Abstract
Cerebral metabolic rate of oxygen (CMRO2) is the rate of oxygen consumption by the brain and is thought to be a direct index of energy homeostasis and brain health. However, in vivo measurement of CMRO2 has been challenging, in particular for neonatal population in whom conventional radiotracer methods are not applicable due to safety concerns. In this study, we propose a method to quantify global CMRO2 in neonates based on arteriovenous differences in oxygen content and employ separate measurements of oxygenation and CBF parameters. Specifically, arterial and venous oxygenation levels were determined with pulse oximetry and a novel T2-Relaxation-Under-Spin-Tagging (TRUST) MRI, respectively. Global CBF was measured with a phase-contrast (PC) flow velocity MRI. The proposed method was implemented on a standard 3T MRI without the need of any exogenous tracers and the total scan duration was less than 5 minutes. We demonstrated the feasibility of this method in twelve healthy neonates within an age range of 35–42 gestational weeks. CMRO2 values were successfully obtained from ten neonates. It was found that average CMRO2 in this age range was 38.3±17.7 μmol/100g/min and was positively correlated with age (p=0.007, slope 5.2 μmol/100g/min per week), although the highest CMRO2 value in this age range was still less than half of the adult level. Test-retest studies showed a coefficient of variation of 5.8±2.2 % between repeated CMRO2 measurements. Additionally, given the highly variable blood flow velocity within this age range, it is recommended that the TRUST labeling thickness and position should be determined on a subject-by-subject basis, and an automatic algorithm was developed for this purpose. Although this method provides a global CMRO2 measure only, the clinical significance of an energy consumption marker and the convenience of this technique may make it a useful tool in functional assessment of neonatal population.
Keywords: CMRO2, brain, fetus, baby, TRUST, energy consumption
Introduction
Cerebral oxidative metabolism plays a critical role in the early development of the brain. Starting from the third trimester and continuing several months after birth, the form of energy production in the human brain shifts from anaerobic glycolysis to the more energy-efficient aerobic metabolism, in order to meet the escalating cerebral energy demands during rapid structural and functional development (1). Therefore, disruption of oxygen supply and metabolism at this stage is particularly detrimental, and has been associated with several cerebral injuries, such as hypoxic-ischemic encephalopathy, stroke, and metabolic disorders, all of which may lead to long-term neurologic deficits (2–4). As a result, quantitative assessment of cerebral oxygen metabolism in the neonate is important for better understanding normal brain development and the etiology of various neonatal brain injuries, as well as for the diagnosis and treatment assessment of these injuries on an individual basis.
There exist considerable challenges in the measurement of cerebral oxygen metabolism in neonates. Positron Emission Tomography (PET) is the gold standard for the measurement of cerebral metabolic rate of oxygen (CMRO2) in adults (5), but its applicability in neonates is limited by the concerns of radiation safety in this population. Near infrared spectroscopy (NIRS) approaches have been proposed to assess CMRO2 (6–10), but have not been widely accepted because assumptions on arteriovenous volume ratio are required, and it is difficult to determine the light penetration depth. Other potential CMRO2 methods, such as the 13C NMR (11), 17O NMR (12), and biophysical model based MRI methods (13–15), have only begun to be explored in adults and are not ready to be used in neonates. As a result of these technical obstacles, little is known about the brain’s metabolic rate in neonates, and normal values of CMRO2 in human neonates are not well established.
The purpose of the present study is to develop a global CMRO2 method that is rapid (<5 minutes), quantitative (in physiologic units), and can be performed on a standard 3T MRI system. This method is based on arteriovenous differences in oxygen content and does not require any exogenous tracers. Feasibility of the proposed method is demonstrated in 12 healthy neonatal subjects with an age range of 35 to 42 gestational weeks. Intra-session test-retest reproducibility is also evaluated. CMRO2 values are reported for 10 of the participants examined in this study.
Materials and Methods
Framework of CMRO2 quantification
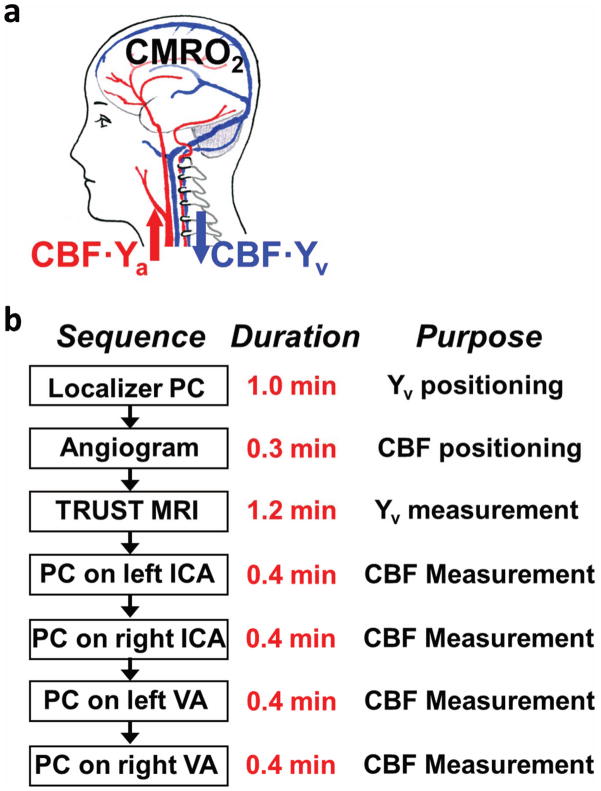
The framework of our CMRO2 measurement is based on the Fick Principle (Figure 1a), in which global CMRO2 can be quantified from arteriovenous differences in oxygen content (16):
| [1] |
where CBF is global cerebral blood flow, Ya and Yv are oxygen saturation fraction in arterial and venous blood, respectively, and Ca is a constant representing the capacity of blood to carry O2 and is well established in physiology literature (0.204 μmol O2/ml blood per hematocrit unit (17)). Thus, once Ya, Yv and CBF are experimentally determined, CMRO2 in units of μmol O2/100 g/min can be calculated.
Figure 1.
