Abstract
The ovaries are susceptible to damage following treatment with gonadotoxic chemotherapy, pelvic radiotherapy, and/or ovarian surgery. Gonadotoxic treatments have also been used in patients with various nonmalignant systemic diseases. Any women of reproductive age with a sufficiently high risk of developing future ovarian failure due to those medical interventions may benefit from embryo cryopreservation though the tools of assessment of such a risk are still not very precise. Furthermore, the risk assessment can be influenced by many other factors such as the delay expected after chemotherapy and the number of children desired in the future. Embryo cryopreservation is an established and most successful method of fertility preservation when there is sufficient time available to perform ovarian stimulation. This publication will review the current state, approach, and indications of embryo cryopreservation for fertility preservation.
Keywords: Embryo cryopreservation, fertility preservation, ovarian damage
In the U.S., the estimated number of new cases of invasive cancer expected among women in the year 2012 is 790,740 (1). Early detection and improvements in screening have increased the number of premenopausal women diagnosed with cancer. As a result, it is estimated that a malignancy will be diagnosed in one among 46 females under the age of 40 years. Based on the cancer diagnosis, we have estimated that approximately half of these females will receive a form of gonadotoxic treatment hence approximately 1% of females with reproductive potential are at risk. With recent advances in cancer therapy, many of these patients will be cured by combination treatment with chemotherapy, radiotherapy, and/or surgery (2). In fact, during the most recent 5 years for which there are data (2004–2008), cancer death rates in women decreased by more than 1.6 % per year (1). However, these treatments have also long-term sequelae and patients must be informed of the possible risks of developing premature ovarian failure and infertility.
As the existing literature based on surveys (3) as well as qualitative and exploratory studies have revealed, fertility is a clear issue for cancer patients (4). Fertility preservation education is not only needed for those involved in reproductive health. Despite the fact that affected patients and their families are interested in information about fertility issues, only a few receive information prior to treatment for different reasons (Fig. 1) (5). Therefore, it is important for cancer care professionals to be familiar with the current techniques for fertility preservation in women with cancer.
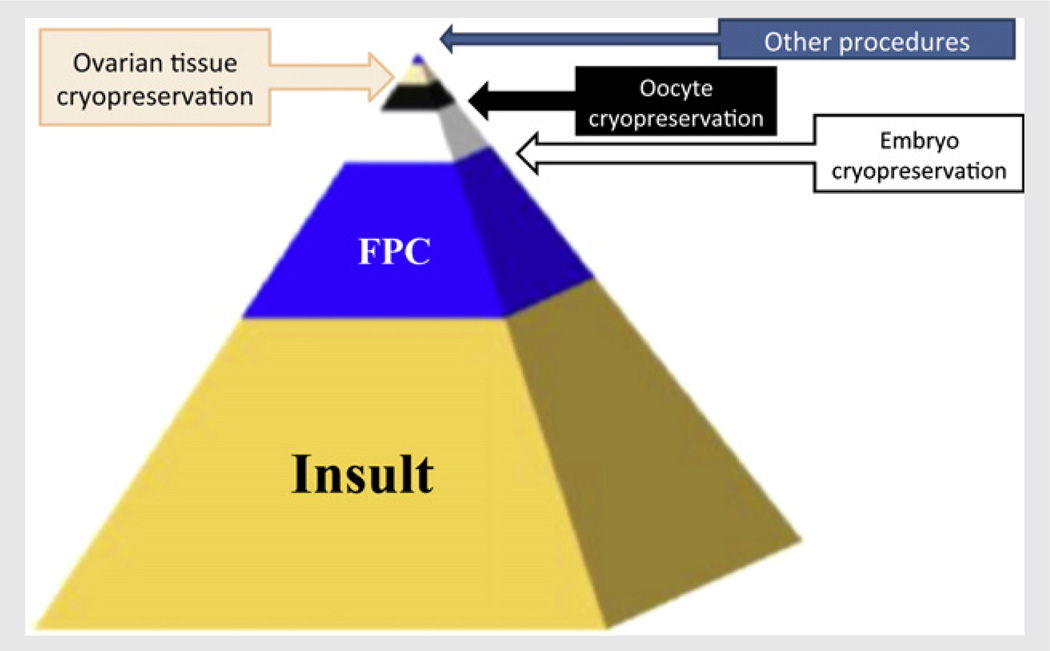
FIGURE 1.
“Pyramid” of fertility preservation. Medical interventions including chemotherapy, radiotherapy, and surgery act as insults to ovarian reserve and may result in premature ovarian failure and infertility. However, of all the patients at risk for premature ovarian failure, only a fraction will be referred to fertility preservation consultation (FPC) (5). Of those even a smaller fraction will be undergoing fertility preservation due to social, economic, or technical hurdles. Of all techniques offered, embryo cryopreservation is most commonly used, followed by oocyte cryopreservation, ovarian tissue freezing, and other methods, in that order.
Fertility preservation is not limited to cancer patients. Similar to cancer, there are some non-oncological systemic diseases which are treated with chemotherapy or radiotherapy, such as autoimmune and hematological conditions (6). In addition, there are other interventions that may impair fertility, such as recurrent ovarian surgery for benign disease or prophylactic oophorectomy in women with BRCA mutations. Therefore, fertility preservation is also commonly utilized in non-cancer conditions, increasing the number of females who benefit from this discipline even further.
The available fertility preservation methods range from established techniques such as embryo and oocyte cryopreservation to experimental techniques such as ovarian tissue cryopreservation (Fig. 2) (7, 8, 66). This publication will review the current state, approach, and indications of embryo freezing for fertility preservation.
