Abstract
Myocardial ischemia/reperfusion (I/R) is the most common cause of myocardial inflammation, which is primarily a manifestation of the innate immune responses. Innate immunity is activated when pattern recognition receptors (PRRs) responds to molecular patterns common to microbes and to danger signals expressed by injured or infected cells, so called pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). The expression of various PRRs in cardiomyocytes and the release of DAMPs from cardiomyocytes subjected to I/R injury, through active mechanisms as well as passive processes, enable cardiomyocytes to generate innate immune responses. Studies in isolated heart and cardiomyocytes have confirmed the inflammatory and functional effects of cardiac PRRs especially toll-like receptors in response to I/R-derived DAMPs, such as heat shock proteins. This review addresses the active role of cardiomyocytes in mediating innate inflammatory responses to myocardial I/R. We propose that cardiomyocytes act as innate immune cells in myocardial I/R injury.
Keywords: heart, cardiomyocytes, pattern recognition receptor, innate immunity, inflammation, ischemia/reperfusion, TLR4, TLR2, NF-κB
1. Introduction
Ischemic heart disease is the leading cause of heart failure. Growing evidence supports that innate immunity plays a critical role in myocardial ischemia and the development of heart failure. A mild to moderate innate immune response may limit the extent of cardiac injury and facilitate tissue repair, whereas an excessive response is likely to be deleterious (Mann, 2011). The persistent activation of innate immune responses, as characterized by progressive increases in serum inflammatory cytokines such as tumor necrosis factor (TNF) and interleukin (IL)-6, is associated with the development of heart failure.
The innate immunity, which manifests as inflammation, is typically generated by innate immune cells, including neutrophils, monocytes, macrophages and dendritric cells. It is activated when pattern recognition receptors (PRRs) in immune cells respond to conserved motifs of invading pathogens and nonself elements, namely pathogen-associated molecular patterns (PAMPs). PPRs may also respond to endogenous molecular patterns released during cellular injury or death, namely damage-associated molecular patterns (DAMPs), and subsequently induce sterile inflammation (Rock et al., 2010).
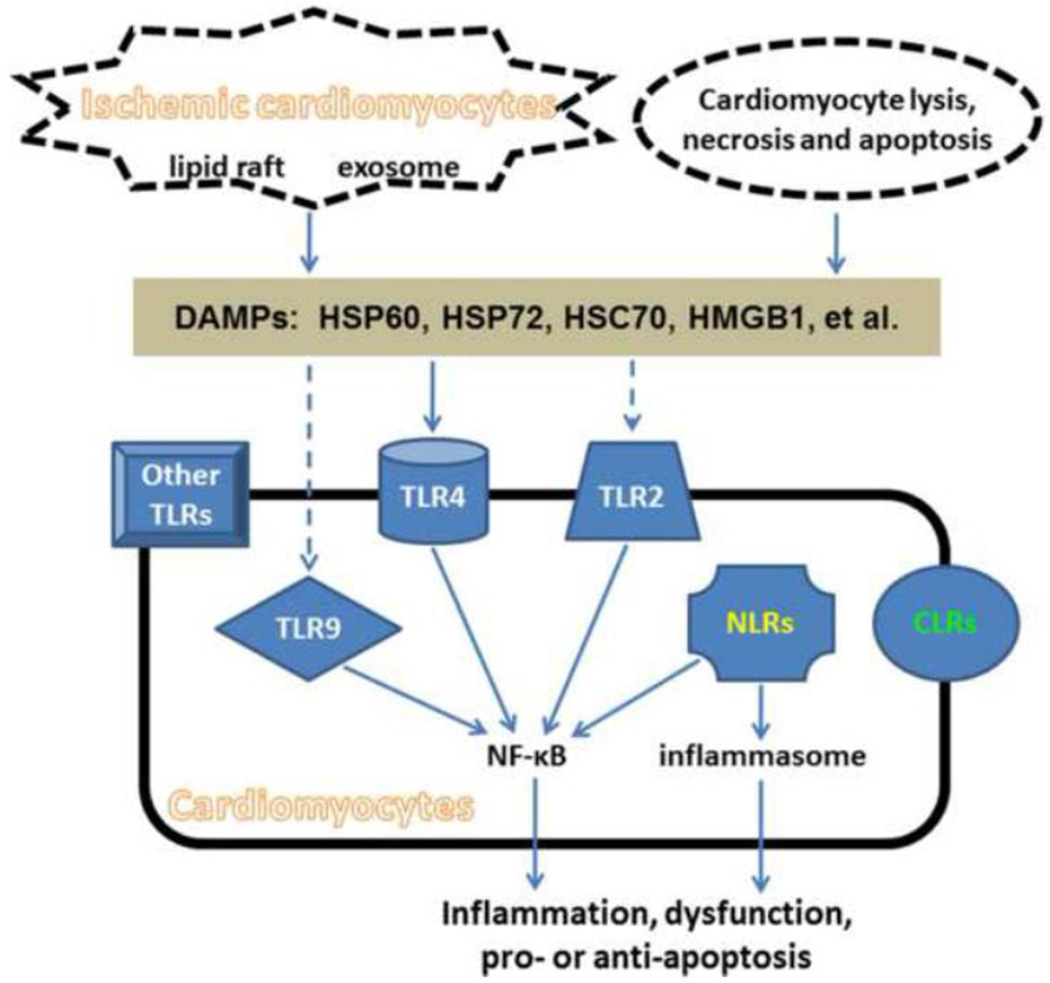
Recently, several lines of data have suggested that cardiomyocytes can be a significant source of innate immune responses. First, the release of multiple DAMPs from stressed cardiomyocytes through active pathways has been found. Second, the expression of a variety of PRRs has been identified in both basal and stressed cardiomyocytes. Third, activation of cardiomyocyte PRRs by either PAMPs or DAMPs leads to inflammatory signaling and cytokine expression, similar to the case for immune cells. The current review focuses on the active role of the heart in inducing and coordinating innate responses to myocardial ischemia/reperfusion (I/R). (Figure 1)
Figure 1.
Activation of PPRs in cardiomyocytes by DAMPs during myocardial I/R injury. Cardiomyocytes express a variety of PRRs including transmembrane receptors such as TLRs and CLRs, and cytoplasmic receptors such as NLRs. During myocardial I/R, DAMP molecules including HSP60, HSP72, HSC70 and HMGB1 can be released from ischemic cardiomyocytes, as well as cardiomyocytes undergoing lysis, necrosis and apoptosis. The release of HSP60, HSP72 and HSC70 is dependent on exosomes and/or lipid rafts, but not the classical secretory pathway. All the above DAMPs have been demonstrated to be able to activate TLR4 in cardiomyocytes. HSP72, but not HSP60, were shown to be able to activate TLR2 in cardiomyocytes. The activation of TLR2 and TLR9 by HMGB1 was demonstrated by studies in immune cells, but remains to be examined in cardiomyocytes. Endogenous DAMPs for other TLR subtypes, NLRs and CLRs remain unclear, though their expression has been recognized. The activation of cardiomyocyte TLR4, TLR2 and TLR9 results in NF-kB activation, which subsequently leads to inflammation, cardiac dysfunction and apoptotic effects. The activation of NLRs majorly results in the formation of inflammasome, which activates caspase-1 and trigger inflammation.
2. Overview of innate immunity and the heart
The immune system was originally described to function by making a distinction between self and nonself. In the relatively recent ‘danger model’ of immunity, the system is believed to react to ‘danger signals’, either self or nonself (Matzinger, 2002). The exogenous ‘danger signals’, so called PAMPs, are highly conserved motifs in microbial pathogens, such as lipopolysaccharide (LPS), peptidoglycan, lipoteichoic acid and flagellin of bacteria, mannan of yeast, chitin and ergosterol of fungi, and single- and double-stranded RNA of viruses. The endogenous 'danger signals', so called DAMPs, may come from distressed or injured cells, such as ischemic cardiac myocytes (Seong and Matzinger, 2004). Both PAMPs and DAMPs can activate the immune system through PRRs, a class of germline-encoded receptors, and lead to innate and adaptive immune responses (Akira et al., 2006). Innate immunity is a rapid response serving as the first line of host defense against danger signals. It is actually not nonspecific, as was originally thought, due to the specificity of PRRs for PAMPs and DAMPs. Furthermore, innate immunity is the major contributor to acute inflammation induced by PAMPs and DAMPs, and important for the activation of acquired immunity (Takeuchi and Akira, 2010). The processes of innate and adaptive immune responses following PRR activation have been addressed in several excellent reviews (Akira et al., 2006; Medzhitov, 2007; Kawai and Akira, 2010). Notably, the activation of PRRs, with the exception of some nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), leads to the production of inflammatory mediators including cytokines and chemokines (Takeuchi and Akira, 2010). Although PRRs are essential for protective immunity against danger signals, inappropriate PRR responses contribute to acute and chronic inflammation, and autoimmune diseases.