Framework of CMRO2 measurement using MRI. (a) Illustration of the relationship among different physiologic parameters associated with oxygen demand and supply of the brain. (b) Proposed MRI procedure for a complete CMRO2 dataset in neonates.
In Equation [1], global CBF can be measured by phase-contrast (PC) MRI performed at the feeding arteries of the brain, which are the left and right internal carotid arteries, and left and right vertebral arteries. PC MRI utilizes the phase of an image to encode the velocity of moving spins and has been validated for angiogram and quantitative flow measurements (18–20). Ya can be obtained using pulse oximetry with the optical sensor attached to the toe of the neonates, which is continuously monitored in neonatal scans. The most challenging component, venous oxygenation (Yv), is measured by a T2-relaxation-under-spin-tagging (TRUST) MRI technique that has recently been developed and validated in our laboratory (21–23). TRUST MRI utilizes the spin-tagging principle on the venous side to separate the pure venous blood signal from the surrounding tissue signal by subtracting a labeled image from a control image. The venous blood signal is then modulated with different T2 weightings using various numbers of flow-insensitive T2-preparation pulses. The mono-exponential fitting of the blood signal to the T2-preparation duration (termed effective echo time [eTE]) gives the T2 value of the venous blood, which is further converted to Yv via the relationship between blood T2 and oxygenation (22,24). TRUST MRI technique has been optimized (23,25) and validated in adults against a gold-standard pulse oximetry method (22), but has not been performed in neonates previously.
Participants
Twelve healthy neonates (8 males and 4 females) were recruited from the Perinatal-Neonatal Clinic in the Parkland Memorial Hospital. The sample was drawn from an ongoing research project at the University of Texas Southwestern Medical Center (UTSW) to study prenatal and perinatal human brain development with MRI. The study protocol was approved by the UTSW Institutional Review Board of the UTSW. Parents of the neonates gave informed written consent before participating in the study. The mean and standard deviation of gestational age of recruited neonates at birth was 34.5±4.8 weeks, ranging from 28.9 to 41.3 weeks. The mean and standard deviation of gestational age of recruited neonates at the time of the study was 37.4± 2.6 weeks, ranging from 34.7 to 41.6 weeks. The recruited neonates had no neurological event at birth, no presence of known or suspected congenital anomalies such as chromosomal anomalies, no major congenital heart disease or congenital infection, and no substance abuse. The neonates were also excluded for a grade>1 on intraventricular hemorrhage (IVH), and the presence of cystic periventricular leukomalacia (PVL), both measured by cranial ultrasound. None of the neonates required respiratory support at the time of study.
General MRI data acquisition
All MRI scans were performed on a 3T MRI system (Philips Medical Systems, Best, the Netherlands) using a body coil for transmission and an eight-channel phased-array coil for reception. No sedation was used. The neonates were well fed before MRI scan and wrapped with a vacuum immobilizer to minimize motion.
The MRI session began with a series of clinical sequences including T2 and FLAIR. A pediatric neuroradiologist read the images to confirm the absence of radiographic abnormalities.
The protocol for a complete set of CMRO2 data is shown in Figure 1b. Measurement of Yv requires two scans, a localizer PC MRI and a TRUST MRI. Measurement of CBF requires five scans, an angiogram and four quantitative PC MRI. The details of these scans are described in the following two sub-sections.
MRI scans to measure Yv
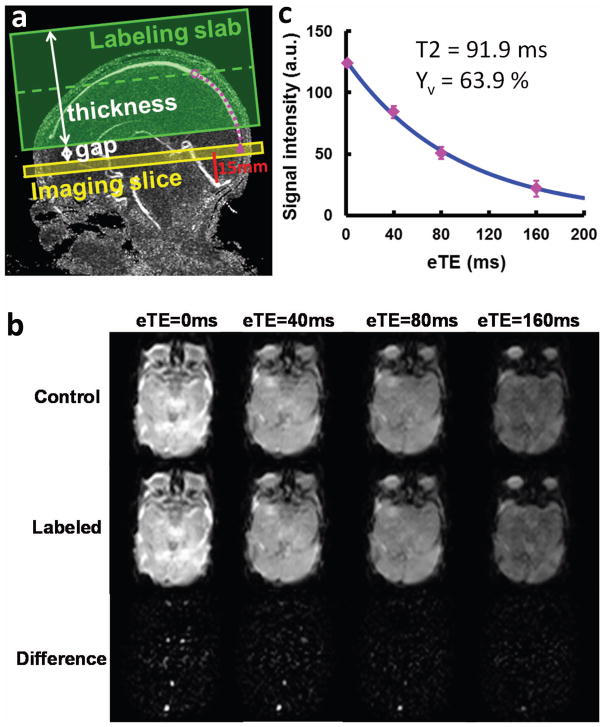
A localizer PC MRI scan was performed with the imaging slab centered at the mid-sagittal plane to provide a quantitative map of venous flow velocity along the entire path of the superior sagittal sinus (Figure 2a). This information was used to determine the optimal labeling position for the TRUST MRI sequence. The imaging parameters of the localizer PC MRI were: FOV=130 × 130 × 8 mm3, voxel size=0.6 × 0.8 × 2 mm3, TR=20ms, Venc=15 cm/s, flow encoding in three orthogonal directions in separate TRs, scan duration = 54 s.
Figure 2.
Measurement of venous oxygenation (Yv) using T2-Relaxation-Under-Spin-Tagging (TRUST) MRI. (a) Imaging slice (yellow) and labeling slab (green) of the TRUST MRI scan illustrated on the velocity map of superior sagittal sinus (SSS). The imaging slice was positioned to be parallel to anterior-commissure posterior-commissure line with a distance of 15 mm from the sinus confluence. The labeling thickness and gap were determined by an automatic algorithm based on identifying the location of the blood (open purple circle) at 1022 ms prior to reaching the imaging slice (filled purple circle). (b) MR images under control (top row) and labeled (middle row) conditions. Each type of image is acquired at four different T2-weightings, denoted by the effective echo time (eTE). The bottom row is the difference image, i.e. Control-Labeled. (c) Monoexponential fitting of the signal intensity in SSS as a function of eTE yields the CPMG T2 of the venous blood, which is then converted to Yv via a calibration plot. Error bar indicates standard error of the eTE measurements. The standard error of this Yv estimation was 3.3%.
