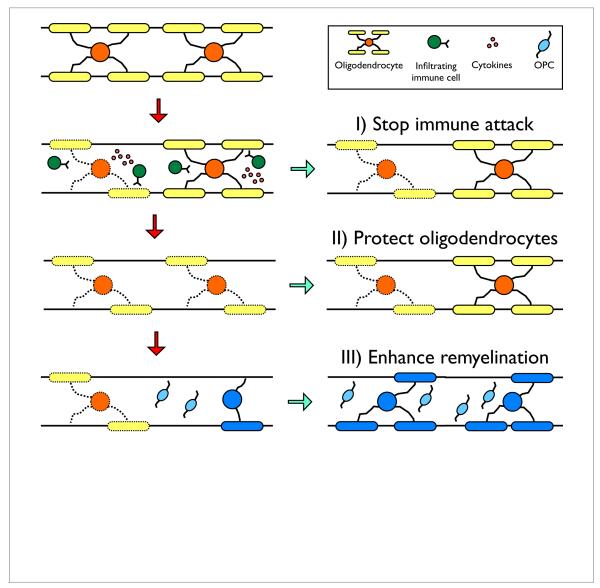
There are three principal approaches to treating MS: 1) halting the pathologic immune response, 2) protecting the CNS from further damage, and 3) repairing the damage through the regeneration of new myelin sheaths, with the overarching goals being to restore conduction and prevent further axonal loss (Figure 1). Currently, all FDA-approved drugs for MS are exclusively immunomodulatory therapies. These drugs are relatively effective at preventing new demyelinated lesions from forming and significantly impeding relapsing-remitting disease progression, but are ineffective at preventing the transition to or advancement of progressive MS. At this chronic stage neurodegeneration becomes increasingly evident leading to the accumulation of irreversible clinical disability (Compston, 2006). At present approximately 50% of people affected by MS are at the progressive stage of the disease illustrating the critical need for developing additional therapeutic strategies to protect oligodendrocytes and bolster regeneration in combination with current immunotherapies.
Figure 1.
Three therapeutic targets for combinatorial treatment strategies for multiple sclerosis. In the experimental autoimmune encephalomyelitis model of MS, CNS-infiltrating, autoreactive CD4+ T cells secrete cytokines that activate resident and infiltrating inflammatory immune cells leading to oligodendrocyte damage. The release of myelin antigens perpetuates the inflammatory process and subsequent oligodendrocyte destruction. The CNS is capable of significant regeneration. Endogenous oligodendrocyte progenitor cells (OPCs) proliferate, migrate to sites of inflammation, and differentiate to synthesize new myelin sheaths. To improve clinical outcome of MS patients we present three principal approaches: 1) halt pathologic immunity ideally by inhibiting antigen-specific responses rather than employing generalized immunosuppression, 2) protect oligodendrocytes from further damage, and 3) enhance remyelination either by transplanted exogenous cells or promote repair via endogenous OPCs.
Immune Modulation
The ten existing disease-modifying treatments for MS target the immune compartment (Derwenskus, 2011). Early approaches aimed to limit activation of pathologic immune cells and were relatively nonspecific in their scope. Early drugs – interferon-beta (Avonex, Rebif, Betaseron, Extavia) and glatiramer acetate (Copaxone), a synthetic copolymer, are both administered subcutaneously to suppress multiple cell types including antigen presenting cells and TH1 and TH17 helper subsets cells and shift the immune system towards a regulatory phenotype (Lalive et al., 2011). Mitoxantrone (Novatrone), a synthetic antineoplastic drug, induces apoptosis in highly proliferative cells and suppresses macrophages, B cells, and TH cells (Derwenskus, 2011). Recent work in autoimmunity has sought to refine the therapeutic approach in new ways, more specifically targeting the pathologic immune compartment without compromising the entire arm of the adaptive immune response thus minimizing side effects and any risks of opportunistic infection. Two current therapies limit T cell migration: fingolimod (Gilenya) constrains T cell migration from lymph nodes, whereas natalizumab (Tysabri) blocks T cell infiltration across the blood-brain barrier into the CNS. Other drugs such as dimethyl fumarate (Tecfidera) have been purported to have neuroprotective effects by modulating oxidative stress in addition to immunomodulatory effects. We also recently published the favorable results in both MS animal model studies (Getts et al., 2013; 2011) and in a phase I clinical trial that induces antigen-specific immune tolerance to myelin epitopes without excessively compromising the adaptive immune system (Lutterotti et al., 2013). Nine MS patients who were off-treatment for standard therapies received a single infusion of autologous peripheral blood mononuclear cells chemically coupled with seven myelin peptides (MOG1-20, MOG35-55, MBP13-32, MBP83-99, MBP111-129, MBP146-170, and PLP139-154). Administration of antigen-coupled cells was feasible and well tolerated, and patients receiving the higher doses (>1 × 109) had a decrease in antigen-specific T cell responses after peptide-coupled cell therapy. We are hopeful this approach may fill a critical need of antigen-specificity for immunomodulatory therapies.
Oligodendrocyte Protective Therapies
The majority of drugs under investigation to protect oligodendrocytes from apoptosis were first discovered for their immunomodulatory or neuroprotective capabilities in other capacities. In the context of MS, it is often poorly understood whether the beneficial effects are due to immune modulation, neuroprotection, or both. Indeed uncoupling these effects is particularly difficult as CNS degeneration is so intimately associated with inflammation. In light of a combination therapeutic approach, drugs that protect oligodendrocytes from apoptosis are likely to be most effective when used in combination with systemic immune modulation whether in cases of acute or chronic inflammation. Current data highlight the importance of proper timing in CNS-protective drug treatments as some prove to be most efficacious in early relapsing-remitting disease associated with deleterious inflammation. In this vein neuroprotective drugs may be unnecessary once immune tolerance has been established, particularly if tolerance can be induced early in disease and proves to have long-lasting effects.
Anti-apoptotic drugs
Minocycline, the most lipophilic of the broad-spectrum tetracycline antibiotics, is commonly used for its potent systemic antibacterial and immunomodulatory actions (Kloppenburg et al., 1996). Its ability to penetrate the CNS and modulate local inflammatory responses however makes it an attractive drug for neurological disorders (Kim and Suh, 2009). Minocycline was shown to be effective in attenuating experimental autoimmune encephalomyelitis (EAE), an immune-mediated rodent model that bears many semblances to MS, both prophylactically and therapeutically (Brundula et al., 2002; Popovic et al., 2002). It is difficult to attribute the therapeutic success of minocycline to either immunomodulation or anti-apoptotic oligodendrocyte effects and it may likely be due to both. Minocycline has a long half-life and is safe for long-term use (Klein and Cunha, 1995), making it an ideal add-on treatment for MS. It has been tested in MS patients in three separate clinical trials in combination with current FDA-approved MS therapies and alone (Luccarini et al., 2008; Metz et al., 2009; 2004; Ruggieri et al., 2008). There were modest positive results (Metz et al., 2004) leading to a current clinical trial recruiting for earlier stage in disease when significant inflammation is present.