From an immunological perspective, the heart is not as active as other organs like the skin, gut, lung and liver, which undergo constant surveillance of pathogens from the external environment. The normal heart does not constitutively express inflammatory cytokines (Kapadia et al., 1995 & 1997). However, a variety of physical and chemical stresses, including pathogen infection, ischemia and mechanical stretch, can cause innate and adaptive immune responses in the heart. Most commonly, the heart becomes involved by innate immunity and acute inflammation as a result of I/R injury (Taqueti et al., 2006). Based on the fact that PRRs are expressed in virtually all cell types, the cardiomyocytes themselves, as well as immune cells that migrate into the myocardium, can respond to DAMPs generated by I/R. Thus, the heart acts as an immune organ in initiating cardiac immunity and inflammation, not just as a target organ affected by immunity.
The capacity of the heart to tolerate inflammatory processes is limited by the anatomy, function and limited regenerative capacity of the myocardium (Taqueti et al., 2006). As reviewed before (Mann, 2011), short-term innate immune activation confers adaptive and protective effects in the heart, whereas a long-lasting activation likely leads to maladaptive and detrimental effects. It has been well-documented that a brief episode of ischemia confers protection against subsequent sustained ischemia, a phenomenon termed as preconditioning, which was first described in the heart (Murry et al., 1986) and later extended to other organs. In contrast, prolonged ischemia leads to significant loss of cardiomyocytes and irreversible damage to the structure and function of the heart. In addition, deleterious remodeling occurs in both the infarcted and non-infarcted myocardium, which can impair cardiac function and increase the incidence of arrhythmias.
3. PRRs in cardiomyocytes
The discovery of PRRs has greatly advanced our understanding of how the body recognizes pathogens and starts immune responses. PRRs are a large family, including transmembrane receptors such as toll-like receptors (TLRs) and C-type lectin receptors (CLRs), as well as cytoplasmic receptors such as the retinoic acid-inducible gene (RIG)-I-like receptors (RLRs), NLRs, and absent-in-melanoma (AIM) 2 receptors (Rathinam et al., 2010; Takeuchi and Akira, 2010). These PRRs are expressed not only in immune cells, but also in various nonprofessional immune cells including cardiomyocytes. Among the PRRs expressed in the heart, TLRs have been most studied. This review focuses on the innate immune responses and phenotypic effects mediated by TLRs in cardiomyocytes. Cardiac CLRs and NLRs are also reviewed below. Other PRRs including RLRs and AIM2 are not included herein since so far little is known about those PRRs in the heart.
3.1 Toll-like receptors in cardiomyocytes
The first and best known PRRs are TLRs, which were originally discovered as a protein responsible for dorsal-ventral polarity in the fly (Anderson et al., 1985), and later identified as a critical component of the immune system (Lemaitre et al., 1996; Medzhitov et al., 1997). Ten functional TLRs have been identified in humans and 13 in mice. Among them, TLR 1/2/4/5/6/10/11/12/13 are expressed on cell surface, whereas TLR3/7/8/9 are localized in intracellular organelles, primarily endosomes and endoplasmic reticulum, with the ligand-binding domains facing the lumen of the organelles (Mann, 2011; Valeur and Valen, 2009). All TLRs are type I transmembrane proteins composed of a leucine-rich repeat (LRR) ectodomain involved in ligand recognition, a single transmembrane domain, and a cytoplasmic domain similar to that of IL-1 receptor, known as toll/IL-1 receptor (TIR) domain. Upon activation, TLRs recruit a specific set of TIR domain–containing adaptors, including myeloid differentiation primary-response gene 88 (MyD88), MyD88 adaptor-like protein (Mal), TIR-domain-containing adaptor protein inducing interferon-β (TRIF) and TRIF-related adaptor molecule (TRAM). Based on the recruited adaptors, TLR signaling can be largely divided to two pathways, MyD88- and Trif-dependent pathways. The MyD88-dependent pathway is universally used by all TLRs except TLR3, leading to the activation of nuclear factor-kappa B (NF-κB) for the induction of inflammatory cytokines. The Trif-dependent pathway is used by TLR3 and TLR4, leading to the activation of interferon regulatory factor (IRF) 3 and NF-κB for the induction of type I interferon (interferon-α/β) as well as inflammatory cytokines (Kawai and Akira, 2010).
Although the expression of different TLRs have not been verified in human cardiomyocytes, the mRNAs for all the 10 human TLRs have been detected in adult human heart tissue, with the relative levels as follows: TLR4 > TLR2 > TLR3 >TLR5 > TLR1 > TLR6 > TLR7 > TLR8 > TLR9 > TLR10 (Nishimura and Naito, 2005). Boyd et al. (2006) reported the mRNA expression of TLR2, TLR3, TLR4, TLR5, TLR7 and TLR9 in mouse heart tissue and a mouse cardiomyocyte cell line HL-1, though they did not examine the other subtypes of TLRs. Frantz et al. (2001) detected the mRNAs for TLR2, TLR3, TLR4, and TLR6, but not for TLR1 or TLR5 in neonatal rat cardiac myocytes, using the method of RT-PCR. Inconsistently, we detected the mRNAs for TLR1∼9 in cardiomyocytes isolated from adult rats, as well as a rat cardiomyocyte cell line H9c2 (unpublished data), using the method of real-time PCR. The expression of TLR2 and TLR4 proteins in cardiomyocytes have been demonstrated by immunological methods including immunohistochemistry and Western blot (Frantz et al., 1999; Tian et al., 2013), and by functional studies using chimeric mice deficient for TLR2 (Arslan et al., 2010) or TLR4 (Avlas et al., 2011) in the heart. However, the expression of other TLR proteins in cardiomyocytes needs further examination. Despite the above mentioned discrepancy for TLR mRNA expression and the lack of data for many TLR proteins, the confirmed expression of at least TLR2 and TLR4 in cardiomyocytes suggests the ability of cardiomyocytes to directly respond to PAMPs and DAMPs.
Changes of TLR expression have been observed in the ischemic and failing heart. Up-regulated expression of both TLR2 and TLR4 mRNA and proteins were observed in H9c2 cells exposed to ischemia, as well as rat heart tissue subjected to 4-h ischemia (Tian et al., 2013). Enhanced TLR4 protein expression was observed in remodeling murine myocardium remote from ischemic area and in heart tissue from patients with idiopathic dilated cardiomyopathy (Frantz et al., 1999). Enhanced TLR4 mRNA expression was also observed in the myocardium of deteriorating patients requiring left ventricular assist devices, compared to patients with less severe heart failure (Birks et al., 2004). In addition, PAMPs and DAMPs may change TLR expression in cardiomyocytes. The PAMP ligand LPS was observed to increase TLR4 mRNA expression, but not TLR4 protein level in neonatal rat ventricular myocytes (Frantz et al., 1999). Similarly, intraperitoneal injection of LPS increased TLR4 mRNA expression in both male and female mouse hearts, with a higher increase in male mouse hearts (Zhu et al., 2009). The mRNA and protein levels of both TLR2 and TLR4 were increased by a DAMP ligand heat shock protein (HSP) 60 in H9c2 cell line (Tian et al., 2013). However, the functional significance of these changes remains to be clarified.