The imaging slice of TRUST MRI was positioned to be parallel to anterior-commissure posterior-commissure line with a distance of 15 mm from the sinus confluence where the superior sagittal sinus (SSS), straight sinus and transverse sinus join (Figure 2a). The distance value of 15 mm was chosen empirically for protocol standardization purposes and was shorter than the value (20 mm) used for adult TRUST MRI protocol (25). This criterion allowed the imaging slice to intersect SSS at an angle close to 90°. Note that the imaging slice is not required to be perfectly perpendicular to the SSS since the spin-tagging principle utilized in TRUST separates the venous blood from static tissue with minimal partial voluming effect. Details of the TRUST pulse sequence has been described extensively in the literature (21,23). In this study, a postsat TRUST sequence (23) was used. Imaging parameters for the neonatal TRUST MRI scan were modified from the adult scan parameters (23,25) to account for the smaller brain size in the neonates. The neonate scan used the following values: TR = 3000 ms, TI = 1022 ms, FOV = 120 × 120 × 5 mm3, matrix size = 64 × 56, SENSE factor = 3, voxel size = 1.88 × 2.14 × 5 mm3, four different T2-weightings with eTEs of 0 ms, 40 ms, 80 ms and 160 ms, with a τCPMG = 10 ms, 3 pairs of control and label images for each eTE, scan duration = 72 s.
Since TRUST MRI uses the spin-tagging principle to separate pure venous blood from tissue, effective labeling of the blood is important to obtain high quality results. In the TRUST sequence, a labeling slab is placed above the imaging plane, so that at the time of acquisition, the blood in the imaging slice has previously experienced the inversion pulse (21). In adults, this requirement can be met effectively in virtually all subjects with a fixed setting of 100 mm thick labeling slab, and a 22.5 mm gap between the labeling slab and the imaging plane(25). However, due to rapid brain development in neonates, blood flow velocity could vary by up to 3 fold across individuals. Thus, an individual-specific labeling parameter set (including labeling slab thickness and labeling gap) may be preferable over a fixed labeling scheme. Therefore, an automatic algorithm was developed to determine the TRUST MRI labeling parameters during the scan session from the localizer PC MRI scan described above.
The gist of the algorithm is to determine where the imaged blood spins are originating from. The spatial location of the blood spins at the time 1022 ms (i.e. the post-labeling delay TI) prior to arriving at the imaging plane, i.e. at the time right after the labeling pulse, is somewhere upstream in the SSS. Once this point of origin is found, it will determine the placement of the center of the labeling slab. The inputs to the algorithm are the velocity map of the SSS obtained from the localizer PC MRI scan (Figure 2a) and the coordinates of the imaging location on the SSS (filled circle in Figure 2a). The algorithm first identifies the trajectory of the SSS based on the highest flow velocity in the upwards neighboring voxels. Next, the time required to traverse adjacent voxels on the trajectory was calculated. Then, the location of the blood at 1022 ms prior to reaching the imaging slice is determined, which is set to be the center of labeling slab. Finally, the thickness of the labeling slab and the gap between labeling slab and imaging plane are determined, such that the gap is 10% of the labeling thickness. This gap is used to minimize direct saturation effect from the labeling RF pulse on the imaging slice.
To evaluate the efficacy of this automatic algorithm, in five neonates, we compared the TRUST MRI results between the automatic method and a method using a fixed parameter set. The fixed parameter set used a labeling thickness of 60 mm and a gap of 6 mm.
MRI scans to measure CBF
First, a Time-Of-Flight angiogram was performed with axial slices encompassing a slab covering foramen magnum (Figure 3a). The resulting images allowed visualization of the feeding arteries (Figure 3b) and were used for the positioning of the arterial PC MRI scans. The imaging parameters of the angiogram were: TR/TE/flip angle = 20 ms/3.45 ms/18°, FOV = 100 × 100 × 20 mm3, voxel size = 1.0 × 1.0 × 2 mm3, one 60 mm saturation slab positioned above the imaging slab, scan duration = 19 s.
Figure 3.
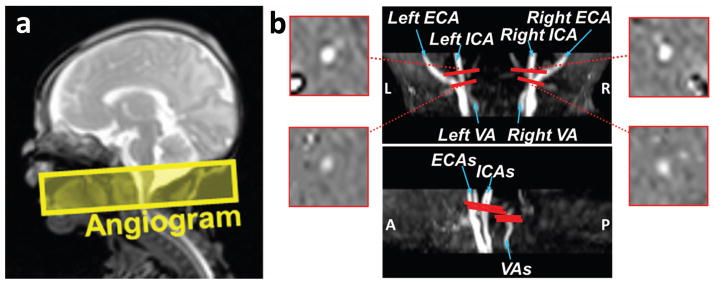
Measurement of cerebral blood flow (CBF) using Phase-Contrast (PC) MRI. (a) Slice position of the 3D angiogram scan that was used to visualize the feeding arteries. (b) Results of the angiogram scan with slice positions of the PC MRI scans. Center: coronal and sagittal maximum-intensity-projection (MIP) views of the angiogram shown on the scanner (See Supplementary Figure S1 for the post-processed MIP images of ICAs and VAs separately). Four PC MRI scans (red bars) are positioned perpendicular to the respective feeding arteries based on the MIP image of the angiogram. The corresponding phase images of the target arteries from the PC MRI scans are shown on the two sides.
The planning of the four quantitative PC MRI scans was based on the maximum-intensity-projection (MIP) images from the angiogram. Each scan was planned on one of the four feeding arteries of the brain, left internal carotid artery (left ICA), right internal carotid artery (right ICA), left vertebral artery (left VA) and right vertebral artery (right VA) (Figure 3b). The slices for the left and right ICAs were positioned at the level of foramen magnum where the arteries enter the skull. However, the placement of the imaging plane for the VAs requires more care, since the VAs have a complex anatomy at the neck region. These arteries are known to make two turns within their V3 segment, one below and the other above the C1 vertebral column (26). Thus, we chose to place the imaging slice between the two turns in V3 segments, at approximately the level of C1 vertebral column. Imaging parameters of PC MRI were: single slice, voxel size = 0.6 × 0.8 × 3 mm3, FOV = 120 × 120 × 3 mm3, maximum velocity encoding = 10 cm/s, non-gated, 4 averages, scan duration of each artery is 24s.