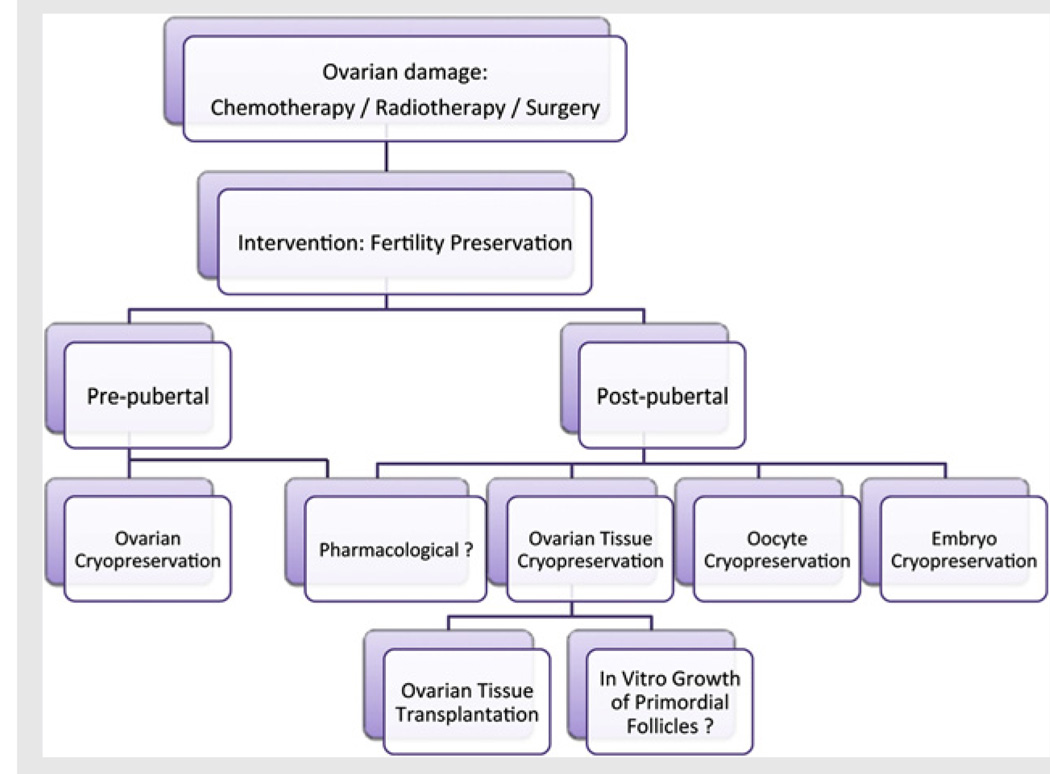
FIGURE 2.
A simplified scheme for fertility preservation options. In pre-pubertal girls, ovarian cryopreservation may be the only practical option. In post-pubertal females, a wider range of options is available with embryo cryopreservation being the most established method for patients with a male partner or who wish to use donor sperm. Oocyte cryopreservation, now considered an established method of fertility preservation by the American Society for Reproductive Medicine (7), is an option for older post-pubertal female children and single women. In cases where there is insufficient time for ovarian stimulation, ovarian cryopreservation as well as immature oocyte retrieval for in vitro maturation (followed by oocyte or embryo cryopreservation) may also be considered. In vitro growth (IVG) of isolated immature follicles is a theoretical option that may offer advantages in the future for females who have undergone ovarian freezing when there is a risk of ovarian involvement with cancer. The simplest approach to fertility preservation could have been a pharmacological intervention; however there is no proven hormonal treatment to preserve fertility. In the future, with the discovery of the mechanisms responsible for the chemotherapy-induced damage to the primordial follicles (8, 66), targeted pharmacological methods may be developed.
EMBRYO CRYOPRESERVATION FOR FERTILITY PRESERVATION
Embryo cryopreservation is an established technique that has been proven to be safe and effective in couples undergoing in vitro fertilization (IVF) treatment. Since the introduction of this technique in assisted reproductive technology (ART) (9), it became apparent that it also held a potential for fertility preservation purposes (10, 11). The first case of embryo cryopreservation for fertility preservation took place in 1996, with the application of a natural IVF cycle prior to chemotherapy in a woman diagnosed with breast cancer (12). Since then, embryo cryopreservation has become the most established technique for fertility preservation.
The procedure can be offered to women in reproductive age with available partner or for women using donor semen. Standard protocols for ovarian stimulation and oocyte retrieval usually requires 2 to 6 weeks of time commitment, depending on where in the menstrual cycle the patient presents.
Special considerations should be given to ovarian stimulation for fertility preservation patients. Ovarian stimulation protocols using gonadotropin-releasing hormone (GnRH) antagonists should be preferred, as they are associated with a lower risk of ovarian hyperstimulation syndrome (OHSS) (13). The risk of OHSS can further be decreased by triggering final oocyte maturation by GnRH agonists (14, 15) and in our center, this is the routine approach we take for cancer patients. Furthermore, to our experience, the use of GnRH agonists can also speed the interval from oocyte retrieval to next menses as well as reducing the likelihood and extent of residual ovarian cyst formation. This in turn improves the chances of multiple back-to-back cycles before initiating cancer treatment (16). In many instances there may not be sufficient time to wait for the menses to begin before initiating ovarian stimulation and random start protocols can be used with good results (17, 18). Patients with hormone sensitive tumors can also benefit from specific protocols that reduce estrogen exposure (16, 19–21).
Alternatively, immature oocytes can be harvested in an unstimulated cycle and fertilized following in vitro maturation (IVM) though the effectiveness of this approach in comparison to embryo freezing with mature oocytes remains to be determined. On the other hand, since a fraction of oocytes retrieved during IVF are immature and typically discarded, these germinal vesicle oocytes can be subjected to IVM to increase the oocyte and embryo yield in fertility preservation cycles (22).