Caspases, a family of cysteine proteases whose activity is essential for apoptosis, are known to be up-regulated in the CNS during acute EAE disease (Das et al., 2008). Treatment of EAE with FK506, a caspase inhibitor, reduced the level of demyelination (Gold et al., 2004). FK506 reduction of oligodendrocyte apoptosis has also been verified in the mouse spinal cord injury (SCI) model and in vitro suggesting that caspase inhibition protects oligodendrocytes from apoptosis (Craighead et al., 1999; Nottingham et al., 2002). However, given that FK506 is also a potent immunosuppressant commonly used to regulate transplant rejection (Liu et al., 1991) and that caspase-mediated apoptosis is a necessary process in anti-tumor immunity (van de Loosdrecht et al., 1993), the use of this drug and other caspase inhibitors for long-term MS treatment is not desirable.
Cardiovascular drugs
Statins are a commonly-used class of drugs that lower cholesterol levels (Alberts, 1988). Besides the ability to reduce atherosclerosis, statins have been shown to have a plethora of anti-inflammatory and neuroprotective effects in the CNS (Garcia, 2005). In a SCI model accompanied by demyelination, researchers found that atorvastatin treatment reduced oligodendrocyte apoptosis and demyelination resulting in a significant improvement in locomotor activity (Pannu et al., 2005). The mechanism was largely attributed to anti-inflammatory effects, reduced inflammatory cytokines, and improved blood-brain barrier integrity; however, it has also been suggested that atorvastatin inhibited apoptosis (Dery et al., 2009; Pannu et al., 2005). Atorvastatin was shown to produce a TH2 helper cell bias and ameliorate clinical disease in EAE (Youssef et al., 2002) and was first investigated for its effects in MS in combination with interferon-beta and minocycline. The results revealed beneficial effects yet minimally above interferon-beta or minocycline alone (Luccarini et al., 2008; Paul et al., 2008). A recent phase 2 clinical trial of atorvastatin for clinically isolated syndrome (CIS) reported a significant decrease in the number of new lesions in patients who began treatment shortly after they experienced their first episode of neurological symptoms (Waubant et al., 2012).
Amiloride, a pyrazine-carbonyl-guanidine originally discovered for its ability to block epithelial sodium channels, is approved for the treatment of hypertension and congestive heart failure. Amiloride has been shown to block other ion channels and exchangers, including acid-sensing ion channel 1 (ASIC1) that is expressed on oligodendrocytes (Feldman et al., 2008) and is up-regulated by oligodendrocytes in MS and EAE lesions (Vergo et al., 2011). Amiloride treatment during EAE reduced disease severity (Friese et al., 2007; Vergo et al., 2011) and these effects were shown to be CNS-protective, reducing tissue acidosis, rather than immunomodulatory (Friese et al., 2007). Thus amiloride is a promising oligodendrocyte protective drug that is currently being tested in a phase II clinical trial for MS.
Cannabinoids
Cannabinoids (CB) are a family of compounds, originating from the plant Cannabis sativa, known for numerous effects on the CNS and immune systems (Zajicek and Apostu, 2011). These effects are mediated by cannabinoid receptors, CB1 and CB2, with restricted expression patterns; whereas CB1 exerts psychotropic effects in the CNS, CB2 receptors are expressed by immune and glial cells and exert immunomodulatory effects (Arevalo-Martin et al., 2008; Galiegue et al., 1995). In EAE, CB1 activation in neurons and CB2 activation in CD4+ T cells have both been shown to ameliorate disease (Croxford and Miller, 2004; Croxford et al., 2008; Maresz et al., 2007). Cannabinoids may also induce neuroprotective effects in oligodendrocytes and oligodendrocyte progenitor cells (OPCs) which both express CB2 (Molina-Holgado et al., 2002). Cannabidiol was shown to protect OPCs from cytokine-mediated apoptosis by attenuating ER stress (Mecha et al., 2012). Additionally WIN55,212-2, a synthetic cannabinoid, stimulated OPC proliferation as well as survival in vivo (Solbrig et al., 2010), and there is evidence that cannabinoids can enhance remyelination by promoting oligodendrocyte maturation (Gomez et al., 2010; 2011). Thus cannabinoids may be a unique means to stimulate neuroprotection as well as regeneration. Several clinical trials of MS patients treated with cannabinoids found significant decreases in neurological symptoms and pain (Zajicek and Apostu, 2011). Currently the cannabinoid oral spray nabiximols is available in Canada and parts of Europe, but has yet to be approved in the U.S.
Remyelination Enhancing Therapies
The third component to a combinatorial therapeutic approach for MS is to enhance regeneration in the absence of the pathologic immune response and primary CNS damage. Early regenerative efforts focused on exogenous cell sources transplanted into the demyelinated CNS to form new myelin sheaths. Characterization of the robust nature of spontaneous remyelination, the process whereby demyelinated axons are ensheathed by new myelin membrane to functionally restore compromised axonal conduction, has refocused efforts towards promoting endogenous repair. Here we review exogenous cell therapies, current understanding on the nature of remyelination, and recent encouraging data employing endogenous remyelinating strategies.
Cell transplantation strategies
Brain OPCs can efficiently myelinate denuded axons when transplanted into a demyelinated lesion forming the basis for decades of cell transplantation research for MS (Duncan et al., 2008; Franklin and Ffrench-Constant, 2010). Promising cellular sources have included adult OPCs, Schwann cells, olfactory ensheathing cells, multipotent mesenchymal and hematopoietic progenitors from bone marrow, and adult and embryonic neural stem cells (Einstein et al., 2003; Huang and Franklin, 2012; Pluchino et al., 2003; Uccelli et al., 2011). To date, these approaches have raised more concerns rather than viable cellular therapies. Confounds include limited availability of source cells, limited migration of transplanted cells in models with multifocal sites of injury, immune rejection of transplanted cells, potential tumorigenesis, and determination of optimal route of administration for maximal delivery into the CNS (Chu et al., 2004; Miron et al., 2011). Finally the viability of cellular transplantation therapies for chronic progressive MS proves problematic in that transplanted OPCs are less likely to regenerate myelin in chronically demyelinated lesions than in the acutely demyelinated environment (Franklin, 2002). Migration and differentiation of transplanted progenitors in the EAE model directly corresponds to the peak of inflammation, and it may be the decrease in inflammation and thus pro-regenerative stimuli that underlie the failure in chronic demyelinated lesions (Foote and Blakemore, 2005; Setzu et al., 2006). An additional concern exists in that transplanted OPCs and newly generated myelin sheaths are subject to the same immune-mediated injury that resulted in the initial demyelinating insult (Blakemore et al., 2002).
Cellular therapy has lately taken an interesting turn in that many cell types under investigation are now thought to influence the injury environment through immunomodulation or trophic support to promote endogenous remyelination and neuroprotection, rather than direct cell replacement (Freedman et al., 2010; Munoz et al., 2005; Reynolds and Rietze, 2005; Robinson et al., 2011). Neural stem cells and bone marrow-derived mesenchymal stem/stromal cells (MSCs) have both been shown to act through anti-inflammatory mechanisms in animal models of demyelination and are purported to act through distinct immunomodulatory mechanisms (Pluchino and Martino, 2008; Uccelli et al., 2008). Recently a phase IIa clinical trial of intravenously infused MSCs into patients with secondary progressive MS was completed. Despite pre-clinical data suggesting MSCs do not engraft long-term in the CNS, patients exhibited improved CNS structure, function, and physiology (Connick et al., 2012).