With the above protocol, the total duration to obtain a neonatal CMRO2 dataset is approximately 4.1 minutes. In three subjects, the CMRO2 protocol was repeated once without repositioning to provide an assessment of intra-session test-retest reproducibility of the results.
Data analysis
Data processing of TRUST MRI and arterial PC MRI followed methods described previously (25,27). Briefly, the TRUST MRI data was motion corrected using the software Statistical Parametric Mapping (SPM2, University College London, UK). Then a pairwise subtraction between control and labeled images results in pure blood signal, on which a preliminary region-of-interest (ROI) was manually drawn to include the superior sagittal sinus. The six voxels with the largest signal intensity were chosen within the ROI for spatial averaging. The averaged venous blood signal for each eTE was then fit to a mono-exponential model to obtain a blood T2. A goodness-of-fit index, ΔR2 (where R2=1/T2), was also computed in the fitting procedure, which is defined as the 95% confidence interval of R2. ΔR2 is a measure of the estimation uncertainty (95% confidence interval) of the mono-exponential fitting. Smaller ΔR2 indicates better precision of the T2 quantification. The T2 was in turn converted to Yv via a calibration plot established by in vitro blood experiments using subject-specific hematocrit values. For PC MRI data, each PC MRI scan generated three images, an anatomic image, a magnitude image and a velocity map. A ROI was manually drawn on the magnitude image of the target artery from each PC MRI scan. The operator was instructed to trace the boundary of only the target artery based on the intensity difference between the vessel region and background tissue. The ROI drawn on the magnitude image was then applied to the velocity map. The velocity values from individual voxels within the ROI were summed to yield the total blood flow of each artery. To account for brain size differences, the unit volume CBF (in ml/100 g/min) was obtained by normalizing the total CBF (in ml/min) of all four arteries to brain parenchyma volume, which was estimated from the clinical T2 image (resolution 0.4 × 0.4 × 3.5 mm3, 27 slices) using the software FSL (FMRIB Software Library, Oxford University). To estimate the brain parenchyma volume, manual skull-stripping was done in 3D slicer (28), and then the FAST tool in FSL was used to segment the T2 image of the brain into gray matter, white matter, and CSF. The brain’s parenchyma volume was given by the sum of gray and white matter volumes, and then converted to the weight of the brain by assuming a parenchyma density of 1.06 g/ml (29).
As the blood flow quantification involves manual ROI selection, inter-rater reliability was evaluated by having two raters (PL and HL) analyze the same datasets independently and calculating the correlation of the blood flow values.
Statistical analysis
Linear regression between the gestational age at the time of the scan and CMRO2 was performed to evaluate the brain metabolic changes in the first few weeks of life. Comparison between TRUST MRI results using fixed labeling parameters and subject-specific parameters was conducted using paired Student t-test. A p value < 0.05 was considered statistically significant.
Results
Data from two neonates (2 males. 35 weeks and 39 weeks of gestational age at the time of the scan) were not usable due to excessive motion artifacts. Thus, the results are reported from 10 remaining subjects. Figure 2b shows representative images of neonatal TRUST MRI. It can be seen that the control and label images are visually similar, but the subtraction clearly highlights the vessel signals. Note that the images have a low resolution (1.88 × 2.14 mm2) because of the use of single-shot Echo-Planar-Imaging (EPI) acquisition. However, we point out that partial volume between blood and tissue is accounted for by the TRUST subtraction scheme, thus the difference signal contains only pure blood. One can also see a fat artifact in the image. This is expected because fat saturation was not employed in the sequence in order to minimize the time gap between the tip-up of the T2-preparation and the excitation RF pulse. This fat artifact does not overlap with our area of interest (i.e. SSS), and does not negatively impact the quality of our data. With increasing eTE, image intensities in the control, labeled, and difference images all show attenuation. The difference signal in the SSS is plotted as a function of eTE in Figure 2c. The resulting blood T2 and the estimated Yv are also shown in the figure. The average Yv value of this neonatal cohort was 63.9±8.2% (mean±SD, N=10). Interestingly, these values are similar to the adult Yv of 64.8±6.3% (21). The goodness-of-fit index, ΔR2, was found to be 4.62 ±2.46. This range is larger than the adult values of 2.44 ±1.10 (30). Ya in this cohort was 95.8±2.2%.
Figure 3b shows representative images of the angiogram (middle) and PC MRI velocity maps of the feeding arteries (corners). The average CBF value in the neonates was 14.9±4.9 ml/100g/min. In contrast to Yv, neonatal CBF values were considerably lower than the adult values of 60.6±9.7 ml/100g/min (25) using similar measurement techniques. Note that these CBF values have been normalized to per unit mass, thus are not attributable to the smaller brain volume in neonates. For CBF processing which involves manual ROI drawing, we observed a high inter-rater reliability of r = 0.98 and P < 0.0001.
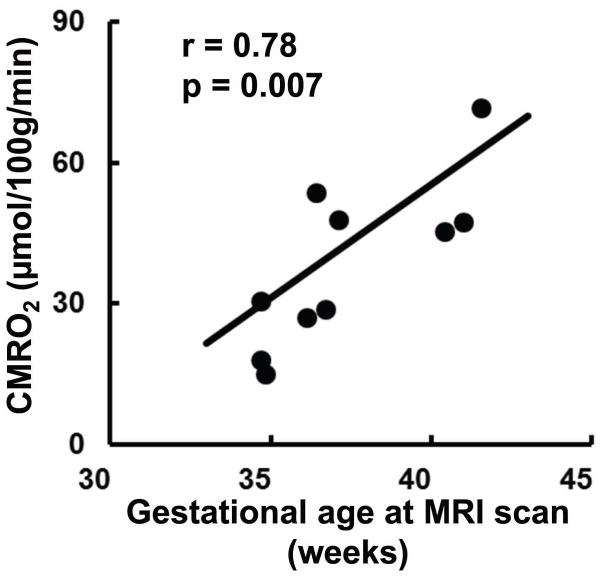
CMRO2 as calculated from the measured CBF, Yv, and Ya was 38.3±17.7 μmol/100g/min. Linear regression analysis showed a significant positive relationship between the gestational age of the neonates at the time of the scan and the measured CMRO2 (r = 0.78, p = 0.007), as shown in Figure 4. Thus, a considerable portion of the intersubject variation in estimated CMRO2 appears to be of physiologic origin and can be attributed to age-related alterations in brain metabolic rate. For reference, adult CMRO2 has been shown to be in the range of 150–200 μmol/100g/min (31).