SUCCESS RATES
As an established technique embryo, cryopreservation has reliable success rates. Even though pregnancy rates with frozen embryos appear to be lower than with fresh embryos in infertility patients, this is likely to be an embryo selection bias due to better embryos being utilized during the fresh attempt. When embryos are frozen such as in the case of ovarian hyperstimulation (23) as well as to our experience in fertility preservation (11), the pregnancy outcomes appear to be similar. In fact there is a recent meta-analysis that suggests that the frozen embryo transfer success rates are higher than with fresh embryo transfer. The latter is attributed to improved embryo-endometrium synchrony (24). Furthermore, despite the advent in oocyte cryopreservation success rates, overall, embryo cryopreservation still appears to offer higher success rates though this difference may be negligible in very young patients (25–28). Given the larger published evidence regarding outcomes, when feasible, embryo cryopreservation in general is offered as the primary method of fertility preservation. However some couples may still elect oocyte cryopreservation because of ethical, religious or practical reasons (such as possibility of future separation, see below) over embryo freezing.
LEGAL ASPECTS
When using fertility preservation procedures, a specialized informed consent is essential. Couples, or when using donor sperm the patient, have the right to know their options concerning fertility preservation and the risks and costs involved in each procedure. A controversial legal aspect is the use of embryos after patient's death, also termed posthumous reproduction. There are wide legal differences internationally concerning this subject, ranging from complete prohibition in some countries to permissive rules in others, often intersecting with religious belief. It should be documented whether the remaining partner is entitled to use the embryos for his/her own reproductive wishes or whether they are to be donated to a third party and used for research or discarded (29). Furthermore, a proportion of couples may be separated. In such a case, neither partner will have full rights over the embryos, and will need to reach a legal agreement prior a decision regarding the utility or disposition of embryos. Given that making such decisions can be particularly difficult for the patient who has been recently diagnosed with a life-threatening disease and is facing a demanding treatment period, they should be given the appropriate counseling using a multidisciplinary approach involving a psychologist and possibly a legal advisor (30).
WHO ARE THE CANDIDATES FOR EMBRYO FREEZING?
Gonadotoxic chemotherapy, radiotherapy, and/or ovarian surgery have been used to treat not only patients with malignant conditions, but also those with various nonmalignant systemic diseases. Any women in reproductive age with a sufficiently high risk of developing future ovarian failure may benefit from embryo cryopreservation though the tools of assessment of such a risk are still not very precise. Furthermore, the risk assessment can be influenced by many other factors such as the delay expected after chemotherapy and the number of children desired in the future. Embryo cryopreservation may be indicated in women with curable cancer where conception has to be postponed until the resolution of the primary disease and in women with nononcological conditions where reproductive function is threatened.
Breast Cancer
Breast cancer is the most frequent cancer diagnosed in women of reproductive age. In 2012, an estimated 230,000 new cases of invasive breast cancer are expected to be diagnosed in American women, whose lifetime risk of developing the disease is one in eight (1). Fortunately, breast cancer lends itself to early diagnosis and treatment when appropriate screening procedures are followed. However, breast cancer in young women presents with a high prevalence of ductal infiltration and most of those patients are likely to undergo adjuvant systemic chemotherapy with recognized gonadotoxic effects (31).
Embryo cryopreservation is an attractive strategy for fertility preservation in breast cancer patients who have a partner or who are willing to use donor semen. The process of embryo cryopreservation requires ovarian stimulation, oocyte retrieval, and IVF, which typically requires a delay of 2 to 6 weeks. Because women with breast cancer generally have a window of approximately 6 to 8 weeks between surgery and the initiation of adjuvant chemotherapy, it is feasible to undergo controlled ovarian hyperstimulation (32, 33).
Since the elevation of estradiol levels is undesirable in women diagnosed with breast cancer, those patients have been historically excluded from conventional ovarian stimulation and IVF. As a result, breast cancer patients were usually offered natural-cycle IVF, which resulted in a single embryo in approximately 60% of the preservation cycles (34). As the rise in estradiol is directly proportional to number of follicles recruited to grow, alternative and potentially safer protocols have been developed for fertility preservation in breast cancer patients including stimulation protocols with tamoxifen or aromatase inhibitors, alone or combined with gonadotropins, to reduce the estrogen production (16, 35). This topic has recently been reviewed in this Journal (16). Stimulation protocols using letrozole combined with gonadotropins are currently preferred over tamoxifen protocols, as treatment with letrozole has shown to be more effective and it is associated to a higher number of oocytes obtained and fertilized when compared to tamoxifen protocols (20). Furthermore, studies suggest that in the short term, aromatase inhibitor letrozole plus gonadotropin protocol is safe and effective for ovarian stimulation in fertility preservation cycles (36).
Endometrial Cancer
Endometrial cancer is another estrogen-sensitive malignancy, which can be encountered in reproductive age women. The accepted treatment of endometrial cancer in young women requires total abdominal hysterectomy and bilateral salpingoophorectomy. However, many of these patients have not initiated or completed childbearing and progestin treatment has been used to preserve fertility in women with stage 1, grade 1–2 endometrial carcinoma (37). Because some patients will not qualify for conservative management or will not respond to progestin treatment and will require surgical treatment, fertility preservation by embryo cryopreservation raises as a possibility before surgery. In earlier studies where assisted reproductive technologies were used in cases with existing endometrial cancer, typically a high-dose progestin treatment was performed prior to attempting IVF with conventional stimulation protocols. Those stimulation regimens generally expose patients to high estrogen levels, and no attempt was made to protect the endometrium against the effects of estrogen. As the elevation of estradiol levels is undesirable, the use of aromatase inhibitors has been developed for ovarian stimulation in patients with endometrial cancer (38). Because tamoxifen is stimulatory on the endometrium, it cannot be used in endometrial cancer for ovarian stimulation.