In cardiac myocytes, activation of TLRs induces innate immune responses, manifested as the activation of NF-κB and inflammation, as well as functional changes of the heart. In the HL-1 cell line, transfection of a NF-κB-luciferase reporter plasmid showed increased NF-κB transcriptional activity in response to individual activation of TLR2, TLR4 and TLR5, but not TLR3, TLR7 or TLR9, by PAMP ligands. Also, activation of TLR2, TLR4 and TLR5 increased the expression of IL-6, keratinocyte-derived chemokine (KC), macrophage inflammatory protein (MIP)-2 and intercellular adhesion molecule (ICAM)-1, but decreased cardiomyocyte contractility (Boyd et al., 2006). Inconsistently, in wild-type mice, TLR9 mediated the increase of NF-κB activity and inflammatory cytokine production in the heart after intraperitoneal injection of synthetic bacterial DNA (CpG-ODN). Also, TLR9 mediated the decrease of cell shortening amplitude in isolated mouse cardiomyocytes exposed to CpG-ODN, suggesting a role of TLR9 in cardiac inflammation and contractile dysfunction (Knuefermann et al., 2008). In isolated mouse cardiomyocytes, a PAMP ligand for TLR2, peptidoglycan-associated lipoprotein, induced MyD88-dependent production of inflammatory cytokines, including TNFα, which subsequently depressed cell shortening and Ca2+ transients (Zhu et al., 2007). The TLR4 ligand LPS reduced sarcomere shortening amplitude and prolonged duration of relaxation in isolated mouse cardiomyocytes, which could be prevented by TLR4 deficiency, inhibition of TLR4 by E5564, as well as inhibition of inducible nitric oxide synthase (iNOS) (Baumgarten et al., 2006). In cardiomyocytes isolated from NF-κB-luciferase knock-in mice, DAMP ligands HSP60, HSP72 and high-mobility group box 1 protein (HMGB1) exclusively increased NF-κB transcriptional activity. Among them, HSP72 was the most active. Further observations on HSP72 showed that HSP72 induced expression of IL-6, KC and ICAM-1, and reduced myocyte contractility with no effect upon calcium flux, through the TLR2-MyD88 pathway (Mathur et al., 2011). In contrast, HSP60 was observed to induce cardiac inflammation through the TLR4-MyD88 pathway, whereas neither TLR2 nor Trif were involved (Kim et al., 2009; Li et al., 2011; Tian et al., 2013).
The above in vitro studies showed direct responses of TLRs on cardiomyocytes to PAMPs and DAMPs. Other work in the Langendorff-perfused heart (Ao et al., 2009; Binck et al., 2005; Zou et al., 2008) and in in vivo models (Avlas et al., 2011) support that TLRs in cardiomyocytes mediate cardiac responses to PAMPs and DAMPs, although the mediative role of TLRs in leukocytes has not been completely excluded (Tavener et al., 2004; Arslan et al., 2010). In Langendorff-perfused heart, both LPS (Binck et al., 2005) and the 70-kDa heat shock cognate protein (HSC70) (Ao et al., 2009; Zou et al., 2008) were observed to depress myocardial contractility in a TLR4-dependent manner. In an isolated mouse heart model of global I/R, infusion of neutrophils during reperfusion resulted in greater infiltration in TLR4-competent hearts than that in TLR4-defective hearts. However, infusion of TLR4-defective neutrophils did not influence infiltration in TLR-4 competent hearts. This supports that myocardial TLR4, rather than neutrophil TLR4, is the determinant of neutrophil infiltration after myocardial ischemia (Ao et al., 2009). Avlas et al. (2011) observed that chimeric mice deficient for TLR4 in the heart, but not in the immunohematopoietic system, were resistant to LPS injection, and cardiac function was significantly less depressed following coronary artery ligation, similar to TLR4-knockout mice. These findings suggest that cardiac TLR4, rather than leukocyte TLR4, plays a greater role in cardiac depression caused by both insults. In contrast, Tavener et al. (2004) reported that leukocyte TLR4 was critical for cardiomyocyte impairment, since chimeric mice with TLR4-deficient leukocytes, rather than TLR4-deficient cardiomyocytes, had no myocardial impairment in response to LPS. Arslan et al. (2010) reported that leukocyte TLR2 mediated myocardial I/R injury based on experiments in chimeric mice deficient for TLR2 either in the heart or in hematopoietic cells. Despite the discrepancies, direct responses of cardiomyocyte TLRs to PAMPs and DAMPs clearly have a role in cardiac injury and dysfunction.
In addition to causing inflammatory and functional changes, TLR signaling modulates cardiomyocyte apoptosis. TLR2 and TLR4 have been linked to both proapoptotic and survival pathways. An early report showed that TLR2 mediates an antiapoptotic effect and a proinflammatory pathway in neonatal rat ventricular myocytes exposed to hydrogen peroxide. Blocking TLR2 with a specific antagonistic antibody enhanced the cytotoxicity induced by hydrogen peroxide (Frantz et al., 2001). In Chagas disease, TLR2 expression is greatly increased in cardiac myocytes, and activation of TLR2 leads to increased IL-6, which in this setting has an anti-apoptotic effect. (Ponce et al., 2012). Thus, TLR2 activation can be beneficial in the setting of cardiac infection. In contrast, TLR2-knockout attenuated cardiac apoptosis, inflammation and dysfunction in mice treated with doxorubicin, suggesting a proapoptotic effect of TLR2 (Nozaki et al., 2004). With respect to TLR4, an early report indicated that LPS injection in rats resulted in induction of early apoptotic and survival pathways, as well as a very modest late-stage apoptosis at 24 h in the heart, while myocardial contractility dramatically decreased at 6 h (McDonald et al., 2000). A more recent study indicated that LPS pretreatment in mice reduced myocardial apoptosis and infarct size induced, through activation of the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway (Ha et al., 2008). In cultured rat cardiomyocytes, LPS induced a dose-dependent antiapoptotic effect against the combination of hypoxia and serum deprivation for up to 24 h, which was dependent on the activation of PI3K/Akt, ERK and IκB kinase β (Chao et al., 2005). Both TLR4 and MyD88 were required for the LPS-induced beneficial effects as demonstrated by improved survival and function in wild-type but not in TLR4−/− or MyD88−/− cardiomyocytes. Moreover, induction of iNOS was involved in the survival and functional rescue of serum-deprived cardiomyocytes treated with LPS (Zhu et al., 2006). Zhu et al. (2006) reported that in adult mouse cardiomyocytes, LPS , when added at the time of serum deprivation, led to a significant reduction in the number of apoptotic cells. LPS failed to exhibit anti-apoptotic effect when added 3–6 h after serum deprivation . Although TLR4-MyD88 pathway was anti-apoptotic in response to relatively low doses of LPS, the same pathway was found to mediate cardiomyocyte apoptosis induced by the DAMP ligand HSP60. Despite the fact that intracellular HSP60 in mitochondria and cytosol protect cardiomyocytes from apoptosis (Kirchhoff et al., 2002), translocation of HSP60 to the plasma membrane correlated with apoptosis (Gupta et al., 2002; Lin et al., 2007). Furthermore, extracellular HSP60, which was released from injured adult rat cardiomyocytes after hypoxia/reoxygenation, induced significant apoptosis in cardiomyocytes through the TLR4/NF-κB pathway (Kim et al., 2009). Similarly, exogenous recombinant, low endotoxin HSP60 induced apoptosis via TLR4 in cardiomyocytes (Kim et al., 2009; Li et al., 2011). The reason why TLR4 signaling activated by either LPS or translocated HSP60 differentially regulates apoptosis is unclear. Competitive binding studies showed that neither LPS nor HMGB1 interfered with HSP60 binding (Kim et al., 2009), but the TLR4 blocking antibody reduced HSP60 binding (unpublished data). Distinct binding sites for HSP60 and LPS are also supported by the fact that LPS required CD14 for binding to TLR4, whereas this was not the case for HSP60 (Kim et al., 2009). The different binding sites may indicate different receptor activity and/or downstream signaling of TLR4. Besides, the different effects of LPS and HSP60 are likely dependent on experimental models and protocols.
The contribution of cardiomyocyte TLRs to cardiac inflammation and dysfunction typically manifested in inflammatory heart diseases, including myocardial I/R, viral myocarditis and septic cardiomyopathy. A recent study showed that mitochondrial DNA that escaped from cells undergoing autophagy led to TLR9-mediated inflammatory responses in cardiomyocytes and was capable of inducing myocarditis and dilated cardiomyopathy (Oka et al., 2012). As has been thoroughly reviewed recently (Feng and Chao, 2011), a number of studies showed that mice with the inactive mutant TLR4 or genetically deficient for TLR2, TLR4 or MyD88 exhibited smaller infarct size after myocardial I/R, as well as reduced myocardial inflammation evidenced by reduced cytokine production and neutrophil infiltration. Others have found that the inactive TLR4 mutant reduced infarct size and serum cytokine levels, but did not preserve heart function after myocardial I/R (Kim et al., 2007). Studies of genetic deficiency for TLR3, TLR4, TLR7, TLR9, MyD88 and Trif revealed their involvement in viral myocarditis, although both protective and detrimental pathological effects have been reported (Feng and Chao, 2011). Considering the complexity of systemic deficiency, the exact role of cardiomyocyte TLRs (vs. immune TLRs) in inflammatory heart diseases remains to be completely defined, but much progress has been made.