Figure 4.
Correlation between measured CMRO2 and gestational age at scan in 10 neonates (r = 0.78, p = 0.007). Each symbol represents data from one subject. The curve indicates the linear trend line.
Results of test-retest assessment in 3 subjects are shown in Figure 5. The coefficient of variation (CoV), defined as standard deviation divided by mean across repetitions, of Yv, CBF, and CMRO2 were 2.54 ±1.54 %, 6.89±4.38 % and 5.78±2.17 %, respectively. The CoV of Yv is similar to that in adults (1.88% (25)). But the CoV values of CBF and CMRO2 were considerably higher than those in adults (2.77% for CBF and 3.84% for CMRO2 (25)). Since CoV is a ratio term, this can be partly attributed to the lower physiologic values (i.e. smaller denominator in the CoV calculation) of these parameters in neonates. For standard deviation across repetitions, it consists of both physiological and thermal noise. The thermal noise does not scale with the mean value, thus the numerator in the CoV calculation is expected to be relatively constant. Therefore, the higher CoV in neonates can be explained by a lower mean value in the face of constant standard deviation.
Figure 5.
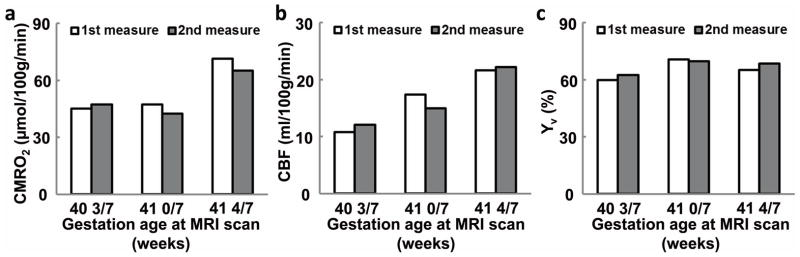
Test-retest reliability of the experimental measures of (a) CMRO2, (b) Yv, and (c) CBF in three neonates. Data from each subject are plotted individually with their age specified.
The proposed automatic algorithm to determine the subject-specific labeling parameter set revealed that the average travel distance from the time of labeling to acquisition was 48.6±12.4 mm. This resulted in an optimal labeling thickness and gap of 57.2±13.8 mm and 5.7±1.4 mm, respectively. The 95% confidence interval of ΔR2 using subject-specific labeling parameters (4.0±0.8 s−1) was significantly smaller (p = 0.028, N=5) than that using fixed parameter set (6.8±2.4 s−1), suggesting an improved estimation precision using the automatic algorithm. However, the estimated blood T2 values were not different between the two types of TRUST MRI data (p = 0.30, N=5), indicating that there is no systematic bias between the two methods.
Discussion
We have proposed a method to quantify global CMRO2 in human neonates without the use of exogenous tracers. We have demonstrated the feasibility of this method in subjects within an age range of 35–42 gestational weeks. It was further found that CMRO2 has a significant dependence on age in this cohort. Test-retest studies showed reproducible CMRO2 results using the proposed approach. Additionally, given the highly variable blood flow velocity within this age range, it is recommended that the labeling thickness and position should be determined on a subject-by-subject basis, and we have developed an automatic algorithm for this purpose.
Although there is no previous literature on neonatal CMRO2 measurement using MRI, a total of four previous reports using other imaging modalities (1 using PET and 3 using NIRS) have quantified absolute CMRO2 values in neonates (8–10,32). Findings from these studies are summarized in Table 1. The first absolute CMRO2 measurement in neonates was reported by Skov et al. in 1993 (9). Using NIRS combined with 133Xe injection and head tilting, the investigators reported a mean CMRO2 of 44.7±17.9 μmol/100g/min from 9 preterm neonates with respiratory distress syndrome and a mean CMRO2 of 62.6±35.8 μmol/100g/min from 10 asphyxiated, term neonates, with a 59% success rate using their technique. In the same year, Altman et al. used PET and measured CMRO2 in 10 sick newborns (32). They reported CMRO2 of 2.7 to 24.1 μmol/100g/min from five preterm neonates and 17.9 to 58.1 μmol/100g/min for five termed neonates. Using NIRS with partial jugular venous occlusion, Yoxall et al. reported CMRO2 values of 23.2 to 78.7 μmol/100g/min from 20 neonates under intensive care aged between 24 and 41 gestational weeks, with 8 neonates under sedation during measurement, and 3 taking medication for seizure treatment (10). More recently, Elwell et al. proposed a model based NIRS method and reported CMRO2 of 30.8 to 68.4 μmol/100g/min from 9 sick neonates between 23 to 37 gestational weeks (8). The CMRO2 values we observed (38.3±17.7 μmol/100g/min) are in general agreement with this scarce and highly variable literature. A difference between the present study and the previous reports is that this study used healthy neonates only, whereas the other reports were conducted on a variety of diseased populations. Thus, the values reported in the present study may be a better representation of reference CMRO2 values in healthy neonates.
Table 1.