Hematologic Cancers
Because hematological cancers, particularly Hodgkin lymphoma and acute lymphoblastic leukemia tend to occur in a younger population, a large proportion of patients will be candidates for fertility preservation. Each hematological malignancy has a unique constellation of fertility considerations that relates to the disease itself, the gonadotoxic potential of common treatment protocols, and the age of the patient population (39). One serious complication is that urgent cancer treatment may not allow for a delay to perform ovarian stimulation. Therefore, patients due to undergo immediate cancer treatment are not candidates for embryo or oocyte cryopreservation and should, instead, be offered alternative methods of fertility preservation. Furthermore, if these patients are exposed to any class of chemotherapy agents prior to ovarian stimulation, there are concerns that these oocytes are DNA damaged and may not be ideal for IVF (8).
Fertility Preservation in Lymphoma Patients
Both Hodgkin (HL) and nonHodgkin lymphomas (NHL) are rare cancers with an incidence of 2 to 3 per 100,000 for HD and 7 to 12 per 100,000 for NHL (40). The overall 5-year survival rates are 85 % for HD and 50% to 60 % for NHL (40). Chemotherapy induced gonadal dysfunction depends on the age at first treatment and the treatment protocols. The younger the patient, the lower the risk of acute premature ovarian failure (POF). However, because gonadotoxic treatment will reduce ovarian reserve, most will experience early menopause when followed for sufficient amount of time (41).
There are several chemotherapeutic regimens for HL that includes adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) and regimens containing alkylating agents (bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, prednisone, procarbazine [BEACOPP]; mechlorethamine, vincristine, procarbazine, prednisone [MOPP]; and cyclophosphamide, doxorubicin, vincristine, prednisone [CHOP]). Treatment protocols like ABVD, without alkylating agents, very rarely result in premature ovarian failure (POF) (42–45) and may not necessarily require fertility preservation. Treatments following protocols that contain alkylating agents, especially procarbazine and cyclophosphamide in cumulative doses, induce POF more often, varying from 20% to 85% depending on the protocol (46). In some cases, hematopoietic stem cell transplantation (HSCT) may be required, associated with high risk of POF, especially if treated as adults.
Most treatment regimens for NHL include alkylating agents. CHOP acutely induces POF in approximately 5% of women with a mean age of 28 ± 7 years and pregnancy rates after treatment are 50% (47). Hyper-cyclophosphamide, vincristine, doxorubicin, dexamethasone, cytarabine, and methotrexate (CVAD) induces POF in approximately 14% of women with a mean age of 25 years and pregnancy rates after treatment are 43% (48). Again, all of those exposed to gonadotoxic agents will experience reduction in the reproductive life span. As in HL, HSCT may be required in some cases, associated with high risk of POF.
While some women may be receiving less gonadotoxic treatments for the initial treatment, treatment failures and recurrences may necessitate more toxic treatments at which time performing ovarian stimulation may not be practical due to recent exposure to chemotherapy and/or lack of sufficient time. As refractory diseases and relapse cannot be predicted, to our opinion, embryo cryopreservation and other fertility preservation options should be discussed with all reproductive-age patients diagnosed with either HL or NHL.
Fertility Preservation in Leukemia Patients
The rate of treatment-induced infertility in leukemia patients depends upon whether HSCT is required (49). The risk of infertility in patients with acute lymphocytic leukemia (ALL) (50) or acute myeloid leukemia (AML), unless treated with HSCT, is very low as contemporary treatment protocols entail either lower doses of alkylating agents or are devoid of alkylating agents. However, as discussed before, refractory diseases and relapse cannot be predicted and fertility preservation procedures, including embryo cryopreservation, should be discussed. Unlike in male leukemia, there is no evidence of pre-treatment fertility impairment (51).
Hematopoietic Stem Cell Transplantation
Hematopoietic stem cell transplantation has been critical in the treatment of numerous malignant and non-malignant systemic diseases. The risk of developing infertility is greatly influenced by the high gonadotoxicity of the preconditioning regimens that are used to ablate the pre-existing bone marrow (52). Preconditioning regimens utilize multiple alkylating agents, with or without total body irradiation (TBI), which are highly gonadotoxic (53–55). Overall pregnancy rates after HSCT remain low, ranging from 0.6 to 11 % (52, 53, 55, 56). Furthermore, women undergoing TBI have higher rates of preterm deliveries, cesarean sections and low birth-weight babies (53, 56), if TBI was performed during childhood. Chemotherapy exposure alone does not seem to affect uterine or endometrial function.
Because of the high risk of premature ovarian failure and infertility, it should be the standard of care to discuss fertility preservation options with women requiring HSCT. If there is sufficient time before treatment, embryo or oocyte cryopreservation can be offered. Ovarian cryopreservation is the only choice to preserve fertility in pediatric patients, and in patients who cannot postpone their treatment.
Pelvic Irradiation
Pelvic/abdominal radiotherapy is a well-established cause of premature ovarian failure and infertility. Radiotherapy to the ovaries causes DNA damage of somatic and germ cells that is not amenable to repair (57). Furthermore, the estimated lethal dose to destroy 50% of non-growing follicles present in the ovary is <2 Grays (58). Gonadal damage occurs not only by direct exposure of the ovaries following total body, abdominal or pelvic irradiation, but also due to scattering radiation. Age, dose, extent, and type of radiotherapy are important prognostic indicators for development of ovarian failure. Single dose radiotherapy also seems to be more toxic than fractionated doses (59). These patients can benefit from fertility preservation procedures before treatment including embryo cryopreservation, or alternatively oophoropexy may be considered, especially if an abdominal surgery is already necessary for the treatment of the primary disease.
Benign Ovarian Conditions Requiring Radical Surgery
Surgery on the ovary due to endometriosis or any other benign ovarian condition may diminish ovarian reserve and lead to premature ovarian failure. Ovarian reserve can be further compromised, either due to extensive or progressive disease, or because of bilateral occurrence and repeated surgery. Several studies reported a lower ovarian reserve after ovarian surgery, especially in patients with ovarian endometriomas, due to incidental excision of normal ovarian tissue during cystectomy or due to damage of healthy tissue by electrosurgical coagulation (60–62). Therefore, fertility preservation procedures, such as embryo cryopreservation, should be considered before surgery in reproductive-age women at risk of ovarian failure.