3.2 NOD-like receptors in cardiomyocytes
NLRs act as cytosolic sensors to intracellular PAMPs and DAMPs. The human NLR family has 22 members, with most sharing a conserved tripartite structure consisting of an N-terminal caspase recruitment domain (CARD) or pyrin domain (PYD), a central nucleotide-binding domain with NTPase activity (NACHT), and a C-terminal LRR domain which mediates ligand sensing. A major inflammatory pathway downstream of NLRs is the activation of the inflammasome, a multiprotein platform that activates caspase-1. Once activated, caspase-1 induces the maturation of pro-cytokines, most notably IL1β and IL-18, which trigger inflammatory responses. Multiple types of NLRs, including CARD-containing NLR4 (NLRC4), NLRC5, PYD-containing NLR1 (NLRP1), NLRP3 and NLRP6, can form inflammasomes. (Franchi et al., 2012; Mason et al., 2012)
A data-mining study analyzed the expression profiles of human mRNA transcripts of NLRs and inflammasome components (Yin et al., 2009). The authors reported that the heart expresses only a few types of NLRs, including NLRC2 (NOD2), NLRC5 (NOD4) and NLRP2 (NALP2). However, immune and defense tissues including blood, lymph nodes, thymus and trachea express more types of NLRs. This study also described the heart as a tissue far from ready to express inflammasomes, since the heart requires upregulation of at least two components to assemble functional inflammasomes. In contrast, brain, placenta, blood and thymus were described as “ready-to-go” tissues since they constitutively express all the inflammasome components. Although this data-mining study reduced the potential contribution of the heart to inflammation mediated by NLR/inflammasome signaling, the results need further biological verification. At least, it is puzzling why TLR2 was the only type of heart-expressed TLRs identified by this study, while many biological studies have confirmed the expression of other types of TLRs such as TLR4, as discussed above. In contrast to the aforementioned data-mining study, a study of human autopsy ventricles from healthy young people who died in accidents revealed the expression of NLRC1, NLRP3 using RT-PCR, and detected 84 inflammasome signaling pathway genes using cDNA array analysis. More interestingly, significant upregulation of NLRC1, NLRP3 and of 29/84 inflammasome genes was found to be associated with air pollution (Villarreal-Calderon et al., 2012).
The NLR inflammasomes have been demonstrated to be involved in the context of myocardial I/R injury. The formation of NLRP3 inflammasome was found to be induced by myocardial ischemia. The exposure of cultured mouse HL-1 cardiomyocytes to simulated ischemia induced the formation of NLRP3 inflammasome, in association with increased cell death (Mezzaroma et al., 2011). In a mouse model of acute myocardial infarction (AMI), all the three components of NLRP3 inflammasome, NLRP3 (also known as cryopyrin), caspase-1 and the adaptor protein apoptosis associated speck-like protein containing a caspase-recruitment domain (ASC), localized to the granulation tissue and cardiomyocytes bordering the infarct area. The inhibition of NLRP3 using silencing RNA or a pharmacologic inhibitor prevented inflammasome formation, and limited infarct size and cardiac enlargement after AMI (Mezzaroma et al., 2011). Similarly, the inhibition of NLRP3 expression attenuated myocardial inflammation and fibrosis in the mice subjected to transverse aortic constriction (TAC) (Wang et al., 2013). Kawaguchi et al. reported increased ASC expression in the hearts of patients following myocardial infarction, and less inflammation and cardiac injury following I/R injury in mice deficient either for ASC or for caspase-1. However, inflammasome activation, likely triggered by reactive oxygen species and K+ efflux, was found in cardiac fibroblasts and infiltrated leukocytes, but not in cardiomyocytes in vitro (Kawaguchi et al., 2011). Although this study did not support the involvement of cardiomyocytes in inflammasome activation, it demonstrated the ability of heart-resident cells other than cardiomyocytes to form inflammasomes. Overall the limited literature in this area suggests that within the area of infarct inflammasomes form in fibroblasts and leukocytes, and only form in cardiac myocytes in the border zone.
In addition to the prominent activity in inflammasome signaling, NLRs regulate a wide range of cellular functions including NF-κB signaling, RLR signaling, autophagy, major histocompatibility complex gene regulation, reproduction and development (Mason et al., 2012). The activation of cardiac NLRC1 (NOD1) was demonstrated to induce cardiac dysfunction concomitantly with cardiac fibrosis and apoptosis in the murine heart, which resulted from the activation of the NF-κB and TGF-β pathways. At the cellular level, both native cardiomyocytes and cardiac fibroblasts expressed NLRC1. NLRC1 activation was linked to NF-κB activation and induction of apoptosis in cardiomyocytes, and to NF-κB activation and pro-fibrotic effects in cardiac fibroblasts (Fernández-Velasco et al., 2012).
3.3 C-type lectin receptors in cardiomyocytes
CLRs are calcium-dependent carbohydrate-binding receptors that contain one or more C-type lectin–like domains. They consist of a large family and recognize a diverse array of structurally unrelated molecules. Although various CLR members have been demonstrated in the context of immune responses, relatively little is known about cardiac CLRs. Lech et al. (2012) examined the mRNA expression profiles of CLRs and CLR-related signaling molecules in healthy adult human and murine solid organs using real time RT-PCR. Among the examined CLR mRNAs including Dectin-1, mannose receptor (MR) 1, MR2, DC-SIGN (CD209), Dec-205 (CD205), Galectin-1, T cell immunoglobulin mucin-3 (Tim-3), and triggering receptor expressed on myeloid cells (TREM)-1, all molecules were constitutively expressed in human and murine heart, except that Dec-205 was not detected in human heart. Signaling molecules for CLRs including Syk, Card-9, Bcl-10, Malt-1, Src and DAP-12 were detected in normal heart. Furthermore, all the above identified human CLR mRNAs , except for Dec-205 and Tim-3, were more abundant in the heart than in the immune organ spleen. The expression of CLRs and CLR-related signaling molecules in healthy heart supports the possible expression of CLRs in cardiomyocytes. Further study of the cellular distribution and function of cardiac CLRs is needed.
LOX-1, a well-known scavenger receptor for oxidized low-density lipoprotein which is highly expressed on endothelial cells, is a member of the ‘Dectin-1 cluster’ of CLRs. LOX-1 was also expressed in cardiomyocytes. Kang et al. (2009) reported that LOX-1 deletion altered immunoglobulin expression and its response to angiotensin II in mouse heart as well as cultured HL-1 cardiomyocytes. Thus, this study demonstrated the function of cardiomyocyte LOX-1 in immune responses. In the conditioned media derived from ischemic human cardiac tissue, which induced differentiation of human mesenchymal stem cells into early stage cardiomyocytes, C-type lectin superfamily member 13 (Clecsf13) was identified by a proteomics analysis as one of the three differentially expressed proteins (Ramesh et al., 2012). Although the exact role of Clecsf13 in cardiac regeneration needs further analysis, its differential expression in ischemic media at least suggests a role of Clecsf13 in myocardial ischemia. In sum, the CLRs are associated with important cardiac processes, such as cardiac regeneration, but much more work is needed to fully define the function of these proteins.
4. DAMPs generated by ischemic cardiomyocytes
Most of our current knowledge of endogenous DAMPs is limited to the DAMPs linked to TLRs, while little is known about the DAMPs linked to other PRRs. Theoretically, every molecule that normally localizes within cells can potentially be a DAMP when released to the extracellular space. The dynamic alterations of extracellular matrix (ECM) resulting from myocardial I/R can also generate DAMP molecules. Either ECM degradation products or de novo synthesized matrix molecules may function as endogenous DAMPs. Previous reviews summarized most of the DAMPs for TLRs (Arslan et al., 2011; Feng and Chao, 2011). This review focuses on the DAMPs generated by myocardial I/R injury, primarily intracellular molecules released from both dying and ischemia-stressed cardiomyocytes.
4.1 HSP60
Well-documented DAMP molecules released from ischemic cardiomyocytes are HSPs, especially HSP60 and HSP72. HSPs are highly conserved molecular chaperones that localize in various intracellular compartments. A variety of stresses including ischemia can induce HSP expression and cause cellular translocation and release of HSPs into extracellular milieu. The concept that specific HSPs can be actively secreted was first suggested by the observation that heat-shocked rat embryo cells rapidly released HSP72 and HSP110 (Hightower and Guidon, 1989).While it is well accepted that intracellular HSPs can be passively released through nonspecific processes, such as cell lysis, necrosis and apoptosis, accumulative evidence has demonstrated that HSPs can be secreted through specific active processes, both lipid raft-dependent (Broquet et al., 2003; Gupta and Knowlton, 2007) and the exosome-dependent pathways (Gupta and Knowlton, 2007; Lancaster and Febbraio, 2005) .