Comparison of the CMRO2 obtained from this study with previous reports.
| Study | Method | Number of subjects | Gestational age (weeks) | Subject condition | Yv (%) | CBF (ml/100g/min) | CMRO2 (μmol/100g/min) |
|---|---|---|---|---|---|---|---|
| Skov et al, 1993 (9) | NIRS | 32 | 26 – 39 | Asphyxiated; RDS | 53.44±15.36 (preterm) 67.30±9.38 (term) |
12.6±6.4 (preterm) 26.5±17.9 (term) |
44.7±17.9 (preterm) 62.6±35.8 (term) |
| Altman et al, 1993 (32) | PET | 11 | 26 – 41 | HIE and other conditions | - | 21.6±21.0 | 21.4±16.4 |
| Yoxall and weindling, 1998 (10) | NIRS | 20 | 24 – 41 | Seizure and other conditions | 64.6 (76.1 – 46.8) | 9.3 (4.5 – 28.3) | 23.1 (8.6 – 78.5) |
| Elwell et all, 2005 (8) | NIRS | 9 | 23 – 37 | Ventilatory support | - | - | 45.9±12.3 |
| This study | MRI | 12 | 35 – 42 | Healthy | 62.6±8.3 | 13.4±4.2 | 38.3±17.7 |
NIRS - near infrared spectroscopy.
PET - positron emission tomography.
MRI - magnetic resonance imaging.
RDS – Respiratory distress syndrome
HIE – Hypoxic-ischemic encephalopathy
An important technical advantage of the proposed method is that it is clinically practical. Therefore, once optimized, this technique can be readily translated to patient studies. Compared to prior studies that used exogenous tracers and/or required the compression of cerebral veins (9,10,32), the present method is completely non-invasive and can be performed on a standard 3T MRI. The scan duration of less than 5 minutes is also practical for most neonatal subjects.
Neonatal CMRO2 values obtained in the present study, as well as in prior studies (8–10,32), showed that the brain metabolic rate is much lower in neonates compared to adults, which are typically 150–200 μmol/100g/min (31). Since these CMRO2 values have been normalized to per unit mass, they are not attributable to the smaller brain volume in neonates. Furthermore, it is also interesting to note that this CMRO2 variability is primarily attributed to CBF difference, but not oxygenation. Specifically, neonates have Yv and Ya values similar to those in adults, but their CBF value is much lower. Thus, Yv of ~60% seems to be a critical target value for tissue function and the brain seems to have a system to adjust its blood supply to meet this target regardless the age. This indicates that the cerebral metabolism and blood supply is well coupled. Our data also suggests that the increase in CMRO2 from neonate to adult values is not linear. This is evidenced by our data that a significant age-dependence can already be observed within a narrow age range of 35–42 gestational weeks. The rate of CMRO2 change according to our data is 5.2 μmol/100g/min per week. Assuming that this trend continues in the following few months, it is estimated that an infant of 6 months old may already approach the adult CMRO2 level.
The reason that neonatal CMRO2 values are smaller than adult values may be due to lower energy demands during the neonatal period. Although there is an escalating cerebral energy demand in the third trimester (i.e., 28 weeks to delivery), the energy required by the neonatal brain is still much lower than that required by the adult brain. Previous studies have shown that the nerve cell density is much higher in the neonate than in adults (33,34), but the neonate brain has also been found to have less neuronal processes, synapses, myelination, neurotransmitters and receptors (35–37). This would lead to fewer neuronal activities in the neonate brain. In the adult brain, the majority of the oxygen delivered is consumed for neuronal activity to maintain and restore the neuronal ionic gradients required for synaptic and neural activation (38). In neonates, the electrocortical activity is also associated with concurrent changes in cerebral oxygenation (39). Therefore, less oxygen consumption is required by the neonatal brain to meet its energy demands compared to an adult brain.
The main source of error in our measurement was subject motion. As stated earlier, no sedation was used in our scan session. Thus, the approaches to minimize motion relied on sufficient feeding the neonate prior to the scan, waiting for the subject to fall asleep, and by using the vacuum immobilizer. This was not always sufficient, which resulted in the data from two subjects to be deemed unusable. The remaining 10 subjects were included in the quantitative analysis, but it should be noted that certain amount of motion was still present in these data sets. This factor may have contributed to the greater CoV observed in the neonatal data compared to adult CMRO2 data.
This initial report should be viewed as a proof-of-principle study only, and further development of several technical aspects should be conducted before it can become a standard tool. First, the conversion of blood T2 to blood oxygenation requires a calibration plot that specifies the relationship between these two parameters. At present, the calibration plot used in our study is based on data from adult blood samples (22). However, the human neonate is known to have an additional type of hemoglobin called fetal hemoglobin, which has a slightly different molecular structure (thus oxygen affinity) compared to the adult form (40). Although no studies have shown this, it is possible that the T2 value of the neonatal blood is different from that of the adult blood, even at identical oxygen saturation and hematocrit levels. Therefore, future studies are needed to establish a calibration plot based specifically on neonatal blood, and to examine whether a difference exists in the T2 relaxometry of neonatal and adult blood. Another limitation of the present study is that the venous oxygenation was based on measurement in the superior sagittal sinus above the location of sinus confluence. While SSS is a major draining vein and its oxygenation can be considered as a global value averaged over the cortical areas, subcortical regions are drained by another vein called the straight sinus, which was not included in the SSS measurement. Thus, future work should aim to measure both SSS and straight sinus simultaneously in a single scan, which could be possible by tilting the image slice to intersect both vessels. Moreover, the intra-session test-retest reproducibility evaluated in this study only examined the measurement errors caused by physiological variation and instrument noise. Therefore, more comprehensive evaluation should be performed in the future to assess the repositioning error and scan operator dependence. Finally, the proposed technique provides a global measure only but lacks spatial information. Region-specific measurement of CBF can be achieved with several non-invasive techniques, such as Arterial-Spin-Labeling (ASL) MRI (41–44). However, techniques to map venous oxygenation in the brain are still underdeveloped, especially for the neonatal population. Therefore, future technical development should focus on this direction. In the meanwhile, given the importance of the CMRO2 marker and the convenience of the proposed method, the present global technique may be a useful tool to assess brain function in the neonatal population.