Prophylactic Oophorectomy in BRCA-Mutation Carriers
Women with BRCA 1 and BRCA 2 mutations have a markedly higher cumulative lifetime risk of developing breast and ovarian cancer. While women with BRCA 1 mutation have an estimated 40% to 90% lifetime risk of breast cancer and a 10% to 40% lifetime risk of ovarian cancer, women with BRCA 2 mutations have an estimated 40% to 50% lifetime risk of breast cancer and a 10% to 20% lifetime risk of ovarian cancer (63). Therefore, prophylactic oophorectomy is suggested as soon as childbearing is completed, or by the age 35 to 40 years depending on the family history, to decrease the risk of ovarian and breast cancer (64).
Embryo cryopreservation can be offered for women in reproductive age with available partner or for women willing to use donor semen who wish to delay childbearing beyond the age of 35 to 40 years. Moreover, women with BRCA mutation may have lower ovarian reserve, requiring multiple cycles to increase the embryo yield and improve subsequent pregnancy chances (65, 66). We have recently shown that serum anti-müllerian hormone levels are lower in BRCA 1 mutation carriers compared to those who tested negative for those mutations. Furthermore, BRCA 1 mutant mice have smaller litter size and have fewer primordial follicles (66). Moreover, women with BRCA mutations experience menopause earlier than those who tested negative (67). These data leave little doubt that BRCA mutations are associated with diminished ovarian reserve.
The possibility of preimplantation genetic diagnosis during IVF treatment to avoid transmitting the mutation has to be discussed with the patient and is an added advantage of fertility preservation by embryo cryopreservation (21, 68). However, use of preimplantation genetic diagnosis to select out BRCA carriers may not be a straightforward decision and may carry an emotional burden, as these mutations do not necessarily have lethal consequences.
Autoimmune and Hematological Diseases Requiring Gonadotoxic Therapy
Systemic lupus erythematosus (SLE) typically affects women in reproductive age, with an overall incidence between 40 and 250 per 100,000 people (69). Cyclophosphamide, with or without HSCT, is used in the treatment of severe manifestations of SLE, such as proliferative nephritis, affection of the central nervous system, pneumonitis, or severe thrombocytopenia (70) and can result in premature ovarian failure in rates of up to 50% in women younger than 30 years of age and 60% in women between 30 and 40 years of age (71). As there is a concern that high levels of estrogen may worsen disease activity in women with SLE, aromatase inhibitors may be used in a manner similar to its use in estrogen sensitive cancers.
Other severe systemic autoimmune diseases may require imminent gonadotoxic treatment with alkylating agents. Examples are refractory glomerulonephritis, inflammatory bowel diseases, Wegener's granulomatosis, and pemphigus vulgaris (72–75).
CONCLUSIONS
Fertility preservation with embryo cryopreservation is a safe and effective option in women at risk of premature ovarian failure due to medical treatment and interventions. As predicting the likelihood of infertility following gonadotoxic treatments is extremely difficult and this likelihood can be affected by unforeseen factors, fertility counseling should be offered to all females with reproductive potential. In most cases, ovarian stimulation protocols using GnRH antagonists and GnRH agonists for trigger should be preferred in fertility preservation cycles because of time limitations and to reduce the risk of OHSS. Patients with estrogen positive receptor cancers can benefit from specific protocols of ovarian stimulation with aromatase inhibitors. Mental Health Professional should be included in a team approach to fertility preservation. Reproductive Endocrinologists should be able to communicate and coordinate with oncologists and other medical specialists who are involved in the care of these young females considering fertility preservation to optimize the care and maximize safety.
Acknowledgments
Supported in part by National Institute of Child Health and Human Development grants R01 HD053112 and R21 HD061259.
Footnotes
Discuss: You can discuss this article with its authors and with other ASRM members at http://fertstertforum.com/bedoschig-fertility-preservation-embryo-cryopreservation/
G.B. has nothing to disclose. K.O. has nothing to disclose.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22:4174–4183. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 4.Tschudin S, Bitzer J. Psychological aspects of fertility preservation in men and women affected by cancer and other life-threatening diseases. Hum Reprod Update. 2009;15:587–597. doi: 10.1093/humupd/dmp015. [DOI] [PubMed] [Google Scholar]
- 5.Lee S, Ozkavukcu S, Heytens E, Moy F, Oktay K. Value of early referral to fertility preservation in young women with breast cancer. J Clin Oncol. 2010;28:4683–4686. doi: 10.1200/JCO.2010.30.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gidoni Y, Holzer H, Tulandi T, Tan SL. Fertility preservation in patients with non-oncological conditions. Reprod Biomed Online. 2008;16:792–800. doi: 10.1016/s1472-6483(10)60144-7. [DOI] [PubMed] [Google Scholar]
- 7.Practice Committees of American Society for Reproductive Medicine, Society for Assisted Reproductive. Mature oocyte cryopreservation: a guide-line. Fertil Steril. 2013;99:37–43. [Google Scholar]