True cellular replacement therapies may be best suited for treating developmental dysmyelination disorders such as the leukodystrophies, where the unrelenting immunological attack and exhaustion of transplanted cells is not an issue, as opposed to MS (Windrem et al., 2002; 2008). Replacement strategies have recently been bolstered by the demonstration of direct induction of OPCs from embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) (Czepiel et al., 2011; Hu et al., 2009; Liu et al., 2011; Najm et al., 2013; 2011). Induced OPCs can be rapidly expanded in culture and in two recent studies have been shown to functionally restore myelin in animal models of congenital hypomyelination (Wang et al., 2013; Yang et al., 2013).
Remyelination naturally follows demyelinating insult and disease
Generation of new myelin in the MS demyelinated lesion has been extensively documented by neuropathologic data (Prineas et al., 1984). Perhaps not surprisingly, clinical remissions or recovery from relapses in MS are correlated with remyelination in the lesions, both older lesions with diminished inflammatory activity and in new lesions with ongoing inflammation (Prineas et al., 1993; Raine and Wu, 1993). Indeed remyelination seems to be limited not by the acute, hostile inflammatory environment, but instead gradually dissipates over time (Sim et al., 2002). In addition to the restoration of conduction, multiple mechanisms have been associated with clinical remissions including the resolution of inflammation, sparing of neural and oligodendroglial cells, and the functional reorganization of myelin components (Mahad et al., 2008). Once a patient transitions to progressive MS, there is a strong correlation between the failure to remyelinate and axonal loss, and both show steady progression (Kornek et al., 2000).
Overwhelming evidence from the past 20 years suggests that new myelin in the MS lesion is synthesized by OPCs rather than the previously myelinating oligodendrocyte (Franklin and Ffrench-Constant, 2008; Keirstead and Blakemore, 1997). OPCs are cells randomly distributed throughout the CNS that are proliferative, motile, and capable of differentiating into oligodendrocytes and forming functional myelin sheaths. OPCs have been identified in the adult human brain comprising 5-8% of glial cells. Their function is presumably for normal myelin turnover as well as remyelination in response to injury (Jones et al., 2003). Current thinking posits that a complex series of coordinated steps is necessary for OPC “activation” and effective remyelination (Franklin and Kotter, 2008). Parenchymal OPCs migrate to sites of demyelination and undergo a vigorous proliferation to repopulate cells lost in the lesion (Gensert and Goldman, 1997; Lucchinetti et al., 1999). Next OPCs differentiate, a process characterized by down-regulation of OPC markers and up-regulation of markers associated with mature oligodendrocytes, coincident with exit from the cell cycle, a loss of motility, and an increasingly complex arborization (Crockett et al., 2005). In possessing all the cellular machinery for myelin formation, the final step involves axon contact and wrapping.
The pivotal question then is: why does remyelination fail? Therapeutic strategies have to date sought to stimulate all facets of OPC activation, though currently promoting OPC differentiation is the most prevalent approach. OPCs can be detected in chronic MS lesions suggesting that remyelination failure is not solely due to exhaustion of the progenitor pool or the breakdown of migration but rather may be primarily a dysregulation of OPC differentiation and axon ensheathment (Kuhlmann et al., 2008). As we elucidate the molecular underpinnings of the failure of remyelination and the activation of OPCs, several promising antibodies directed against CNS targets and pharmacological targets have been identified to promote OPC maturation and/or remyelination.
Antibody-based strategies to promote remyelination
Recombinant antibodies
LINGO-1 is a recently discovered leucine-rich repeat protein that is expressed in the CNS and functions as a negative regulator of OPC differentiation (Mi et al., 2005). Human antibodies generated against LINGO-1 were shown to block signaling in cultured OPCs and stimulate OPC differentiation and function in myelinating co-cultures. In animal models of demyelination, anti-LINGO-1 purportedly promotes remyelination by creating a hospitable environment for OPC activation (Mi et al., 2009). Early stage clinical trials are currently underway (Rudick et al., 2008). Restricted expression of the target protein in the CNS makes anti-LINGO-1 a particularly attractive target minimizing the chance of non-neural tissue complications (Mi et al., 2008); however, delivering the antibody in sufficient concentrations to the CNS may prove problematic.
A recombinant human IgM, termed rHIgM22, was identified from screens of antibodies for oligodendrocyte binding and remyelination promotion and has been shown to very effectively stimulate remyelination in an animal model of demyelination (Warrington et al., 2000; 2007). Mechanistic studies indicate that rHIgM22 drives OPC proliferation in conjunction with other glial-derived factors by inhibiting apoptotic signaling in OPCs (Watzlawik et al., 2010; 2013). rHIgM22 is currently the only antibody-based approach targeted to promote OPC proliferation.
Naturally occurring antibodies
Naturally occurring autoantibodies represent another promising source for antibody-based therapy to promote remyelination. Myelin- and oligodendrocyte-reactive autoantibodies have been identified from serum, CSF, and CNS tissue from MS patients and have been shown to promote remyelination in animal models of demyelination (Bieber et al., 2002; Miller et al., 1996). The antigenic targets have in some cases been identified as demonstrated by description of anti-myelin basic protein (MBP) antibodies that promote CNS remyelination (Rodriguez et al., 1996). The observation that remyelination-promoting oligodendrocyte-specific antibodies are polyreactive, binding to both extracellular and intracellular antigens, argues against a direct activation mechanism via specific cell surface receptor. Instead IgM binding to damaged oligodendrocytes via the common μ-heavy chain has been hypothesized to enhance debris clearance by scavenger macrophages and microglia thus indirectly enhancing remyelination (Asakura et al., 1998).
Antibodies reactive to viral peptides, exogenous and endogenous, have also been identified for their ability to promote remyelination. Semliki Forest Virus (SFV) encephalomyelitis produces CNS inflammation and subsequent demyelination (Safavi et al., 2011). Antibodies reactive to an SFV peptide were demonstrated to promote disease recovery, and when administered in EAE promote remyelination and clinical amelioration (Mokhtarian et al., 2012). Another monoclonal antibody reactive against an envelope protein from the human endogenous retrovirus type W family was initially identified because the viral particles were isolated from brain tissue from MS patients (Curtin et al., 2012; Perron et al., 1997). The viral protein, named Multiple Sclerosis-Associated Retrovirus (MSRV), was found to potentiate inflammatory responses in a humanized mouse model (Firouzi et al., 2003). The MSRV-reactive antibody, named GNbAC1, was shown to ameliorate EAE, and a phase I clinical trial was recently completed with favorable results (Curtin et al., 2012). For both viral protein reactive antibodies, the direct oligodendroglial or CNS mechanism of action has yet to be identified.
Molecular and pharmacological strategies to promote remyelination
Wnt inhibitors
Wnt proteins are a family of signaling glycoproteins that promote accumulation and activation of the transcription factor beta-catenin. Beta-catenin signaling is a known regulator of embryonic neural development, cellular proliferation, and differentiation. In OPCs beta-catenin negatively regulates differentiation (Feigenson et al., 2009) as an excess of beta-catenin in OPCs delays developmental myelination and remyelination during EAE (Fancy et al., 2009; Ye et al., 2009). XAV939, a small molecule inhibitor, enhances oligodendrocyte differentiation and remyelination by stabilizing Axin2, an intracellular target of Wnt transcriptional activation (Fancy et al., 2011). Exploiting the function of Axin2 is likely to enhance remyelination in EAE and MS, but further research is needed.