Conclusions
The present study demonstrated the feasibility to quantify global CMRO2 in neonates using the Fick principle, with separate measurements of blood oxygenation and CBF parameters. It was shown that this method can be performed on a standard 3T MRI in less than 5 minutes without the need of any exogenous tracers. CMRO2 values were obtained from 10 neonates within an age range of 35–42 gestational weeks. It was found that CMRO2 increases rapidly during this period of life, although the highest value was still less than half of the adult level.
Supplementary Material
Acknowledgments
Grant sponsors: NIH R01 MH092535 (to HH), MH092535-S1 (to HH), NIH R01 MH084021 (to HL), NIH R01 NS067015 (to HL), NIH R01 AG042753 (to HL), and K23HD069521-01A11 (to LC).
The authors are grateful to Jared Mosher for assistance with the MRI experiments and to the nurses of the transporting team in Division of Perinatal-Neonatal Medicine in Parkland Memorial Hospital. The authors are also grateful to Lisa Krishnamurthy for scientific editing of the manuscript.
Abbreviations used
- CMRO2
cerebral metabolic rate of oxygen
- TRUST
T2-Relaxation-Under-Spin-Tagging
- CBF
cerebral blood flow
- PC
phase-contrast
- PET
positron emission tomography
- NIRS
near infrared spectroscopy
- eTE
effective echo time
- IVH
intraventricular hemorrhage
- PVL
periventricular leukomalacia
- FOV
field of view
- TR
time of repetition
- TE
echo time
- TI
time of inversion
- SSS
superior sagittal sinus
- MIP
maximum-intensity-projection
- ICA
internal carotid artery
- VA
vertebral artery
- ROI
region-of-interest
- SD
standard deviation
- CoV
coefficient of variation
- ASL
Arterial-Spin-Labeling
- RDS
respiratory distress syndrome
- HIE
hypoxic-ischemic encephalopathy
References
- 1.du Plessis AJ. Cerebral blood flow and metabolism in the developing fetus. Clin Perinatol. 2009;36(3):531–548. doi: 10.1016/j.clp.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Kiechl-Kohlendorfer U, Ralser E, Pupp Peglow U, Reiter G, Trawoger R. Adverse neurodevelopmental outcome in preterm infants: risk factor profiles for different gestational ages. Acta paediatrica. 2009;98(5):792–796. doi: 10.1111/j.1651-2227.2009.01219.x. [DOI] [PubMed] [Google Scholar]
- 3.Trescher WH, Lehman RA, Vannucci RC. The influence of growth retardation on perinatal hypoxic-ischemic brain damage. Early human development. 1990;21(3):165–173. doi: 10.1016/0378-3782(90)90115-y. [DOI] [PubMed] [Google Scholar]
- 4.Pape KE, Wigglesworth JS. Clinics in Developmental Medicine. Mac Keith Press; 1993. Haemorrhage, Ischaemia and the Perinatal Brain; pp. 1–196. [Google Scholar]
- 5.Mintun MA, Raichle ME, Martin WR, Herscovitch P. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. J Nucl Med. 1984;25(2):177–187. [PubMed] [Google Scholar]
- 6.Brown DW, Hadway J, Lee TY. Near-infrared spectroscopy measurement of oxygen extraction fraction and cerebral metabolic rate of oxygen in newborn piglets. Pediatr Res. 2003;54(6):861–867. doi: 10.1203/01.PDR.0000090928.93045.BE. [DOI] [PubMed] [Google Scholar]
- 7.Tichauer KM, Elliott JT, Hadway JA, Lee DS, Lee TY, St Lawrence K. Using near-infrared spectroscopy to measure cerebral metabolic rate of oxygen under multiple levels of arterial oxygenation in piglets. J Appl Physiol. 2010;109(3):878–885. doi: 10.1152/japplphysiol.01432.2009. [DOI] [PubMed] [Google Scholar]
- 8.Elwell CE, Henty JR, Leung TS, Austin T, Meek JH, Delpy DT, Wyatt JS. Measurement of CMRO2 in neonates undergoing intensive care using near infrared spectroscopy. Adv Exp Med Biol. 2005;566:263–268. doi: 10.1007/0-387-26206-7_35. [DOI] [PubMed] [Google Scholar]
- 9.Skov L, Pryds O, Greisen G, Lou H. Estimation of cerebral venous saturation in newborn infants by near infrared spectroscopy. Pediatr Res. 1993;33(1):52–55. doi: 10.1203/00006450-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Yoxall CW, Weindling AM. Measurement of cerebral oxygen consumption in the human neonate using near infrared spectroscopy: cerebral oxygen consumption increases with advancing gestational age. Pediatr Res. 1998;44(3):283–290. doi: 10.1203/00006450-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Hyder F, Chase JR, Behar KL, Mason GF, Siddeek M, Rothman DL, Shulman RG. Increased tricarboxylic acid cycle flux in rat brain during forepaw stimulation detected with 1H[13C]NMR. Proc Natl Acad Sci U S A. 1996;93(15):7612–7617. doi: 10.1073/pnas.93.15.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu XH, Zhang Y, Tian RX, Lei H, Zhang N, Zhang X, Merkle H, Ugurbil K, Chen W. Development of (17)O NMR approach for fast imaging of cerebral metabolic rate of oxygen in rat brain at high field. Proc Natl Acad Sci U S A. 2002;99(20):13194–13199. doi: 10.1073/pnas.202471399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An H, Lin W, Celik A, Lee YZ. Quantitative measurements of cerebral metabolic rate of oxygen utilization using MRI: a volunteer study. NMR Biomed. 2001;14(7–8):441–447. doi: 10.1002/nbm.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gauthier CJ, Hoge RD. Magnetic resonance imaging of resting OEF and CMRO(2) using a generalized calibration model for hypercapnia and hyperoxia. Neuroimage. 2012;60(2):1212–1225. doi: 10.1016/j.neuroimage.2011.12.056. [DOI] [PubMed] [Google Scholar]
- 15.He X, Yablonskiy DA. Quantitative BOLD: mapping of human cerebral deoxygenated blood volume and oxygen extraction fraction: default state. Magn Reson Med. 2007;57(1):115–126. doi: 10.1002/mrm.21108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kety SS, Schmidt CF. The Effects of Altered Arterial Tensions of Carbon Dioxide and Oxygen on Cerebral Blood Flow and Cerebral Oxygen Consumption of Normal Young Men. J Clin Invest. 1948;27(4):484–492. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guyton AC, Hall JE. Respiration. In: Guyton AC, Hall JE, editors. Textbook of medical physiology. Saunders/Elsevier; Philadelphia: 2005. [Google Scholar]