- 8.Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY) 2011;3:782–793. doi: 10.18632/aging.100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trounson A, Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature. 1983;305:707–709. doi: 10.1038/305707a0. [DOI] [PubMed] [Google Scholar]
- 10.Ethics Committee of the American Society for Reproductive. Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;83:1622–1628. doi: 10.1016/j.fertnstert.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 12.Brown JR, Modell E, Obasaju M, King YK. Natural cycle in-vitro fertilization with embryo cryopreservation prior to chemotherapy for carcinoma of the breast. Hum Reprod. 1996;11:197–199. doi: 10.1093/oxfordjournals.humrep.a019017. [DOI] [PubMed] [Google Scholar]
- 13.Von Wolff M, Montag M, Dittrich R, Denschlag D, Nawroth F, Lawrenz B. Fertility preservation in women: a practical guide to preservation techniques and therapeutic strategies in breast cancer, Hodgkin's lymphoma and borderline ovarian tumours by the fertility preservation network FertiPRO-TEKT. Arch Gynecol Obstet. 2011;284:427–435. doi: 10.1007/s00404-011-1874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kol S, Solt I. GnRH agonist for triggering final oocyte maturation in patients at risk of ovarian hyperstimulation syndrome: still a controversy? J Assist Reprod Genet. 2008;25:63–66. doi: 10.1007/s10815-008-9198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oktay K, Turkcuoglu I, Rodriguez-Wallberg KA. GnRH agonist trigger for women with breast cancer undergoing fertility preservation by aromatase inhibitor/FSH stimulation. Reprod Biomed Online. 2010;20:783–788. doi: 10.1016/j.rbmo.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy J, Oktay K. Ovarian stimulation and fertility preservation with the use of aromatase inhibitors in women with breast cancer. Fertil Steril. 2012;98:1363–1369. doi: 10.1016/j.fertnstert.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 17.Von Wolff M, Thaler CJ, Frambach T, Zeeb C, Lawrenz B, Popovici RM, et al. Ovarian stimulation to cryopreserve fertilized oocytes in cancer patients can be started in the luteal phase. Fertil Steril. 2009;92:1360–1365. doi: 10.1016/j.fertnstert.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Bedoschi GM, De Albuquerque FO, Ferriani RA, Navarro PA. Ovarian stimulation during the luteal phase for fertility preservation of cancer patients: case reports and review of the literature. J Assist Reprod Genet. 2010;27:491–494. doi: 10.1007/s10815-010-9429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oktay K. Further evidence on the safety and success of ovarian stimulation with letrozole and tamoxifen in breast cancer patients undergoing in vitro fertilization to cryopreserve their embryos for fertility preservation. J Clin Oncol. 2005;23:3858–3859. doi: 10.1200/JCO.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23:4347–4353. doi: 10.1200/JCO.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Wallberg KA, Oktay K. Fertility preservation and pregnancy in women with and without BRCA mutation-positive breast cancer. Oncologist. 2012;17:1409–1417. doi: 10.1634/theoncologist.2012-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oktay K, Buyuk E, Rodriguez-Wallberg KA, Sahin G. In vitro maturation improves oocyte or embryo cryopreservation outcome in breast cancer patients undergoing ovarian stimulation for fertility preservation. Reprod Biomed Online. 2010;20:634–638. doi: 10.1016/j.rbmo.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 23.D'angelo A, Amso N. Embryo freezing for preventing ovarian hyperstimulation syndrome. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD002806.pub2. CD002806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roque M, Lattes K, Serra S, Sola I, Geber S, Carreras R, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. 2013;99:156–162. doi: 10.1016/j.fertnstert.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Cil AP, Rosen M, Oktay K. Impact of number of oocytes thawed and the day of embryo transfer on ivf success rates with vitrified donor oocytes: an individual patient data meta-analysis from 508 thaw cycles. Fertil Steril. 2012;98(Suppl):S75. [Google Scholar]
- 26.Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2011;96:277–285. doi: 10.1016/j.fertnstert.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 27.Cobo A, Meseguer M, Remohi J, Pellicer A. Use of cryo-banked oocytes in an ovum donation programme: a prospective, randomized, controlled, clinical trial. Hum Reprod. 2010;25:2239–2246. doi: 10.1093/humrep/deq146. [DOI] [PubMed] [Google Scholar]
- 28.Oktay K, Cil AP, Bang H. Efficiency of oocyte cryopreservation: a meta-analysis. Fertil Steril. 2006;86:70–80. doi: 10.1016/j.fertnstert.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 29.Shah DK, Goldman E, Fisseha S. Medical, ethical, and legal considerations in fertility preservation. Int J Gynaecol Obstet. 2011;115:11–15. doi: 10.1016/j.ijgo.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Ata B, Chian RC, Tan SL. Cryopreservation of oocytes and embryos for fertility preservation for female cancer patients. Best Pract Res Clin Obstet Gynaecol. 2010;24:101–112. doi: 10.1016/j.bpobgyn.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Wallberg KA, Oktay K. Options on fertility preservation in female cancer patients. Cancer Treat Rev. 2012;38:354–361. doi: 10.1016/j.ctrv.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Baynosa J, Westphal LM, Madrigrano A, Wapnir I. Timing of breast cancer treatments with oocyte retrieval and embryo cryopreservation. J Am Coll Surg. 2009;209:603–607. doi: 10.1016/j.jamcollsurg.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Madrigrano A, Westphal L, Wapnir I. Egg retrieval with cryopreservation does not delay breast cancer treatment. Am J Surg. 2007;194:477–481. doi: 10.1016/j.amjsurg.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Oktay K, Buyuk E, Davis O, Yermakova I, Veeck L, Rosenwaks Z. Fertility preservation in breast cancer patients: IVF and embryo cryopreservation after ovarian stimulation with tamoxifen. Hum Reprod. 2003;18:90–95. doi: 10.1093/humrep/deg045. [DOI] [PubMed] [Google Scholar]