HSP60 is the first endogenous molecule discovered to be a TLR ligand (Ohashi et al., 2000). In normal cells, HSP60 is primarily localized in the mitochondria, with a small part in the cytosol. In isolated adult rat cardiomyocytes, we observed that cytosolic HSP60 complexes with bax in normal states. Reduction in cytosolic HSP60 precipitates translocation of bax to the mitochondria and apoptosis (Kirchhoff et al., 2002). When exposed to hypoxia, the total amount and mitochondrial content of HSP60 remained unchanged. However, cytosolic HSP60 translocated to the plasma membrane. Concurrently, bax translocated to the mitochondria, which was sufficient to trigger apoptosis (Gupta and Knowlton, 2002). We first showed that adult cardiomyocytes released exosomes, which provided an active pathway for the exocytosis of HSP60. Cardiomyocytes release HSP60 in exosomes through a mechanism dependent on the formation of lipid rafts, but not the classic, Golgi-mediated secretory pathway (Gupta and Knowlton, 2007).
The detrimental effects of extracellular HSP60 in myocardial I/R injury have been well documented (Arslan et al., 2011). It is highly notable that studies in vivo and vitro consistently showed that ischemia either alone or followed by reperfusion induce marked release of HSP60 from cardiomyocytes (Gupta and Knowlton, 2007; Li et al., 2011; Tian et al., 2013). HSP60 was also found in the circulation of heart failure rats induced by myocardial infarction, and the plasma membrane fraction of human hearts with dilated and ischemic cardiomyopathy (Lin et al., 2007). All these observations demonstrate the appearance of extracellular HSP60 as a DAMP under ischemia. Previously, exogenous HSP60 was showed to activate TLR2 and TLR4 in innate immune cells (Gobert et al., 2004; Vabulas et al., 2001). The study by Li et al. (2011) and our recent study (Tian et al., 2013) both suggest that HSP60, either exogenously recombinant or endogenously released by ischemic cardiomyocytes, can activate the TLR4-MyD88 signaling and induce innate immune responses in cardiomyocytes. In contrast, neither TLR2 nor Trif can be activated, despite that cardiomyocytes express abundant TLR2 and Trif proteins. Increases of serum HSP60 have been reported in a number of clinical conditions, including coronary artery disease (Mandal et al., 2006) and acute myocardial infarction (Novo et al., 2011). The activation of HSP60 on TLR4-MyD88 signaling in cardiomyocytes may represent an important trigger mechanism for cardiac inflammation in these diseases, and may contribute to continued inflammation and functional decline in ischemic heart failure.
4.2 HSP72 and HSC70
The 70-kDa HSP family is another well-documented TLR ligand associated with myocardial ischemia. Both the inducible HSP72 and the constitutive HSC70 have been identified as ligands of TLR2 and TLR4. While HSC70 is more abundantly expressed in basal state, the expression of HSP72 is more inducible than HSP60 in response to myocardial ischemia and heat stress (Marber et al., 1993). This can be explained, at least in part, by our observation that HSP60 expression was driven by NF-κB activation, as well as by HSF (Wang et al., 2010). Rat HSP60 contains only one heat shock element (HSE) for HSF to bind to, while the HSP72 gene contains two HSE. On the other hand, human HSP60 and 72 each contain 2 HSE. Thus, other factors must have a role in regulating the difference in inducibility of HSP60 and 72.
Various cell types including cardiomyocytes and immune cells may release HSP72 under various stresses. In clinical heart conditions related to ischemia, HSP72 was found to be released into the circulation after coronary artery bypass grafting (Dybdahl et al., 2002) and acute myocardial infarction (Dybdahl et al., 2005). Intracellular HSP72 is well-known for its protective effects in myocardial ischemia, but extracellular HSP72 has been demonstrated to act as a DAMP, activating immune responses. It can activate TLR2 and TLR4 in innate immune cells (Asea et al., 2002; Dybdahl et al., 2002), although its direct action on cardiomyocyte TLRs during myocardial I/R has not been reported, to our knowledge. Exogenous HSP72 was shown to activate NF-κB and induce inflammation in cardiomyocytes through the TLR2-MyD88 pathway (Mathur et al., 2011). Other than ischemia, the stress of pressure overload was observed to induce HSP72 expression and translocation to cardiomyocyte membrane and extracellular space. The extracellular HSP72 co-localized with TLR4, and mediated cardiac macrophage infiltration and inflammatory cytokine expression (Cai et al., 2010).
Zou et al. (2008) first showed that HSC70 was released from the heart during I/R and induced the expression of cardiac depressant cytokines via a TLR4-dependent mechanism. The same group further showed that myocardial TLR4, rather than neutrophil TLR4, mediated the pro-inflammatory effect of extracellular HSC70 released during I/R (Ao et al., 2009). The active secretory pathway for HSP72 and HSC70 has yet to be demonstrated in cardiomyocytes. In other cell types such as human peripheral blood mononuclear cells (PBMCs) in both basal and heat shock states (Lancaster and Febbraio, 2005) and rat endothelial cells challenged with oxidative low density lipoprotein and homocysteine (Zhan et al., 2009), HSP72 was released in exosomes, independent of lipid rafts or the classical secretory pathway. Although HSC70 was considered to be a common component of exosomes (Gupta and Knowlton, 2007), in serum-deprived baby hamster kidney (BHK-21) cells, secretion of HSP72 and HSC70 was reported to be dependent on lipid rafts, but not exosomes or the classical pathway (Evdokimovskaya et al., 2010).
4.3 HMGB1
HMGB1, a nuclear DNA-binding protein, has been identified as a DAMP when released into the extracellular space. It can be passively released during cellular necrosis by all nucleated cells, and actively secreted by stressed immune cells. Viable cardiomyocytes may also actively secrete HMGB1 under stresses, such as the stimulation of LPS (Xu et al., 2010), though the secretory mechanism remains unclear. Translocation of HBMG1 from nuclei to cytosol was observed as well in stressed cardiomyocytes (Funayama et al., 2013; Ha et al., 2011).
Extracellular HMGB1 can activate TLR2, TLR4 and TLR9 in immune cells (Park et al., 2004; Park et al., 2006; Tian et al., 2007). The activation of cardiomyocyte TLR4 by extracellular HMGB1 was suggested by physiological association between HMGB1 and TLR4 in myocardium (Ha et al., 2011), and inhibition of HMGB1-mediated cardiac effects by TLR4 deficiency (Yao et al., 2012). However, more direct evidence is needed to discriminate the activation of HMGB1 on cardiomyocytes from other heart-resident cells and infiltrated immune cells. Treatment with exogenous HMGB1 was observed to decrease contractility in adult cardiomyocytes (Tzeng et al., 2008) and induce hypertrophy in neonatal cardiomyocytes (Su et al., 2012).
It has been well-documented that myocardial I/R resulted in increased levels of HMGB1 in circulation. However, as reviewed by Arslan et al. (2011), conflicting results have been reported for the role of HMGB1 in myocardial I/R injury. A higher peak serum HMGB1 level was reported to be associated with pump failure, cardiac rupture, and in-hospital cardiac death in patients with acute myocardial infarction (Kohno et al., 2009). Pretreatment with HMGB1 1 h before myocardial I/R led to severe inflammation and enlarged infarct size in mice (Andrassy et al., 2008). Increased HMGB1 expression and release was observed in neonatal cardiomyocytes treated with doxorubicin, and pharmacological or genetic inhibition of HMGB1 attenuated doxorubicin-induced apoptosis (Yao et al., 2012). Similar results were reported for neonatal cardiomyocytes treated with hydrogen peroxide, in which increased HMGB1 expression and release mediated cell death (Yu et al., 2012). In contrast, cardiac-specific overexpression of HMGB1 restored heart function and improved survival after myocardial infarction by enhancing angiogenesis (Kitahara et al., 2008). Injection with HMGB1 in the peri-infarcted left ventricle at 4 h after ischemia improved heart function by stimulating the proliferation and differentiation of endogenous cardiac c-kit+ progenitor cells (Limana et al., 2005). Further study in intro showed that the conditioned media of HMGB1-stimulated human primary cardiac fibroblasts induced the migration and proliferation of cardiac c-kit+ progenitor cells, also suggesting the role of HMGB1 to facilitate cardiac regeneration (Rossini et al., 2008). The reason for the above discrepancies remains unknown. Arslan et al. (2011) raised a possible explanation that differences in ischemic models (with or without reperfusion) may reverse the effects of HMGB1-induced inflammation on myocardial I/R injury. In addition to that, we herein suggest that subcellular localization of HMGB1 might be another explanation. Nuclear HMGB1 is well-known for stabilizing nucleosomes and facilitating genome transcription and repair. It is predictable that loss of nuclear HMGB1 in cardiomyocytes could be closely related to cardiac dysfunction. This notion actually has been supported by studies that addressed myocardial HMGB1 translocation (Funayama et al., 2013; Ha et al., 2011). In the case of myocardial I/R, investigation of the subcellular distribution of HMGB1 and evaluation of the functional significance of each fraction (nuclear, cytosol, or extracellular HMGB1), is needed clarify the overall effects of HMGB1 in cardiac injury.