- 18.Bakker CJ, Hoogeveen RM, Viergever MA. Construction of a protocol for measuring blood flow by two-dimensional phase-contrast MRA. J Magn Reson Imaging. 1999;9(1):119–127. doi: 10.1002/(sici)1522-2586(199901)9:1<119::aid-jmri16>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 19.Evans AJ, Iwai F, Grist TA, Sostman HD, Hedlund LW, Spritzer CE, Negro-Vilar R, Beam CA, Pelc NJ. Magnetic resonance imaging of blood flow with a phase subtraction technique. In vitro and in vivo validation. Invest Radiol. 1993;28(2):109–115. doi: 10.1097/00004424-199302000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Zananiri FV, Jackson PC, Goddard PR, Davies ER, Wells PN. An evaluation of the accuracy of flow measurements using magnetic resonance imaging (MRI) J Med Eng Technol. 1991;15(4–5):170–176. doi: 10.3109/03091909109023704. [DOI] [PubMed] [Google Scholar]
- 21.Lu H, Ge Y. Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. Magn Reson Med. 2008;60(2):357–363. doi: 10.1002/mrm.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu H, Xu F, Grgac K, Liu P, Qin Q, van Zijl P. Calibration and validation of TRUST MRI for the estimation of cerebral blood oxygenation. Magn Reson Med. 2012;67(1):42–49. doi: 10.1002/mrm.22970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu F, Uh J, Liu P, Lu H. On improving the speed and reliability of T2-relaxation-under-spin-tagging (TRUST) MRI. Magn Reson Med. 2012;68(1):198–204. doi: 10.1002/mrm.23207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golay X, Silvennoinen MJ, Zhou J, Clingman CS, Kauppinen RA, Pekar JJ, van Zij PC. Measurement of tissue oxygen extraction ratios from venous blood T(2): increased precision and validation of principle. Magn Reson Med. 2001;46(2):282–291. doi: 10.1002/mrm.1189. [DOI] [PubMed] [Google Scholar]
- 25.Liu P, Xu F, Lu H. Test-retest reproducibility of a rapid method to measure brain oxygen metabolism. Magn Reson Med. 2013;69(3):675–681. doi: 10.1002/mrm.24295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber EC, Vilensky JA, Carmichael SW. Netter’s Concise Radiologic Anatomy. Elsevier Health Sciences; Philadelphia, PA, USA: 2009. Head and Neck. [Google Scholar]
- 27.Xu F, Ge Y, Lu H. Noninvasive quantification of whole-brain cerebral metabolic rate of oxygen (CMRO2) by MRI. Magn Reson Med. 2009;62(1):141–148. doi: 10.1002/mrm.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magnetic resonance imaging. 2012;30(9):1323–1341. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herscovitch P, Raichle ME. What is the correct value for the brain--blood partition coefficient for water? Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1985;5(1):65–69. doi: 10.1038/jcbfm.1985.9. [DOI] [PubMed] [Google Scholar]
- 30.Liu P, Dimitrov I, Andrews T, Crane D, Dariotis J, Desmond J, Dumas J, Gilbert G, Kumar A, Leroux J, MacIntosh B, Yang S, Xiao G, Lu H. Multi-site evaluations of a TRUST MRI technique to measure brain oxygenation. Salt Lake City, USA: 2013. p. 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, Hebrank AC, Uh J, Park DC. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex. 2011;21(6):1426–1434. doi: 10.1093/cercor/bhq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altman DI, Perlman JM, Volpe JJ, Powers WJ. Cerebral oxygen metabolism in newborns. Pediatrics. 1993;92(1):99–104. [PubMed] [Google Scholar]
- 33.Cragg BG. The development of synapses in the visual system of the cat. The Journal of comparative neurology. 1975;160(2):147–166. doi: 10.1002/cne.901600202. [DOI] [PubMed] [Google Scholar]
- 34.Tsumoto T, Suda K, Sato H. Postnatal development of corticotectal neurons in the kitten striate cortex: a quantitative study with the horseradish peroxidase technique. The Journal of comparative neurology. 1983;219(1):88–99. doi: 10.1002/cne.902190109. [DOI] [PubMed] [Google Scholar]
- 35.Changeux JP, Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976;264(5588):705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- 36.Cowan WM, Fawcett JW, O’Leary DD, Stanfield BB. Regressive events in neurogenesis. Science. 1984;225(4668):1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- 37.Rao MS, Jacobson M. Developmental neurobiology. Plenum; New York: 2005. [Google Scholar]
- 38.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2001;21(10):1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Roche-Labarbe N, Wallois F, Ponchel E, Kongolo G, Grebe R. Coupled oxygenation oscillation measured by NIRS and intermittent cerebral activation on EEG in premature infants. Neuroimage. 2007;36(3):718–727. doi: 10.1016/j.neuroimage.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Ohls RK. Core Concepts: The Biology of Hemoglobin. NeoReviews. 2011;12(1):e29–e38. [Google Scholar]
- 41.Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60(6):1488–1497. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Detre JA, Alsop DC. Perfusion magnetic resonance imaging with continuous arterial spin labeling: methods and clinical applications in the central nervous system. Eur J Radiol. 1999;30(2):115–124. doi: 10.1016/s0720-048x(99)00050-9. [DOI] [PubMed] [Google Scholar]
- 43.Wong EC. Vessel-encoded arterial spin-labeling using pseudocontinuous tagging. Magn Reson Med. 2007;58(6):1086–1091. doi: 10.1002/mrm.21293. [DOI] [PubMed] [Google Scholar]
- 44.Golay X, Hendrikse J, Lim TC. Perfusion imaging using arterial spin labeling. Topics in magnetic resonance imaging: TMRI. 2004;15(1):10–27. doi: 10.1097/00002142-200402000-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.