- 35.Oktay K, Hourvitz A, Sahin G, Oktem O, Safro B, Cil A, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;91:3885–3890. doi: 10.1210/jc.2006-0962. [DOI] [PubMed] [Google Scholar]
- 36.Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008;26:2630–2635. doi: 10.1200/JCO.2007.14.8700. [DOI] [PubMed] [Google Scholar]
- 37.Ota T, Yoshida M, Kimura M, Kinoshita K. Clinicopathologic study of uterine endometrial carcinoma in young women aged 40 years and younger. Int J Gynecol Cancer. 2005;15:657–662. doi: 10.1111/j.1525-1438.2005.00129.x. [DOI] [PubMed] [Google Scholar]
- 38.Azim A, Oktay K. Letrozole for ovulation induction and fertility preservation by embryo cryopreservation in young women with endometrial carcinoma. Fertil Steril. 2007;88:657–664. doi: 10.1016/j.fertnstert.2006.12.068. [DOI] [PubMed] [Google Scholar]
- 39.Leader A, Lishner M, Michaeli J, Revel A. Fertility considerations and preservation in haemato-oncology patients undergoing treatment. Br J Haematol. 2011;153:291–308. doi: 10.1111/j.1365-2141.2011.08629.x. [DOI] [PubMed] [Google Scholar]
- 40.Storm HH, Klint A, Tryggvadottir L, Gislum M, Engholm G, Bray F, et al. Trends in the survival of patients diagnosed with malignant neoplasms of lymphoid, haematopoietic, and related tissue in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol. 2010;49:694–712. doi: 10.3109/02841861003631495. [DOI] [PubMed] [Google Scholar]
- 41.Sklar C. Maintenance of ovarian function and risk of premature menopause related to cancer treatment. J Natl Cancer Inst Monogr. 2005:25–27. doi: 10.1093/jncimonographs/lgi018. [DOI] [PubMed] [Google Scholar]
- 42.Kiserud CE, Fossa A, Holte H, Fossa SD. Post-treatment parenthood in Hodgkin's lymphoma survivors. Br J Cancer. 2007;96:1442–1449. doi: 10.1038/sj.bjc.6603711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hodgson DC, Pintilie M, Gitterman L, Dewitt B, Buckley CA, Ahmed S, et al. Fertility among female hodgkin lymphoma survivors attempting pregnancy following ABVD chemotherapy. Hematol Oncol. 2007;25:11–15. doi: 10.1002/hon.802. [DOI] [PubMed] [Google Scholar]
- 44.De Bruin ML, Huisbrink J, Hauptmann M, Kuenen MA, Ouwens GM, Van't Veer MB, et al. Treatment-related risk factors for premature menopause following Hodgkin lymphoma. Blood. 2008;111:101–108. doi: 10.1182/blood-2007-05-090225. [DOI] [PubMed] [Google Scholar]
- 45.Brusamolino E, Bacigalupo A, Barosi G, Biti G, Gobbi PG, Levis A, et al. Classical Hodgkin's lymphoma in adults: guidelines of the Italian Society of Hematology, the Italian Society of Experimental Hematology, and the Italian Group for Bone Marrow Transplantation on initial work-up, management, and follow-up. Haematologica. 2009;94:550–565. doi: 10.3324/haematol.2008.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Behringer K, Wildt L, Mueller H, Mattle V, Ganitis P, Van Den Hoonaard B, et al. No protection of the ovarian follicle pool with the use of GnRH-analogues or oral contraceptives in young women treated with escalated BEACOPP for advanced-stage Hodgkin lymphoma. Final results of a phase II trial from the German Hodgkin Study Group. Ann Oncol. 2010;21:2052–2060. doi: 10.1093/annonc/mdq066. [DOI] [PubMed] [Google Scholar]
- 47.Elis A, Tevet A, Yerushalmi R, Blickstein D, Bairy O, Dann EJ, et al. Fertility status among women treated for aggressive non-Hodgkin's lymphoma. Leuk Lymphoma. 2006;47:623–627. doi: 10.1080/10428190500353877. [DOI] [PubMed] [Google Scholar]
- 48.Seshadri T, Hourigan MJ, Wolf M, Mollee PN, Seymour JF. The effect of the Hyper-CVAD chemotherapy regimen on fertility and ovarian function. Leuk Res. 2006;30:483–485. doi: 10.1016/j.leukres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 49.Watson M, Wheatley K, Harrison GA, Zittoun R, Gray RG, Goldstone AH, et al. Severe adverse impact on sexual functioning and fertility of bone marrow transplantation, either allogeneic or autologous, compared with consolidation chemotherapy alone: analysis of the MRC AML 10 trial. Cancer. 1999;86:1231–1239. doi: 10.1002/(sici)1097-0142(19991001)86:7<1231::aid-cncr18>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 50.Kreuser ED, Hetzel WD, Heit W, Hoelzer D, Kurrle E, Xiros N, et al. Reproductive and endocrine gonadal functions in adults following multidrug chemotherapy for acute lymphoblastic or undifferentiated leukemia. J Clin Oncol. 1988;6:588–595. doi: 10.1200/JCO.1988.6.4.588. [DOI] [PubMed] [Google Scholar]
- 51.Hallak J, Kolettis PN, Sekhon VS, Thomas AJ, Jr, Agarwal A. Cryopreservation of sperm from patients with leukemia: is it worth the effort? Cancer. 1999;85:1973–1978. [PubMed] [Google Scholar]
- 52.Jadoul P, Anckaert E, Dewandeleer A, Steffens M, Dolmans MM, Vermylen C, et al. Clinical and biologic evaluation of ovarian function in women treated by bone marrow transplantation for various indications during childhood or adolescence. Fertil Steril. 2011;96:126.e3–133.e3. doi: 10.1016/j.fertnstert.2011.03.108. [DOI] [PubMed] [Google Scholar]