Notch
Notch is a protein involved in cell fate decisions in the CNS as well as regulatory effects in the immune system (Jurynczyk and Selmaj, 2010). Notch has been detected in immature oligodendrocytes of MS lesions and following demyelination in mice (Stidworthy et al., 2004). In addition, cultured human OPCs exposed to the Notch ligand Jagged failed to mature suggesting Notch may inhibit the differentiation of OPCs (John et al., 2002). Indeed EAE mice treated with a γ-secretase inhibitor, MW167, which prevents Notch cleavage and signaling, demonstrated improved myelin repair and axonal survival (Jurynczyk et al., 2005). γ-secretase inhibitors are under investigation for a number of CNS degenerative disorders. An oral formulation was recently tested in clinical trials for Alzheimer’s disease, but yielded disappointing results. Nonetheless the agent is able to gain access to the CNS and was well tolerated and thus remains hopeful for MS trials (Fleisher et al., 2008).
RXR agonists
Retinoid X receptor-γ (RXRγ), is a nuclear receptor that drives oligodendrocyte differentiation and myelin sheath formation by OPCs, and is the only positive regulator of OPC activation showing promising advanced results in studies (Huang et al., 2011b). In MS tissues RXRγ is highly expressed in acute and remyelinating lesions compared to chronic inactive lesions (Huang et al., 2011a). An RXR agonist was shown to improve remyelination in both an ex vivo cerebellar slice culture and EAE (Diab et al., 2004). Additionally RXR agonists have been purported to enhance phagocytic activity and attenuate inflammation in the CNS by regulating macrophage activity suggesting RXR activation may exert dual functions in regulating inflammation and OPC differentiation in the injured CNS (Kotter et al., 2006; Xu and Drew, 2006). Clinical trials evaluating RXR agonists for MS should be forthcoming. Indeed a licensed RXR agonist Targretin (bexarotene) is already in clinical use for the treatment of cutaneous T cell lymphoma (Ballanger et al., 2010).
Progesterone
Progesterone is a well-characterized steroid hormone involved in the female menstrual cycle and reproduction with immunomodulatory effects in several models of neurological diseases including EAE (Garay et al., 2007; 2012). Additionally progesterone is known for neuroprotective effects and progesterone signaling in oligodendrocytes has been shown to promote remyelination. Mice with EAE treated with progesterone had decreased clinical severity and enhanced expression of transcription factors essential for oligodendrocyte differentiation, density of mature oligodendrocytes, and myelin protein transcripts (Garay et al., 2012). Using a non-immune mediated animal model of demyelination researchers demonstrated that progesterone stimulated OPC proliferation and remyelination independent of immunomodulation (Garay et al., 2011). It is common for pregnant MS patients with elevated levels of progesterone to experience fewer relapses, but they often relapse post-partum (Vukusic and Confavreux, 2006). A clinical trial in Europe is currently evaluating postpartum progesterone treatments to reduce the incidence of relapse.
Conclusions
The landscape for MS therapies is undergoing a rapid expansion as knowledge accumulates on how aberrant immune responses produce demyelinating injury and how the CNS is able to regenerate myelin. The early broad-based immunosuppressive drugs are giving way to targeted, antigen-specific approaches minimizing side effects and maximizing clinical benefit. At the same time decades of work suggest that immunomodulation alone will only go so far and that strategies to protect oligodendrocytes and promote remyelination should be considered in parallel, combinatorial therapeutic approaches. Many repurposed drugs are showing promise for protecting oligodendrocytes, and remyelination strategies focusing on antibodies and pharmacological targets have shifted the focus from cellular replacement to enhancing endogenous repair.
Acknowledgments
This work was supported by NIH Grant NS-026543 and a Grant from the Myelin Repair Foundation.
Footnotes
J.M.R. and A.P.R. contributed equally to the work.
Disclosure
The authors have no known or potential conflicts of interest.
References
- Alberts AW. Discovery, biochemistry and biology of lovastatin. Am J Cardiol. 1988;62(15):10J–15J. doi: 10.1016/0002-9149(88)90002-1. [DOI] [PubMed] [Google Scholar]
- Arevalo-Martin A, Garcia-Ovejero D, Gomez O, Rubio-Araiz A, Navarro-Galve B, Guaza C, Molina-Holgado E, Molina-Holgado F. CB2 cannabinoid receptors as an emerging target for demyelinating diseases: from neuroimmune interactions to cell replacement strategies. Br J Pharmacol. 2008;153(2):216–225. doi: 10.1038/sj.bjp.0707466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura K, Miller DJ, Pease LR, Rodriguez M. Targeting of IgMkappa antibodies to oligodendrocytes promotes CNS remyelination. J Neurosci. 1998;18(19):7700–7708. doi: 10.1523/JNEUROSCI.18-19-07700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanger F, Nguyen JM, Khammari A, Dreno B. Evolution of clinical and molecular responses to bexarotene treatment in cutaneous T-cell lymphoma. Dermatology. 2010;220(4):370–375. doi: 10.1159/000305354. [DOI] [PubMed] [Google Scholar]
- Bieber AJ, Warrington A, Asakura K, Ciric B, Kaveri SV, Pease LR, Rodriguez M. Human antibodies accelerate the rate of remyelination following lysolecithin-induced demyelination in mice. Glia. 2002;37(3):241–249. doi: 10.1002/glia.10033. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Chari DM, Gilson JM, Crang AJ. Modelling large areas of demyelination in the rat reveals the potential and possible limitations of transplanted glial cells for remyelination in the CNS. Glia. 2002;38(2):155–168. doi: 10.1002/glia.10067. [DOI] [PubMed] [Google Scholar]
- Brundula V, Rewcastle NB, Metz LM, Bernard CC, Yong VW. Targeting leukocyte MMPs and transmigration: minocycline as a potential therapy for multiple sclerosis. Brain. 2002;125(Pt 6):1297–1308. doi: 10.1093/brain/awf133. [DOI] [PubMed] [Google Scholar]
- Chu K, Kim M, Chae SH, Jeong SW, Kang KS, Jung KH, Kim J, Kim YJ, Kang L, Kim SU, Yoon BW. Distribution and in situ proliferation patterns of intravenously injected immortalized human neural stem-like cells in rats with focal cerebral ischemia. Neurosci Res. 2004;50(4):459–465. doi: 10.1016/j.neures.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Compston A. The basis for treatment in multiple sclerosis. Acta Neurol Scand Suppl. 2006;183:41–47. doi: 10.1111/j.1600-0404.2006.00614.x. [DOI] [PubMed] [Google Scholar]
- Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW, Du MQ, Luan SL, Altmann DR, Thompson AJ, Compston A, Scott MA, Miller DH, Chandran S. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11(2):150–156. doi: 10.1016/S1474-4422(11)70305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craighead MW, Tiwari P, Keynes RG, Waters CM. Human oligodendroglial cell line, MO3.13, can be protected from apoptosis using the general caspase inhibitor zVAD-FMK. J Neurosci Res. 1999;57(2):236–243. doi: 10.1002/(SICI)1097-4547(19990715)57:2<236::AID-JNR9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Crockett DP, Burshteyn M, Garcia C, Muggironi M, Casaccia-Bonnefil P. Number of oligodendrocyte progenitors recruited to the lesioned spinal cord is modulated by the levels of the cell cycle regulatory protein p27Kip-1. Glia. 2005;49(2):301–308. doi: 10.1002/glia.20111. [DOI] [PubMed] [Google Scholar]
- Croxford JL, Miller SD. Towards cannabis and cannabinoid treatment of multiple sclerosis. Drugs Today (Barc) 2004;40(8):663–676. doi: 10.1358/dot.2004.40.8.850469. [DOI] [PubMed] [Google Scholar]
- Croxford JL, Pryce G, Jackson SJ, Ledent C, Giovannoni G, Pertwee RG, Yamamura T, Baker D. Cannabinoid-mediated neuroprotection, not immunosuppression, may be more relevant to multiple sclerosis. J Neuroimmunol. 2008;193(1-2):120–129. doi: 10.1016/j.jneuroim.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Curtin F, Lang AB, Perron H, Laumonier M, Vidal V, Porchet HC, Hartung HP. GNbAC1, a humanized monoclonal antibody against the envelope protein of multiple sclerosis-associated endogenous retrovirus: a first-in-humans randomized clinical study. Clin Ther. 2012;34(12):2268–2278. doi: 10.1016/j.clinthera.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Czepiel M, Balasubramaniyan V, Schaafsma W, Stancic M, Mikkers H, Huisman C, Boddeke E, Copray S. Differentiation of induced pluripotent stem cells into functional oligodendrocytes. Glia. 2011;59(6):882–892. doi: 10.1002/glia.21159. [DOI] [PubMed] [Google Scholar]
- Das A, Guyton MK, Butler JT, Ray SK, Banik NL. Activation of calpain and caspase pathways in demyelination and neurodegeneration in animal model of multiple sclerosis. CNS Neurol Disord Drug Targets. 2008;7(3):313–320. doi: 10.2174/187152708784936699. [DOI] [PubMed] [Google Scholar]
- Derwenskus J. Current disease-modifying treatment of multiple sclerosis. Mt Sinai J Med. 2011;78(2):161–175. doi: 10.1002/msj.20239. [DOI] [PubMed] [Google Scholar]
- Dery MA, Rousseau G, Benderdour M, Beaumont E. Atorvastatin prevents early apoptosis after thoracic spinal cord contusion injury and promotes locomotion recovery. Neurosci Lett. 2009;453(1):73–76. doi: 10.1016/j.neulet.2009.01.062. [DOI] [PubMed] [Google Scholar]
- Diab A, Hussain RZ, Lovett-Racke AE, Chavis JA, Drew PD, Racke MK. Ligands for the peroxisome proliferator-activated receptor-gamma and the retinoid X receptor exert additive anti-inflammatory effects on experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148(1-2):116–126. doi: 10.1016/j.jneuroim.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Duncan ID, Goldman S, Macklin WB, Rao M, Weiner LP, Reingold SC. Stem cell therapy in multiple sclerosis: promise and controversy. Mult Scler. 2008;14(4):541–546. doi: 10.1177/1352458507087324. [DOI] [PubMed] [Google Scholar]
- Einstein O, Karussis D, Grigoriadis N, Mizrachi-Kol R, Reinhartz E, Abramsky O, Ben-Hur T. Intraventricular transplantation of neural precursor cell spheres attenuates acute experimental allergic encephalomyelitis. Mol Cell Neurosci. 2003;24(4):1074–1082. doi: 10.1016/j.mcn.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Fancy SP, Baranzini SE, Zhao C, Yuk DI, Irvine KA, Kaing S, Sanai N, Franklin RJ, Rowitch DH. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23(13):1571–1585. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SP, Harrington EP, Yuen TJ, Silbereis JC, Zhao C, Baranzini SE, Bruce CC, Otero JJ, Huang EJ, Nusse R, Franklin RJ, Rowitch DH. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat Neurosci. 2011;14(8):1009–1016. doi: 10.1038/nn.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenson K, Reid M, See J, Crenshaw EB, 3rd, Grinspan JB. Wnt signaling is sufficient to perturb oligodendrocyte maturation. Mol Cell Neurosci. 2009;42(3):255–265. doi: 10.1016/j.mcn.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Feldman DH, Horiuchi M, Keachie K, Mccauley E, Bannerman P, Itoh A, Itoh T, Pleasure D. Characterization of acid-sensing ion channel expression in oligodendrocyte-lineage cells. Glia. 2008;56(11):1238–1249. doi: 10.1002/glia.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firouzi R, Rolland A, Michel M, Jouvin-Marche E, Hauw JJ, Malcus-Vocanson C, Lazarini F, Gebuhrer L, Seigneurin JM, Touraine JL, Sanhadji K, Marche PN, Perron H. Multiple sclerosis-associated retrovirus particles cause T lymphocyte-dependent death with brain hemorrhage in humanized SCID mice model. J Neurovirol. 2003;9(1):79–93. doi: 10.1080/13550280390173328. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Raman R, Siemers ER, Becerra L, Clark CM, Dean RA, Farlow MR, Galvin JE, Peskind ER, Quinn JF, Sherzai A, Sowell BB, Aisen PS, Thal LJ. Phase 2 safety trial targeting amyloid beta production with a gamma-secretase inhibitor in Alzheimer disease. Arch Neurol. 2008;65(8):1031–1038. doi: 10.1001/archneur.65.8.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote AK, Blakemore WF. Inflammation stimulates remyelination in areas of chronic demyelination. Brain. 2005;128(Pt 3):528–539. doi: 10.1093/brain/awh417. [DOI] [PubMed] [Google Scholar]
- Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3(9):705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9(11):839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Stem cell treatments and multiple sclerosis. BMJ. 2010;340:c1387. doi: 10.1136/bmj.c1387. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Kotter MR. The biology of CNS remyelination: the key to therapeutic advances. J Neurol. 2008;255(Suppl 1):19–25. doi: 10.1007/s00415-008-1004-6. [DOI] [PubMed] [Google Scholar]
- Freedman MS, Bar-Or A, Atkins HL, Karussis D, Frassoni F, Lazarus H, Scolding N, Slavin S, Le Blanc K, Uccelli A. The therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: consensus report of the International MSCT Study Group. Mult Scler. 2010;16(4):503–510. doi: 10.1177/1352458509359727. [DOI] [PubMed] [Google Scholar]
- Friese MA, Craner MJ, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, Vincent A, Fugger L. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med. 2007;13(12):1483–1489. doi: 10.1038/nm1668. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232(1):54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Garay L, Deniselle MC, Lima A, Roig P, De Nicola AF. Effects of progesterone in the spinal cord of a mouse model of multiple sclerosis. J Steroid Biochem Mol Biol. 2007;107(3-5):228–237. doi: 10.1016/j.jsbmb.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Garay L, Tungler V, Deniselle MC, Lima A, Roig P, De Nicola AF. Progesterone attenuates demyelination and microglial reaction in the lysolecithin-injured spinal cord. Neuroscience. 2011;192:588–597. doi: 10.1016/j.neuroscience.2011.06.065. [DOI] [PubMed] [Google Scholar]