5. Conclusion
In terms of the ability to generate DAMPs and express functional PRRs, the heart functions as an active innate immune organ in myocardial I/R. Rather than passively being a target of inflammation, the active involvement of the heart in inducing and regulating inflammation provides important insights in myocardial I/R injury, and opens up a novel field for the investigation of cardiac inflammation, which can lead to new therapeutic interventions.
Acknowledgments
This work was supported by the National Institutes of Health [ grant numbers HL077281, HL079071] and a Merit Award from the Department of Veterans Affairs (all to AAK), and the National Natural Science Foundation of China (grant numbers 31071023 and 81370348, both to LL).
Table of abbreviations
- AIM
absent-in-melanoma
- CARD
caspase recruitment domain
- CLR
C-type lectin receptor
- DAMP
damage-associated molecular pattern
- HMGB1
high-mobility group box 1 protein
- HSC
heat shock cognate protein
- HSP
heat shock protein
- ICAM
intercellular adhesion molecule
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- I/R
ischemia/reperfusion
- IRF
interferon regulatory factor
- KC
keratinocyte-derived chemokine
- LPS
lipopolysaccharide
- LRR
leucine-rich repeat
- Mal
MyD88 adaptor-like protein
- MIP
macrophage inflammatory protein
- MR
mannose receptor
- MyD88
myeloid differentiation primary-response gene 88
- NF-κB
nuclear factor-kappa B
- NLR
NOD-like receptor
- NLRC
CARD-containing NLR
- NLRP
PYD-containing NLR
- NOD
nucleotide-binding oligomerization domain
- PAMP
pathogen-associated molecular pattern
- PI3K
phosphatidylinositol 3-kinase
- PRR
pattern recognition receptor
- PYD
pyrin domain
- RLR
retinoic acid-inducible gene (RIG)-I-like receptor
- RT-PCR
reverse transcription-polymerase chain reaction
- TIR
toll/IL-1 receptor
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- TRAM
TRIF-related adaptor molecule
- TRIF
TIR-domain-containing adaptor protein inducing interferon-β
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None declared.
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Anderson KV, Bokla L, Nüsslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell. 1985;42(3):791–798. doi: 10.1016/0092-8674(85)90275-2. [DOI] [PubMed] [Google Scholar]
- Anderson KV, Jürgens G, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell. 1985;42(3):779–789. doi: 10.1016/0092-8674(85)90274-0. [DOI] [PubMed] [Google Scholar]
- Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK, Bea F, Hardt SE, Humpert PM, Bianchi ME, Mairbäurl H, Nawroth PP, Remppis A, Katus HA, Bierhaus A. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117(25):3216–3226. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- Ao L, Zou N, Cleveland JC, Jr, Fullerton DA, Meng X. Myocardial TLR4 is a determinant of neutrophil infiltration after global myocardial ischemia: mediating KC and MCP-1 expression induced by extracellular HSC70. AJP. 2009;297(1):H21–H28. doi: 10.1152/ajpheart.00292.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan F, de Kleijn DP, Pasterkamp G. Innate immune signaling in cardiac ischemia. Nat Rev Cardiol. 2011;8(5):292–300. doi: 10.1038/nrcardio.2011.38. [DOI] [PubMed] [Google Scholar]
- Arslan F, Smeets MB, O'Neill LA, Keogh B, McGuirk P, Timmers L, Tersteeg C, Hoefer IE, Doevendans PA, Pasterkamp G, de Kleijn DP. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation. 2010;121(1):80–90. doi: 10.1161/CIRCULATIONAHA.109.880187. [DOI] [PubMed] [Google Scholar]
- Avlas O, Fallach R, Shainberg A, Porat E, Hochhauser E. Toll-like receptor 4 stimulation initiates an inflammatory response that decreases cardiomyocyte contractility. Antioxid Redox Signal. 2011;15(7):1895–1909. doi: 10.1089/ars.2010.3728. [DOI] [PubMed] [Google Scholar]
- Baumgarten G, Knuefermann P, Schuhmacher G, Vervölgyi V, von Rappard J, Dreiner U, Fink K, Djoufack C, Hoeft A, Grohé C, Knowlton AA, Meyer R. Toll-like receptor 4, nitric oxide, and myocardial depression in endotoxemia. Shock. 2006;25(1):43–49. doi: 10.1097/01.shk.0000196498.57306.a6. [DOI] [PubMed] [Google Scholar]
- Binck BW, Tsen MF, Islas M, White DJ, Schultz RA, Willis MS, Garcia JV, Horton JW, Thomas JA. Bone marrow-derived cells contribute to contractile dysfunction in endotoxic shock. Am J Physiol Heart Circ Physiol. 2005;288(2):H577–H583. doi: 10.1152/ajpheart.00745.2004. [DOI] [PubMed] [Google Scholar]
- Birks EJ, Felkin LE, Banner NR, Khaghani A, Barton PJ, Yacoub MH. Increased toll-like receptor 4 in the myocardium of patients requiring left ventricular assist devices. J Heart Lung Transplant. 2004;23(2):228–235. doi: 10.1016/S1053-2498(03)00106-2. [DOI] [PubMed] [Google Scholar]
- Boyd JH, Mathur S, Wang Y, Bateman RM, Walley KR. Toll-like receptor stimulation in cardiomyocytes decreases contractility and initiates an NF-κB dependent inflammatory response. Cardiovasc Res. 2006;72(3):384–393. doi: 10.1016/j.cardiores.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Broquet AH, Thomas G, Masliah J, Trugnan G, Bachelet M. Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J Biol Chem. 2003;278(24):21601–21606. doi: 10.1074/jbc.M302326200. [DOI] [PubMed] [Google Scholar]
- Cai WF, Zhang XW, Yan HM, Ma YG, Wang XX, Yan J, Xin BM, Lv XX, Wang QQ, Wang ZY, Yang HZ, Hu ZW. Intracellular or extracellular heat shock protein 70 differentially regulates cardiac remodelling in pressure overload mice. Cardiovasc Res. 2010;88(1):140–149. doi: 10.1093/cvr/cvq182. [DOI] [PubMed] [Google Scholar]
- Chao W, Shen Y, Zhu X, Zhao H, Novikov M, Schmidt U, Rosenzweig A. Lipopolysaccharide improves cardiomyocyte survival and function after serum deprivation. J Biol Chem. 2005;280(23):21997–22005. doi: 10.1074/jbc.M413676200. [DOI] [PubMed] [Google Scholar]
- Dybdahl B, Slørdahl SA, Waage A, Kierulf P, Espevik T, Sundan A. Myocardial ischaemia and the inflammatory response: release of heat shock protein 70 after myocardial infarction. Heart. 2005;91(3):299–304. doi: 10.1136/hrt.2003.028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybdahl B, Wahba A, Lien E, Flo TH, Waage A, Qureshi N, Sellevold OF, Espevik T, Sundan A. Inflammatory response after open heart surgery: release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation. 2002;105(6):685–690. doi: 10.1161/hc0602.103617. [DOI] [PubMed] [Google Scholar]
- Evdokimovskaya Y, Skarga Y, Vrublevskaya V, Morenkov O. Secretion of the heat shock proteins HSP70 and HSC70 by baby hamster kidney (BHK-21) cells. Cell Biol Int. 2010;34(10):985–990. doi: 10.1042/CBI20100147. [DOI] [PubMed] [Google Scholar]
- Feng Y, Chao W. Toll-like receptors and myocardial inflammation. Int J Inflam. 2011;2011:170352. doi: 10.4061/2011/170352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Velasco M, Prieto P, Terrón V, Benito G, Flores JM, Delgado C, Zaragoza C, Lavin B, Gómez-Parrizas M, López-Collazo E, Martín-Sanz P, Boscá L. NOD1 activation induces cardiac dysfunction and modulates cardiac fibrosis and cardiomyocyte apoptosis. PLoS One. 2012;7(9):e45260. doi: 10.1371/journal.pone.0045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Muñoz-Planillo R, Núñez G. Sensing and reacting to microbes via the inflammasomes. Nat Immunol. 2012;13(4):325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz S, Kelly RA, Bourcier T. Role of TLR-2 in the activation of nuclear factor-kappa B by oxidative stress in cardiac myocytes. J Biol Chem. 2001;276(7):5197–5203. doi: 10.1074/jbc.M009160200. [DOI] [PubMed] [Google Scholar]
- Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, Kelly RA. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104(3):271–280. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama A, Shishido T, Netsu S, Narumi T, Kadowaki S, Takahashi H, Miyamoto T, Watanabe T, Woo CH, Abe JI, Kuwahara K, Nakao K, Takeishi Y, Kubota I. Cardiac nuclear high mobility group box 1 prevents the development of cardiac hypertrophy and heart failure. Cardiovasc Res. 2013 Jul 14; doi: 10.1093/cvr/cvt128. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert AP, Bambou JC, Werts C, Balloy V, Chignard M, Moran AP, Ferrero RL. Helicobacter pylori heat shock protein 60 mediates interleukin-6 production by macrophages via a toll-like receptor (TLR)-2-, TLR-4-, and myeloid differentiation 88-independent mechanism. J Biol Chem. 2004;279(1):245–250. doi: 10.1074/jbc.M307858200. [DOI] [PubMed] [Google Scholar]
- Gupta S, Knowlton AA. Cytosolic heat shock protein 60, hypoxia, and apoptosis. Circulation. 2002;106(21):2727–2733. doi: 10.1161/01.cir.0000038112.64503.6e. [DOI] [PubMed] [Google Scholar]
- Gupta S, Knowlton AA. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol. 2007;292(6):H3052–H3056. doi: 10.1152/ajpheart.01355.2006. [DOI] [PubMed] [Google Scholar]
- Ha T, Hua F, Liu X, Ma J, McMullen JR, Shioi T, Izumo S, Kelley J, Gao X, Browder W, Williams DL, Kao RL, Li C. Lipopolysaccharide-induced myocardial protection against ischaemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovasc Res. 2008;78(3):546–553. doi: 10.1093/cvr/cvn037. [DOI] [PubMed] [Google Scholar]
- Ha T, Xia Y, Liu X, Lu C, Liu L, Kelley J, Kalbfleisch J, Kao RL, Williams DL, Li C. Glucan phosphate attenuates myocardial HMGB1 translocation in severe sepsis through inhibiting NF-κB activation. Am J Physiol Heart Circ Physiol. 2011;301(3):H848–H855. doi: 10.1152/ajpheart.01007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower LE, Guidon PT., Jr Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol. 1989;138(2):257–266. doi: 10.1002/jcp.1041380206. [DOI] [PubMed] [Google Scholar]
- Kang BY, Hu C, Prayaga S, Khaidakov M, Sawamura T, Seung KB, Mehta JL. LOX-1 dependent overexpression of immunoglobulins in cardiomyocytes in response to angiotensin II. Biochem Biophys Res Commun. 2009;379(2):395–399. doi: 10.1016/j.bbrc.2008.12.143. [DOI] [PubMed] [Google Scholar]
- Kapadia S, Lee J, Torre-Amione G, Birdsall HH, Ma TS, Mann DL. Tumor necrosis factor-alpha gene and protein expression in adult feline myocardium after endotoxin administration. J Clin Invest. 1995;96(2):1042–1052. doi: 10.1172/JCI118090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia SR, Oral H, Lee J, Nakano M, Taffet GE, Mann DL. Hemodynamic regulation of tumor necrosis factor-alpha gene and protein expression in adult feline myocardium. Circ Res. 1997;81(2):187–195. doi: 10.1161/01.res.81.2.187. [DOI] [PubMed] [Google Scholar]
- Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J, Hongo M, Noda T, Nakayama J, Sagara J, Taniguchi S, Ikeda U. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123(6):594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kim SC, Ghanem A, Stapel H, Tiemann K, Knuefermann P, Hoeft A, Meyer R, Grohé C, Knowlton AA, Baumgarten G. Toll-like receptor 4 deficiency: smaller infarcts, but no gain in function. BMC Physiol. 2007;7:5. doi: 10.1186/1472-6793-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SC, Stice JP, Chen L, Jung JS, Gupta S, Wang Y, Baumgarten G, Trial J, Knowlton AA. Extracellular heat shock protein 60, cardiac myocytes, and apoptosis. Circ Res. 2009;105(12):1186–1195. doi: 10.1161/CIRCRESAHA.109.209643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff SR, Gupta S, Knowlton AA. Cytosolic heat shock protein 60, apoptosis, and myocardial injury. Circulation. 2002;105(24):2899–2904. doi: 10.1161/01.cir.0000019403.35847.23. [DOI] [PubMed] [Google Scholar]
- Kitahara T, Takeishi Y, Harada M, Niizeki T, Suzuki S, Sasaki T, Ishino M, Bilim O, Nakajima O, Kubota I. High-mobility group box 1 restores cardiac function after myocardial infarction in transgenic mice. Cardiovasc Res. 2008;80(1):40–46. doi: 10.1093/cvr/cvn163. [DOI] [PubMed] [Google Scholar]
- Knuefermann P, Schwederski M, Velten M, Krings P, Ehrentraut H, Rüdiger M, Boehm O, Fink K, Dreiner U, Grohé C, Hoeft A, Baumgarten G, Koch A, Zacharowski K, Meyer R. Bacterial DNA induces myocardial inflammation and reduces cardiomyocyte contractility: role of toll-like receptor 9. Cardiovasc Res. 2008;78(1):26–35. doi: 10.1093/cvr/cvn011. [DOI] [PubMed] [Google Scholar]
- Kohno T, Anzai T, Naito K, Miyasho T, Okamoto M, Yokota H, Yamada S, Maekawa Y, Takahashi T, Yoshikawa T, Ishizaka A, Ogawa S. Role of high-mobility group box 1 protein in post-infarction healing process and left ventricular remodelling. Cardiovasc Res. 2009;81(3):565–573. doi: 10.1093/cvr/cvn291. [DOI] [PubMed] [Google Scholar]
- Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem. 2005;280(24):23349–23355. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- Lech M, Susanti HE, Römmele C, Gröbmayr R, Günthner R, Anders HJ. Quantitative expression of C-type lectin receptors in humans and mice. Int J Mol Sci. 2012;13(8):10113–10131. doi: 10.3390/ijms130810113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86(6):973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Li Y, Si R, Feng Y, Chen HH, Zou L, Wang E, Zhang M, Warren HS, Sosnovik DE, Chao W. Myocardial ischemia activates an injurious innate immune signaling via cardiac heat shock protein 60 and toll-like receptor 4. J Biol Chem. 2011;286(36):31308–31319. doi: 10.1074/jbc.M111.246124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limana F, Germani A, Zacheo A, Kajstura J, Di Carlo A, Borsellino G, Leoni O, Palumbo R, Battistini L, Rastaldo R, Müller S, Pompilio G, Anversa P, Bianchi ME, Capogrossi MC. Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac C-kit+ cell proliferation and differentiation. Circ Res. 2005;97(8):e73–e83. doi: 10.1161/01.RES.0000186276.06104.04. [DOI] [PubMed] [Google Scholar]
- Mandal K, Afzal AR, Brecker SJ, Poloniecki J, Xu Q, Jahangiri M. Association of serum soluble heat shock protein 60 with toll-like receptor 4 polymorphism and severity of coronary artery disease. Heart. 2006;92(5):683–685. doi: 10.1136/hrt.2004.059170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res. 2011;108(9):1133–1145. doi: 10.1161/CIRCRESAHA.110.226936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88(3):1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- Mason DR, Beck PL, Muruve DA. Nucleotide-binding oligomerization domain-like receptors and inflammasomes in the pathogenesis of non-microbial inflammation and diseases. J Innate Immun. 2012;4(1):16–30. doi: 10.1159/000334247. [DOI] [PubMed] [Google Scholar]
- Mathur S, Walley KR, Wang Y, Indrambarya T, Boyd JH. Extracellular heat shock protein 70 induces cardiomyocyte inflammation and contractile dysfunction via TLR2. Circ J. 2011;75(10):2445–2452. doi: 10.1253/circj.cj-11-0194. [DOI] [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296(5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- McDonald TE, Grinman MN, Carthy CM, Walley KR. Endotoxin infusion in rats induces apoptotic and survival pathways in hearts. Am J Physiol Heart Circ Physiol. 2000;279(5):H2053–H2061. doi: 10.1152/ajpheart.2000.279.5.H2053. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449(7164):819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388(6640):394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF, Abbate A. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A. 2011;108(49):19725–19730. doi: 10.1073/pnas.1108586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol Pharm Bull. 2005;28(5):886–892. doi: 10.1248/bpb.28.886. [DOI] [PubMed] [Google Scholar]