- 53.Salooja N, Szydlo RM, Socie G, Rio B, Chatterjee R, Ljungman P, et al. Pregnancy outcomes after peripheral blood or bone marrow transplantation: a retrospective survey. Lancet. 2001;358:271–276. doi: 10.1016/s0140-6736(01)05482-4. [DOI] [PubMed] [Google Scholar]
- 54.Spinelli S, Chiodi S, Bacigalupo A, Brasca A, Menada MV, Petti AR, et al. Ovarian recovery after total body irradiation and allogeneic bone marrow transplantation: long-term follow up of 79 females. Bone Marrow Transplant. 1994;14:373–380. [PubMed] [Google Scholar]
- 55.Sanders JE, Pritchard S, Mahoney P, Amos D, Buckner CD, Witherspoon RP, et al. Growth and development following marrow transplantation for leukemia. Blood. 1986;68:1129–1135. [PubMed] [Google Scholar]
- 56.Carter A, Robison LL, Francisco L, Smith D, Grant M, Baker KS, et al. Prevalence of conception and pregnancy outcomes after hematopoietic cell transplantation: report from the Bone Marrow Transplant Survivor Study. Bone Marrow Transplant. 2006;37:1023–1029. doi: 10.1038/sj.bmt.1705364. [DOI] [PubMed] [Google Scholar]
- 57.Dobrzynska MM. Assessment of DNA damage in multiple organs from mice exposed to X-rays or acrylamide or a combination of both using the comet assay. In Vivo. 2007;21:657–662. [PubMed] [Google Scholar]
- 58.Wallace WH, Shalet SM, Crowne EC, Morris-Jones PH, Gattamaneni HR. Ovarian failure following abdominal irradiation in childhood: natural history and prognosis. Clin Oncol (R Coll Radiol) 1989;1:75–79. doi: 10.1016/s0936-6555(89)80039-1. [DOI] [PubMed] [Google Scholar]
- 59.Knopman JM, Papadopoulos EB, Grifo JA, Fino ME, Noyes N. Surviving childhood and reproductive-age malignancy: effects on fertility and future parenthood. Lancet Oncol. 2010;11:490–498. doi: 10.1016/S1470-2045(09)70317-1. [DOI] [PubMed] [Google Scholar]
- 60.Ragni G, Somigliana E, Benedetti F, Paffoni A, Vegetti W, Restelli L, et al. Damage to ovarian reserve associated with laparoscopic excision of endometriomas: a quantitative rather than a qualitative injury. Am J Obstet Gynecol. 2005;193:1908–1914. doi: 10.1016/j.ajog.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 61.Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD004992.pub3. CD004992. [DOI] [PubMed] [Google Scholar]
- 62.Donnez J, Lousse JC, Jadoul P, Donnez O, Squifflet J. Laparoscopic management of endometriomas using a combined technique of excisional (cystectomy) and ablative surgery. Fertil Steril. 2010;94:28–32. doi: 10.1016/j.fertnstert.2009.02.065. [DOI] [PubMed] [Google Scholar]
- 63.Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kauff ND, Satagopan JM, Robson ME, Scheuer L, Hensley M, Hudis CA, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609–1615. doi: 10.1056/NEJMoa020119. [DOI] [PubMed] [Google Scholar]
- 65.Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol. 2010;28:240–244. doi: 10.1200/JCO.2009.24.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, et al. Impairment of BRCA1-Related DNA Double-Strand Break Repair Leads to Ovarian Aging in Mice and Humans. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3004925. 172ra21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin WT, Beattie M, Chen LM, Oktay K, Crawford SL, Gold EB, et al. Comparison of age at natural menopause in BRCA1/2 mutation carriers with a non-clinic- based sample of women in northern California. Cancer. 2013 doi: 10.1002/cncr.27952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sagi M, Weinberg N, Eilat A, Aizenman E, Werner M, Girsh E, et al. Preimplantation genetic diagnosis for BRCA1/2–a novel clinical experience. Prenat Diagn. 2009;29:508–513. doi: 10.1002/pd.2232. [DOI] [PubMed] [Google Scholar]
- 69.Michet CJ, Jr, Mckenna CH, Elveback LR, Kaslow RA, Kurland LT. Epidemiology of systemic lupus erythematosus and other connective tissue diseases in Rochester, Minnesota, 1950 through 1979. Mayo Clin Proc. 1985;60:105–113. doi: 10.1016/s0025-6196(12)60294-8. [DOI] [PubMed] [Google Scholar]
- 70.Schroeder JO, Euler HH, Loffler H. Synchronization of plasmapheresis and pulse cyclophosphamide in severe systemic lupus erythematosus. Ann Intern Med. 1987;107:344–346. doi: 10.7326/0003-4819-107-2-344. [DOI] [PubMed] [Google Scholar]
- 71.Manger K, Wildt L, Kalden JR, Manger B. Prevention of gonadal toxicity and preservation of gonadal function and fertility in young women with systemic lupus erythematosus treated by cyclophosphamide: the PREGO-Study. Autoimmun Rev. 2006;5:269–272. doi: 10.1016/j.autrev.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 72.Russell AI, Lawson WA, Haskard DO. Potential new therapeutic options in Behcet's syndrome. BioDrugs. 2001;15:25–35. doi: 10.2165/00063030-200115010-00003. [DOI] [PubMed] [Google Scholar]
- 73.Langford CA, Talar-Williams C, Barron KS, Sneller MC. Use of a cyclophosphamide- induction methotrexate-maintenance regimen for the treatment of Wegener's granulomatosis: extended follow-up and rate of relapse. Am J Med. 2003;114:463–469. doi: 10.1016/s0002-9343(03)00077-9. [DOI] [PubMed] [Google Scholar]
- 74.Nousari CH, Brodsky R, Anhalt GJ. Evaluating the role of immunoablative high-dose cyclophosphamide therapy in pemphigus vulgaris. J Am Acad Dermatol. 2003;49:148–150. doi: 10.1067/mjd.2003.581. [DOI] [PubMed] [Google Scholar]
- 75.Stallmach A, Wittig BM, Moser C, Fischinger J, Duchmann R, Zeitz M. Safety and efficacy of intravenous pulse cyclophosphamide in acute steroid refractory inflammatory bowel disease. Gut. 2003;52:377–382. doi: 10.1136/gut.52.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]