- Garay LI, Gonzalez Deniselle MC, Brocca ME, Lima A, Roig P, De Nicola AF. Progesterone down-regulates spinal cord inflammatory mediators and increases myelination in experimental autoimmune encephalomyelitis. Neuroscience. 2012;226:40–50. doi: 10.1016/j.neuroscience.2012.09.032. [DOI] [PubMed] [Google Scholar]
- Garcia PJ. Pleiotropic effects of statins: moving beyond cholesterol control. Curr Atheroscler Rep. 2005;7(1):34–39. doi: 10.1007/s11883-005-0073-6. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19(1):197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Getts DR, Martin AJ, Mccarthy DP, Terry RL, Hunter ZN, Yap WT, Getts MT, Pleiss M, Luo X, King NJ, Shea LD, Miller SD. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat Biotechnol. 2013;31(6):565. doi: 10.1038/nbt.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getts DR, Turley DM, Smith CE, Harp CT, Mccarthy D, Feeney EM, Getts MT, Martin AJ, Luo X, Terry RL, King NJ, Miller SD. Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. J Immunol. 2011;187(5):2405–2417. doi: 10.4049/jimmunol.1004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BG, Voda J, Yu X, Mckeon G, Bourdette DN. FK506 and a non-immunosuppressant derivative reduce axonal and myelin damage in experimental autoimmune encephalomyelitis: neuroimmunophilin ligand-mediated neuroprotection in a model of multiple sclerosis. J Neurosci Res. 2004;77(3):367–377. doi: 10.1002/jnr.20165. [DOI] [PubMed] [Google Scholar]
- Gomez O, Arevalo-Martin A, Garcia-Ovejero D, Ortega-Gutierrez S, Cisneros JA, Almazan G, Sanchez-Rodriguez MA, Molina-Holgado F, Molina-Holgado E. The constitutive production of the endocannabinoid 2-arachidonoylglycerol participates in oligodendrocyte differentiation. Glia. 2010;58(16):1913–1927. doi: 10.1002/glia.21061. [DOI] [PubMed] [Google Scholar]
- Gomez O, Sanchez-Rodriguez A, Le M, Sanchez-Caro C, Molina-Holgado F, Molina-Holgado E. Cannabinoid receptor agonists modulate oligodendrocyte differentiation by activating PI3K/Akt and the mammalian target of rapamycin (mTOR) pathways. Br J Pharmacol. 2011;163(7):1520–1532. doi: 10.1111/j.1476-5381.2011.01414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BY, Du ZW, Zhang SC. Differentiation of human oligodendrocytes from pluripotent stem cells. Nat Protoc. 2009;4(11):1614–1622. doi: 10.1038/nprot.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JK, Franklin RJ. Current status of myelin replacement therapies in multiple sclerosis. Prog Brain Res. 2012;201:219–231. doi: 10.1016/B978-0-444-59544-7.00011-1. [DOI] [PubMed] [Google Scholar]
- Huang JK, Jarjour AA, Ffrench-Constant C, Franklin RJ. Retinoid X receptors as a potential avenue for regenerative medicine in multiple sclerosis. Expert Rev Neurother. 2011a;11(4):467–468. doi: 10.1586/ern.11.34. [DOI] [PubMed] [Google Scholar]
- Huang JK, Jarjour AA, Nait Oumesmar B, Kerninon C, Williams A, Krezel W, Kagechika H, Bauer J, Zhao C, Baron-Van Evercooren A, Chambon P, Ffrench-Constant C, Franklin RJ. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat Neurosci. 2011b;14(1):45–53. doi: 10.1038/nn.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GR, Shankar SL, Shafit-Zagardo B, Massimi A, Lee SC, Raine CS, Brosnan CF. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat Med. 2002;8(10):1115–1121. doi: 10.1038/nm781. [DOI] [PubMed] [Google Scholar]
- Jones SA, Jolson DM, Cuta KK, Mariash CN, Anderson GW. Triiodothyronine is a survival factor for developing oligodendrocytes. Mol Cell Endocrinol. 2003;199(1-2):49–60. doi: 10.1016/s0303-7207(02)00296-4. [DOI] [PubMed] [Google Scholar]
- Jurynczyk M, Jurewicz A, Bielecki B, Raine CS, Selmaj K. Inhibition of Notch signaling enhances tissue repair in an animal model of multiple sclerosis. J Neuroimmunol. 2005;170(1-2):3–10. doi: 10.1016/j.jneuroim.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Jurynczyk M, Selmaj K. Notch: a new player in MS mechanisms. J Neuroimmunol. 2010;218(1-2):3–11. doi: 10.1016/j.jneuroim.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Blakemore WF. Identification of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. J Neuropathol Exp Neurol. 1997;56(11):1191–1201. doi: 10.1097/00005072-199711000-00003. [DOI] [PubMed] [Google Scholar]
- Kim HS, Suh YH. Minocycline and neurodegenerative diseases. Behav Brain Res. 2009;196(2):168–179. doi: 10.1016/j.bbr.2008.09.040. [DOI] [PubMed] [Google Scholar]
- Klein NC, Cunha BA. Tetracyclines. Med Clin North Am. 1995;79(4):789–801. doi: 10.1016/s0025-7125(16)30039-6. [DOI] [PubMed] [Google Scholar]
- Kloppenburg M, Brinkman BM, De Rooij-Dijk HH, Miltenburg AM, Daha MR, Breedveld FC, Dijkmans BA, Verweij C. The tetracycline derivative minocycline differentially affects cytokine production by monocytes and T lymphocytes. Antimicrob Agents Chemother. 1996;40(4):934–940. doi: 10.1128/aac.40.4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornek B, Storch MK, Weissert R, Wallstroem E, Stefferl A, Olsson T, Linington C, Schmidbauer M, Lassmann H. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol. 2000;157(1):267–276. doi: 10.1016/S0002-9440(10)64537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter MR, Li WW, Zhao C, Franklin RJ. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci. 2006;26(1):328–332. doi: 10.1523/JNEUROSCI.2615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann T, Miron V, Cui Q, Wegner C, Antel J, Bruck W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131(Pt 7):1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- Lajtha A, Toth J, Fujimoto K, Agrawal HC. Turnover of myelin proteins in mouse brain in vivo. Biochem J. 1977;164(2):323–329. doi: 10.1042/bj1640323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalive PH, Neuhaus O, Benkhoucha M, Burger D, Hohlfeld R, Zamvil SS, Weber MS. Glatiramer acetate in the treatment of multiple sclerosis: emerging concepts regarding its mechanism of action. CNS Drugs. 2011;25(5):401–414. doi: 10.2165/11588120-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Farmer JD, Jr., Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jiang P, Deng W. OLIG gene targeting in human pluripotent stem cells for motor neuron and oligodendrocyte differentiation. Nat Protoc. 2011;6(5):640–655. doi: 10.1038/nprot.2011.310. [DOI] [PubMed] [Google Scholar]
- Luccarini I, Ballerini C, Biagioli T, Biamonte F, Bellucci A, Rosi MC, Grossi C, Massacesi L, Casamenti F. Combined treatment with atorvastatin and minocycline suppresses severity of EAE. Exp Neurol. 2008;211(1):214–226. doi: 10.1016/j.expneurol.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. A quantitative analysis of oligodendrocytes in multiple sclerosis lesions. A study of 113 cases. Brain. 1999;122(Pt 12):2279–2295. doi: 10.1093/brain/122.12.2279. [DOI] [PubMed] [Google Scholar]
