Abstract
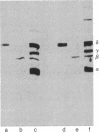
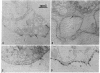
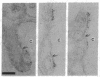
Antibodies were raised against two synthetic peptides whose sequences correspond respectively to the COOH-terminal end (residues 501-516) of the protein encoded by the gene for the delta chain and to a proposed cytoplasmic region (residues 350-358) of the beta chain of the acetylcholine receptor from Torpedo californica. Binding of the COOH-terminal antibody to the acetylcholine receptor in intact, receptor-rich vesicles was tested by radioimmunoassay and by precipitation with immobilized protein A. In both cases, binding was detected only after treatment of the vesicles with detergent, suggesting that the segment of the receptor that is recognized by this antibody is on the cytoplasmic side of the membrane. Electron microscopy of tissue from Torpedo electric organ labeled with colloidal gold-conjugated second antibodies established that both anti-receptor antibodies bind to the cytoplasmic surface of the postsynaptic membrane. These experiments give ultrastructural evidence that the COOH-terminal segment of the delta chain as well as residues 350-358 of the beta chain are on the cytoplasmic surface. They strongly support a model in which each of the receptor subunits crosses the membrane five times in which one transmembrane segment of each chain contributes to the formation of a central ion channel.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Blobel G. In vitro synthesis, glycosylation, and membrane insertion of the four subunits of Torpedo acetylcholine receptor. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5598–5602. doi: 10.1073/pnas.78.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. J., Walter P., Blobel G. Signal recognition protein is required for the integration of acetylcholine receptor delta subunit, a transmembrane glycoprotein, into the endoplasmic reticulum membrane. J Cell Biol. 1982 May;93(2):501–506. doi: 10.1083/jcb.93.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake J., Hagman J., Ramachandran J. Synthesis of human corticotropinyl-thiolglycine and its specific conjugation to bovine serum albumin. Int J Pept Protein Res. 1982 Aug;20(2):97–101. doi: 10.1111/j.1399-3011.1982.tb02659.x. [DOI] [PubMed] [Google Scholar]
- Blake J., Hines K. K., Li C. H. Synthesis of human beta-endorphinyl-thiolglycine and its use for the preparation of affinity columns of beta-endorphin. Int J Pept Protein Res. 1982 Nov;20(5):429–433. doi: 10.1111/j.1399-3011.1982.tb03063.x. [DOI] [PubMed] [Google Scholar]
- Blake J., Li C. H. New segment-coupling method for peptide synthesis in aqueous solution: application to synthesis of human [Gly17]-beta-endorphin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4055–4058. doi: 10.1073/pnas.78.7.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake J. Peptide segment coupling in aqueous medium: silver ion activation of the thiolcarboxyl group. Int J Pept Protein Res. 1981 Feb;17(2):273–274. doi: 10.1111/j.1399-3011.1981.tb01992.x. [DOI] [PubMed] [Google Scholar]
- Buckley D. I., Hagman J., Ramachandran J. A sensitive radioimmunoassay for corticotropin using a fully biologically active 125I-labeled ligand. Endocrinology. 1981 Jul;109(1):10–16. doi: 10.1210/endo-109-1-10. [DOI] [PubMed] [Google Scholar]
- Buckley K. M., Schweitzer E. S., Miljanich G. P., Clift-O'Grady L., Kushner P. D., Reichardt L. F., Kelly R. B. A synaptic vesicle antigen is restricted to the junctional region of the presynaptic plasma membrane. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7342–7346. doi: 10.1073/pnas.80.23.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. W., Bock E. Molecular forms of acetylcholine receptor. Effects of calcium ions and a sulfhydryl reagent on the occurrence of oligomers. Biochemistry. 1977 Oct 4;16(20):4513–4520. doi: 10.1021/bi00639a028. [DOI] [PubMed] [Google Scholar]
- Claudio T., Ballivet M., Patrick J., Heinemann S. Nucleotide and deduced amino acid sequences of Torpedo californica acetylcholine receptor gamma subunit. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1111–1115. doi: 10.1073/pnas.80.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Raftery M. A. The nicotinic cholinergic receptor: correlation of molecular structure with functional properties. Annu Rev Biochem. 1982;51:491–530. doi: 10.1146/annurev.bi.51.070182.002423. [DOI] [PubMed] [Google Scholar]
- Devillers-Thiery A., Giraudat J., Bentaboulet M., Changeux J. P. Complete mRNA coding sequence of the acetylcholine binding alpha-subunit of Torpedo marmorata acetylcholine receptor: a model for the transmembrane organization of the polypeptide chain. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2067–2071. doi: 10.1073/pnas.80.7.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J., Blanchard S. G., Wu W., Miller J., Strader C. D., Hartig P., Moore H. P., Racs J., Raftery M. A. Purification of Torpedo californica post-synaptic membranes and fractionation of their constituent proteins. Biochem J. 1980 Mar 1;185(3):667–677. doi: 10.1042/bj1850667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairclough R. H., Finer-Moore J., Love R. A., Kristofferson D., Desmeules P. J., Stroud R. M. Subunit organization and structure of an acetylcholine receptor. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):9–20. doi: 10.1101/sqb.1983.048.01.004. [DOI] [PubMed] [Google Scholar]