- Novo G, Cappello F, Rizzo M, Fazio G, Zambuto S, Tortorici E, Marino Gammazza A, Corrao S, Zummo G, De Macario EC, Macario AJ, Assennato P, Novo S, Li Volti G. Hsp60 and heme oxygenase-1 (Hsp32) in acute myocardial infarction. Transl Res. 2011;157(5):285–292. doi: 10.1016/j.trsl.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Nozaki N, Shishido T, Takeishi Y, Kubota I. Modulation of doxorubicin-induced cardiac dysfunction in toll-like receptor-2-knockout mice. Circulation. 2004;110(18):2869–2874. doi: 10.1161/01.CIR.0000146889.46519.27. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164(2):558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485(7397):251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290(3):C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of Toll like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279(9):7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- Ponce NE, Cano RC, Carrera-Silva EA, Lima AP, Gea S, Aoki MP. Toll-like receptor-2 and interleukin-6 mediate cardiomyocyte protection from apoptosis during Trypanosoma cruzi murine infection. Med Microbiol Immunol. 2012;201(2):145–155. doi: 10.1007/s00430-011-0216-z. [DOI] [PubMed] [Google Scholar]
- Ramesh B, Bishi DK, Rallapalli S, Arumugam S, Cherian KM, Guhathakurta S. Ischemic cardiac tissue conditioned media induced differentiation of human mesenchymal stem cells into early stage cardiomyocytes. Cytotechnology. 2012;64(5):563–575. doi: 10.1007/s10616-012-9440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11(5):395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol. 2010;28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini A, Zacheo A, Mocini D, Totta P, Facchiano A, Castoldi R, Sordini P, Pompilio G, Abeni D, Capogrossi MC, Germani A. HMGB1-stimulated human primary cardiac fibroblasts exert a paracrine action on human and murine cardiac stem cells. J Mol Cell Cardiol. 2008;44(4):683–693. doi: 10.1016/j.yjmcc.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4(6):469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- Su FF, Shi MQ, Guo WG, Liu XT, Wang HT, Lu ZF, Zheng QS. High-mobility group box 1 induces calcineurin-mediated cell hypertrophy in neonatal rat ventricular myocytes. Mediators Inflamm. 2012;2012:805149. doi: 10.1155/2012/805149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Taqueti VR, Mitchell RN, Lichtman AH. Protecting the pump: controlling myocardial inflammatory responses. Annu Rev Physiol. 2006;68:67–95. doi: 10.1146/annurev.physiol.68.040104.124611. [DOI] [PubMed] [Google Scholar]
- Tavener SA, Long EM, Robbins SM, McRae KM, Van Remmen H, Kubes P. Immune cell Toll-like receptor 4 is required for cardiac myocyte impairment during endotoxemia. Circ Res. 2004;95(7):700–707. doi: 10.1161/01.RES.0000144175.70140.8c. [DOI] [PubMed] [Google Scholar]
- Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An LL, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow MK, Fitzgerald KA, Latz E, Kiener PA, Coyle AJ. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8(5):487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- Tian J, Guo X, Liu XM, Liu L, Weng QF, Dong SJ, Knowlton AA, Yuan WJ, Lin L. Extracellular HSP60 induces inflammation through activating and up-regulating TLRs in cardiomyocytes. Cardiovasc Res. 2013;98(3):391–401. doi: 10.1093/cvr/cvt047. [DOI] [PubMed] [Google Scholar]
- Tzeng HP, Fan J, Vallejo JG, Dong JW, Chen X, Houser SR, Mann DL. Negative inotropic effects of high-mobility group box 1 protein in isolated contracting cardiac myocytes. Am J Physiol Heart Circ Physiol. 2008;294(3):H1490–H1496. doi: 10.1152/ajpheart.00910.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, da Costa C, Miethke T, Kirschning CJ, Häcker H, Wagner H. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276(33):31332–31339. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- Valeur HS, Valen G. Innate immunity and myocardial adaptation to ischemia. Basic Res Cardiol. 2009;104(1):22–32. doi: 10.1007/s00395-008-0756-6. [DOI] [PubMed] [Google Scholar]
- Villarreal-Calderon R, Dale G, Delgado-Chávez R, Torres-Jardón R, Zhu H, Herritt L, Gónzalez-Maciel A, Reynoso-Robles R, Yuan Y, Wang J, Solorio-López E, Medina-Cortina H, Calderón-Garcidueñas L. Intra-city differences in cardiac expression of inflammatory genes and inflammasomes in young urbanites: a pilot study. J Toxicol Pathol. 2012;25(2):163–173. doi: 10.1293/tox.25.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen L, Hagiwara N, Knowlton AA. Regulation of heat shock protein 60 and 72 expression in the failing heart. J Mol Cell Cardiol. 2010;48(2):360–366. doi: 10.1016/j.yjmcc.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu Y, Chen J, Zhao S, Li H. Pirfenidone attenuates cardiac fibrosis in a mouse model of TAC-induced left ventricular remodeling by suppressing nlrp3 inflammasome formation. Cardiology. 2013;126(1):1–11. doi: 10.1159/000351179. [DOI] [PubMed] [Google Scholar]
- Xu H, Su Z, Wu J, Yang M, Penninger JM, Martin CM, Kvietys PR, Rui T. The alarmin cytokine, high mobility group box 1, is produced by viable cardiomyocytes and mediates the lipopolysaccharide-induced myocardial dysfunction via a TLR4/phosphatidylinositol 3-kinase gamma pathway. J Immunol. 2010;184(3):1492–1498. doi: 10.4049/jimmunol.0902660. [DOI] [PubMed] [Google Scholar]
- Yao Y, Xu X, Zhang G, Zhang Y, Qian W, Rui T. Role of HMGB1 in doxorubicin-induced myocardial apoptosis and its regulation pathway. Basic Res Cardiol. 2012;107(3):267. doi: 10.1007/s00395-012-0267-3. [DOI] [PubMed] [Google Scholar]
- Yin Y, Yan Y, Jiang X, Mai J, Chen NC, Wang H, Yang XF. Inflammasomes are differentially expressed in cardiovascular and other tissues. Int J Immunopathol Pharmacol. 2009;22(2):311–322. doi: 10.1177/039463200902200208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Liu M, Zhang L, Cao Q, Zhang P, Jiang H, Zou Y, Ge J. Heat shock transcription factor 1 inhibits H2O2-induced cardiomyocyte death through suppression of high-mobility group box 1. Mol Cell Biochem. 2012;364(1–2):263–269. doi: 10.1007/s11010-012-1226-x. [DOI] [PubMed] [Google Scholar]
- Zhan R, Leng X, Liu X, Wang X, Gong J, Yan L, Wang L, Wang Y, Wang X, Qian LJ. Heat shock protein 70 is secreted from endothelial cells by a non-classical pathway involving exosomes. Biochem Biophys Res Commun. 2009;387(2):229–233. doi: 10.1016/j.bbrc.2009.06.095. [DOI] [PubMed] [Google Scholar]
- Zhu H, Shan L, Peng T. Rac1 mediates sex difference in cardiac tumor necrosis factor-alpha expression via NADPH oxidase-ERK1/2/p38 MAPK pathway in endotoxemia. J Mol Cell Cardiol. 2009;47(2):264–274. doi: 10.1016/j.yjmcc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Zhu X, Bagchi A, Zhao H, Kirschning CJ, Hajjar RJ, Chao W, Hellman J, Schmidt U. Toll-like receptor 2 activation by bacterial peptidoglycan-associated lipoprotein activates cardiomyocyte inflammation and contractile dysfunction. Crit Care Med. 2007;35(3):886–892. doi: 10.1097/01.CCM.0000256723.37586.A2. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhao H, Graveline AR, Buys ES, Schmidt U, Bloch KD, Rosenzweig A, Chao W. MyD88 and NOS2 are essential for toll-like receptor 4-mediated survival effect in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2006;291(4):H1900–H1909. doi: 10.1152/ajpheart.00112.2006. [DOI] [PubMed] [Google Scholar]
- Zou N, Ao L, Cleveland JC, Jr, Yang X, Su X, Cai GY, Banerjee A, Fullerton DA, Meng X. Critical role of extracellular heat shock cognate protein 70 in the myocardial inflammatory response and cardiac dysfunction after global ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2008;294(6):H2805–H2813. doi: 10.1152/ajpheart.00299.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]