- Lutterotti A, Yousef S, Sputtek A, Sturner KH, Stellmann JP, Breiden P, Reinhardt S, Schulze C, Bester M, Heesen C, Schippling S, Miller SD, Sospedra M, Martin R. Antigen-specific tolerance by autologous myelin Peptide-coupled cells: a phase 1 trial in multiple sclerosis. Sci Transl Med. 2013;5(188):188ra175. doi: 10.1126/scitranslmed.3006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahad D, Lassmann H, Turnbull D. Review: Mitochondria and disease progression in multiple sclerosis. Neuropathol Appl Neurobiol. 2008;34(6):577–589. doi: 10.1111/j.1365-2990.2008.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresz K, Pryce G, Ponomarev ED, Marsicano G, Croxford JL, Shriver LP, Ledent C, Cheng X, Carrier EJ, Mann MK, Giovannoni G, Pertwee RG, Yamamura T, Buckley NE, Hillard CJ, Lutz B, Baker D, Dittel BN. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat Med. 2007;13(4):492–497. doi: 10.1038/nm1561. [DOI] [PubMed] [Google Scholar]
- Mecha M, Torrao AS, Mestre L, Carrillo-Salinas FJ, Mechoulam R, Guaza C. Cannabidiol protects oligodendrocyte progenitor cells from inflammation-induced apoptosis by attenuating endoplasmic reticulum stress. Cell Death Dis. 2012;3:e331. doi: 10.1038/cddis.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz LM, Li D, Traboulsee A, Myles ML, Duquette P, Godin J, Constantin M, Yong VW. Glatiramer acetate in combination with minocycline in patients with relapsing–remitting multiple sclerosis: results of a Canadian, multicenter, double-blind, placebo-controlled trial. Mult Scler. 2009;15(10):1183–1194. doi: 10.1177/1352458509106779. [DOI] [PubMed] [Google Scholar]
- Metz LM, Zhang Y, Yeung M, Patry DG, Bell RB, Stoian CA, Yong VW, Patten SB, Duquette P, Antel JP, Mitchell JR. Minocycline reduces gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol. 2004;55(5):756. doi: 10.1002/ana.20111. [DOI] [PubMed] [Google Scholar]
- Mi S, Miller RH, Lee X, Scott ML, Shulag-Morskaya S, Shao Z, Chang J, Thill G, Levesque M, Zhang M, Hession C, Sah D, Trapp B, He Z, Jung V, Mccoy JM, Pepinsky RB. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci. 2005;8(6):745–751. doi: 10.1038/nn1460. [DOI] [PubMed] [Google Scholar]
- Mi S, Miller RH, Tang W, Lee X, Hu B, Wu W, Zhang Y, Shields CB, Miklasz S, Shea D, Mason J, Franklin RJ, Ji B, Shao Z, Chedotal A, Bernard F, Roulois A, Xu J, Jung V, Pepinsky B. Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells. Ann Neurol. 2009;65(3):304–315. doi: 10.1002/ana.21581. [DOI] [PubMed] [Google Scholar]
- Mi S, Sandrock A, Miller RH. LINGO-1 and its role in CNS repair. Int J Biochem Cell Biol. 2008;40(10):1971–1978. doi: 10.1016/j.biocel.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Njenga MK, Murray PD, Leibowitz J, Rodriguez M. A monoclonal natural autoantibody that promotes remyelination suppresses central nervous system inflammation and increases virus expression after Theiler’s virus-induced demyelination. Int Immunol. 1996;8(1):131–141. doi: 10.1093/intimm/8.1.131. [DOI] [PubMed] [Google Scholar]
- Miron VE, Kuhlmann T, Antel JP. Cells of the oligodendroglial line-age, myelination, and remyelination. Biochim Biophys Acta. 2011;1812(2):184–193. doi: 10.1016/j.bbadis.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Mokhtarian F, Safavi F, Sarafraz-Yazdi E. Immunization with a peptide of Semliki Forest virus promotes remyelination in experimental autoimmune encephalomyelitis. Brain Res. 2012;1488:92–103. doi: 10.1016/j.brainres.2012.09.038. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado E, Vela JM, Arevalo-Martin A, Almazan G, Molina-Holgado F, Borrell J, Guaza C. Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J Neurosci. 2002;22(22):9742–9753. doi: 10.1523/JNEUROSCI.22-22-09742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz JR, Stoutenger BR, Robinson AP, Spees JL, Prockop DJ. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci U S A. 2005;102(50):18171–18176. doi: 10.1073/pnas.0508945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm FJ, Lager AM, Zaremba A, Wyatt K, Caprariello AV, Factor DC, Karl RT, Maeda T, Miller RH, Tesar PJ. Transcription factor-mediated reprogramming of fibroblasts to expandable, myelinogenic oligodendrocyte progenitor cells. Nat Biotechnol. 2013;31(5):426–433. doi: 10.1038/nbt.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm FJ, Zaremba A, Caprariello AV, Nayak S, Freundt EC, Scacheri PC, Miller RH, Tesar PJ. Rapid and robust generation of functional oligodendrocyte progenitor cells from epiblast stem cells. Nat Methods. 2011;8(11):957–962. doi: 10.1038/nmeth.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottingham S, Knapp P, Springer J. FK506 treatment inhibits caspase-3 activation and promotes oligodendroglial survival following traumatic spinal cord injury. Exp Neurol. 2002;177(1):242–251. doi: 10.1006/exnr.2002.7975. [DOI] [PubMed] [Google Scholar]
- Pannu R, Barbosa E, Singh AK, Singh I. Attenuation of acute inflammatory response by atorvastatin after spinal cord injury in rats. J Neurosci Res. 2005;79(3):340–350. doi: 10.1002/jnr.20345. [DOI] [PubMed] [Google Scholar]
- Paul F, Waiczies S, Wuerfel J, Bellmann-Strobl J, Dorr J, Waiczies H, Haertle M, Wernecke KD, Volk HD, Aktas O, Zipp F. Oral high-dose atorvastatin treatment in relapsing-remitting multiple sclerosis. PLoS One. 2008;3(4):e1928. doi: 10.1371/journal.pone.0001928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron H, Garson JA, Bedin F, Beseme F, Paranhos-Baccala G, Komurian-Pradel F, Mallet F, Tuke PW, Voisset C, Blond JL, Lalande B, Seigneurin JM, Mandrand B. Molecular identification of a novel retrovirus repeatedly isolated from patients with multiple sclerosis. The Collaborative Research Group on Multiple Sclerosis. Proc Natl Acad Sci U S A. 1997;94(14):7583–7588. doi: 10.1073/pnas.94.14.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Martino G. The therapeutic plasticity of neural stem/precursor cells in multiple sclerosis. J Neurol Sci. 2008;265(1-2):105–110. doi: 10.1016/j.jns.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422(6933):688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Pohl HB, Porcheri C, Mueggler T, Bachmann LC, Martino G, Riethmacher D, Franklin RJ, Rudin M, Suter U. Genetically induced adult oligodendrocyte cell death is associated with poor myelin clearance, reduced remyelination, and axonal damage. J Neurosci. 2011;31(3):1069–1080. doi: 10.1523/JNEUROSCI.5035-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic N, Schubart A, Goetz BD, Zhang SC, Linington C, Duncan ID. Inhibition of autoimmune encephalomyelitis by a tetracycline. Ann Neurol. 2002;51(2):215–223. doi: 10.1002/ana.10092. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Barnard RO, Kwon EE, Sharer LR, Cho ES. Multiple sclerosis: remyelination of nascent lesions. Ann Neurol. 1993;33(2):137–151. doi: 10.1002/ana.410330203. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Kwon EE, Cho ES, Sharer LR. Continual breakdown and regeneration of myelin in progressive multiple sclerosis plaques. Ann N Y Acad Sci. 1984;436:11–32. doi: 10.1111/j.1749-6632.1984.tb14773.x. [DOI] [PubMed] [Google Scholar]
- Raine CS, Wu E. Multiple sclerosis: remyelination in acute lesions. J Neuropathol Exp Neurol. 1993;52(3):199–204. [PubMed] [Google Scholar]