- Finer-Moore J., Stroud R. M. Amphipathic analysis and possible formation of the ion channel in an acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Jan;81(1):155–159. doi: 10.1073/pnas.81.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehner S. C., Douville K., Klink S., Culp W. J. Monoclonal antibodies to cytoplasmic domains of the acetylcholine receptor. J Biol Chem. 1983 Jun 10;258(11):7112–7120. [PubMed] [Google Scholar]
- Guy H. R. A structural model of the acetylcholine receptor channel based on partition energy and helix packing calculations. Biophys J. 1984 Jan;45(1):249–261. doi: 10.1016/S0006-3495(84)84152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig P. R., Raftery M. A. Preparation of right-side-out, acetylcholine receptor enriched intact vesicles from Torpedo californica electroplaque membranes. Biochemistry. 1979 Apr 3;18(7):1146–1150. doi: 10.1021/bi00574a004. [DOI] [PubMed] [Google Scholar]
- Kistler J., Stroud R. M., Klymkowsky M. W., Lalancette R. A., Fairclough R. H. Structure and function of an acetylcholine receptor. Biophys J. 1982 Jan;37(1):371–383. doi: 10.1016/S0006-3495(82)84685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klymkowsky M. W., Stroud R. M. Immunospecific identification and three-dimensional structure of a membrane-bound acetylcholine receptor from Torpedo californica. J Mol Biol. 1979 Mar 5;128(3):319–334. doi: 10.1016/0022-2836(79)90091-3. [DOI] [PubMed] [Google Scholar]
- Kosower E. M. Partial tertiary structure assignment for the acetylcholine receptor on the basis of the hydrophobicity of amino acid sequences and channel location using single group rotation theory. Biochem Biophys Res Commun. 1983 Mar 29;111(3):1022–1026. doi: 10.1016/0006-291x(83)91402-x. [DOI] [PubMed] [Google Scholar]
- Kosower E. M. Partial tertiary structure assignments for the beta-, gamma- and delta-subunits of the acetylcholine receptor on the basis of the hydrophobicity of amino acid sequences and channel location using single group rotation theory. FEBS Lett. 1983 May 8;155(2):245–247. doi: 10.1016/0014-5793(82)80613-3. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Furutani Y., Hirose T., Asai M., Inayama S., Miyata T., Numa S. Primary structure of alpha-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature. 1982 Oct 28;299(5886):793–797. doi: 10.1038/299793a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Furutani Y., Hirose T., Takashima H., Inayama S., Miyata T. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature. 1983 Apr 7;302(5908):528–532. doi: 10.1038/302528a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Hirose T., Asai M., Takashima H., Inayama S., Miyata T. Primary structures of beta- and delta-subunit precursors of Torpedo californica acetylcholine receptor deduced from cDNA sequences. Nature. 1983 Jan 20;301(5897):251–255. doi: 10.1038/301251a0. [DOI] [PubMed] [Google Scholar]
- Raftery M. A., Hunkapiller M. W., Strader C. D., Hood L. E. Acetylcholine receptor: complex of homologous subunits. Science. 1980 Jun 27;208(4451):1454–1456. doi: 10.1126/science.7384786. [DOI] [PubMed] [Google Scholar]
- Rao A. J., Behrens C., Ramachandran J. Immunochemical studies of adrenocorticotropin using tritium labeled hormone. Int J Pept Protein Res. 1980 May;15(5):480–484. doi: 10.1111/j.1399-3011.1980.tb02925.x. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Karlin A. Molecular weight in detergent solution of acetylcholine receptor from Torpedo californica. Biochemistry. 1978 May 30;17(11):2035–2038. doi: 10.1021/bi00604a001. [DOI] [PubMed] [Google Scholar]
- Sargent P. B., Hedges B. E., Tsavaler L., Clemmons L., Tzartos S., Lindstrom J. M. Structure and transmembrane nature of the acetylcholine receptor in amphibian skeletal muscle as revealed by cross-reacting monoclonal antibodies. J Cell Biol. 1984 Feb;98(2):609–618. doi: 10.1083/jcb.98.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John P. A., Froehner S. C., Goodenough D. A., Cohen J. B. Nicotinic postsynaptic membranes from Torpedo: sidedness, permeability to macromolecules, and topography of major polypeptides. J Cell Biol. 1982 Feb;92(2):333–342. doi: 10.1083/jcb.92.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumikawa K., Houghton M., Smith J. C., Bell L., Richards B. M., Barnard E. A. The molecular cloning and characterisation of cDNA coding for the alpha subunit of the acetylcholine receptor. Nucleic Acids Res. 1982 Oct 11;10(19):5809–5822. doi: 10.1093/nar/10.19.5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos S. J., Rand D. E., Einarson B. L., Lindstrom J. M. Mapping of surface structures of electrophorus acetylcholine receptor using monoclonal antibodies. J Biol Chem. 1981 Aug 25;256(16):8635–8645. [PubMed] [Google Scholar]
- Wennogle L. P., Oswald R., Saitoh T., Changeux J. P. Dissection of the 66 000-dalton subunit of the acetylcholine receptor. Biochemistry. 1981 Apr 28;20(9):2492–2497. doi: 10.1021/bi00512a020. [DOI] [PubMed] [Google Scholar]