- Reynolds BA, Rietze RL. Neural stem cells and neurospheres–re-evaluating the relationship. Nat Methods. 2005;2(5):333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- Robinson AP, Foraker JE, Ylostalo J, Prockop DJ. Human stem/progenitor cells from bone marrow enhance glial differentiation of rat neural stem cells: a role for transforming growth factor beta and Notch signaling. Stem Cells Dev. 2011;20(2):289–300. doi: 10.1089/scd.2009.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Miller DJ, Lennon VA. Immunoglobulins reactive with myelin basic protein promote CNS remyelination. Neurology. 1996;46(2):538–545. doi: 10.1212/wnl.46.2.538. [DOI] [PubMed] [Google Scholar]
- Rudick RA, Mi S, Sandrock AW., Jr LINGO-1 antagonists as therapy for multiple sclerosis: in vitro and in vivo evidence. Expert Opin Biol Ther. 2008;8(10):1561–1570. doi: 10.1517/14712598.8.10.1561. [DOI] [PubMed] [Google Scholar]
- Ruggieri M, Pica C, Lia A, Zimatore GB, Modesto M, Di Liddo E, Specchio LM, Livrea P, Trojano M, Avolio C. Combination treatment of Glatiramer Acetate and Minocycline affects phenotype expression of blood monocyte-derived dendritic cells in Multiple Sclerosis patients. J Neuroimmunol. 2008;197(2):140–146. doi: 10.1016/j.jneuroim.2008.04.032. [DOI] [PubMed] [Google Scholar]
- Safavi F, Feliberti JP, Raine CS, Mokhtarian F. Role of gammadelta T cells in antibody production and recovery from SFV demyelinating disease. J Neuroimmunol. 2011;235(1-2):18–26. doi: 10.1016/j.jneuroim.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzu A, Lathia JD, Zhao C, Wells K, Rao MS, Ffrench-Constant C, Franklin RJ. Inflammation stimulates myelination by transplanted oligodendrocyte precursor cells. Glia. 2006;54(4):297–303. doi: 10.1002/glia.20371. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Penderis J, Franklin RJ. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22(7):2451–2459. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solbrig MV, Fan Y, Hermanowicz N, Morgese MG, Giuffrida A. A synthetic cannabinoid agonist promotes oligodendrogliogenesis during viral encephalitis in rats. Exp Neurol. 2010;226(1):231–241. doi: 10.1016/j.expneurol.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stidworthy MF, Genoud S, Li WW, Leone DP, Mantei N, Suter U, Franklin RJ. Notch1 and Jagged1 are expressed after CNS demyelination, but are not a major rate-determining factor during remyelination. Brain. 2004;127(Pt 9):1928–1941. doi: 10.1093/brain/awh217. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011;10(7):649–656. doi: 10.1016/S1474-4422(11)70121-1. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Van De Loosdrecht AA, Ossenkoppele GJ, Beelen RH, Broekhoven MG, Drager AM, Langenhuijsen MM. Apoptosis in tumor necrosis factor-alpha-dependent, monocyte-mediated leukemic cell death: a functional, morphologic, and flow-cytometric analysis. Exp Hematol. 1993;21(13):1628–1639. [PubMed] [Google Scholar]
- Vergo S, Craner MJ, Etzensperger R, Attfield K, Friese MA, Newcombe J, Esiri M, Fugger L. Acid-sensing ion channel 1 is involved in both axonal injury and demyelination in multiple sclerosis and its animal model. Brain. 2011;134(Pt 2):571–584. doi: 10.1093/brain/awq337. [DOI] [PubMed] [Google Scholar]
- Vukusic S, Confavreux C. Pregnancy and multiple sclerosis: the children of PRIMS. Clin Neurol Neurosurg. 2006;108(3):266–270. doi: 10.1016/j.clineuro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, Maherali N, Studer L, Hochedlinger K, Windrem M, Goldman SA. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12(2):252–264. doi: 10.1016/j.stem.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington AE, Asakura K, Bieber AJ, Ciric B, Van Keulen V, Kaveri SV, Kyle RA, Pease LR, Rodriguez M. Human monoclonal antibodies reactive to oligodendrocytes promote remyelination in a model of multiple sclerosis. Proc Natl Acad Sci U S A. 2000;97(12):6820–6825. doi: 10.1073/pnas.97.12.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington AE, Bieber AJ, Ciric B, Pease LR, Van Keulen V, Rodriguez M. A recombinant human IgM promotes myelin repair after a single, very low dose. J Neurosci Res. 2007;85(5):967–976. doi: 10.1002/jnr.21217. [DOI] [PubMed] [Google Scholar]
- Watzlawik J, Holicky E, Edberg DD, Marks DL, Warrington AE, Wright BR, Pagano RE, Rodriguez M. Human remyelination promoting antibody inhibits apoptotic signaling and differentiation through Lyn kinase in primary rat oligodendrocytes. Glia. 2010;58(15):1782–1793. doi: 10.1002/glia.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watzlawik JO, Warrington AE, Rodriguez M. PDGF is required for remyelination-promoting IgM stimulation of oligodendrocyte progenitor cell proliferation. PLoS One. 2013;8(2):e55149. doi: 10.1371/journal.pone.0055149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waubant E, Pelletier D, Mass M, Cohen JA, Kita M, Cross A, Bar-Or A, Vollmer T, Racke M, Stuve O, Schwid S, Goodman A, Kachuck N, Preiningerova J, Weinstock-Guttman B, Calabresi PA, Miller A, Mokhtarani M, Ikle D, Murphy S, et al. Randomized controlled trial of atorvastatin in clinically isolated syndrome: the STAyCIS study. Neurology. 2012;78(15):1171–1178. doi: 10.1212/WNL.0b013e31824f7fdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem MS, Roy NS, Wang J, Nunes M, Benraiss A, Goodman R, Mckhann GM, 2nd, Goldman SA. Progenitor cells derived from the adult human subcortical white matter disperse and differentiate as oligodendrocytes within demyelinated lesions of the rat brain. J Neurosci Res. 2002;69(6):966–975. doi: 10.1002/jnr.10397. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Schanz SJ, Guo M, Tian GF, Washco V, Stanwood N, Rasband M, Roy NS, Nedergaard M, Havton LA, Wang S, Goldman SA. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2(6):553–565. doi: 10.1016/j.stem.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Drew PD. 9-Cis-retinoic acid suppresses inflammatory responses of microglia and astrocytes. J Neuroimmunol. 2006;171(1-2):135–144. doi: 10.1016/j.jneuroim.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Zuchero JB, Ahlenius H, Marro S, Ng YH, Vierbuchen T, Hawkins JS, Geissler R, Barres BA, Wernig M. Generation of oligodendroglial cells by direct lineage conversion. Nat Biotechnol. 2013;31(5):434–439. doi: 10.1038/nbt.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Chen Y, Hoang T, Montgomery RL, Zhao XH, Bu H, Hu T, Taketo MM, Van Es JH, Clevers H, Hsieh J, Bassel-Duby R, Olson EN, Lu QR. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci. 2009;12(7):829–838. doi: 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, Bravo M, Mitchell DJ, Sobel RA, Steinman L, Zamvil SS. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420(6911):78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- Zajicek JP, Apostu VI. Role of cannabinoids in multiple sclerosis. CNS Drugs. 2011;25(3):187–201. doi: 10.2165/11539000-000000000-00000. [DOI] [PubMed] [Google Scholar]