Cui Y, Song Y, Wang J, et al. Clinical Pharmacology: Advances and Applications. 2013;5(1):177–184.
Note that on page 182, Figure 3 should be corrected as follows, with the line for mean anti-Xa activity extended through to 72 hours post dose on Day 9, and the Day 1 plasma apixaban concentration-time profile rendering error bars and symbols more visually apparent.
Figure 3.
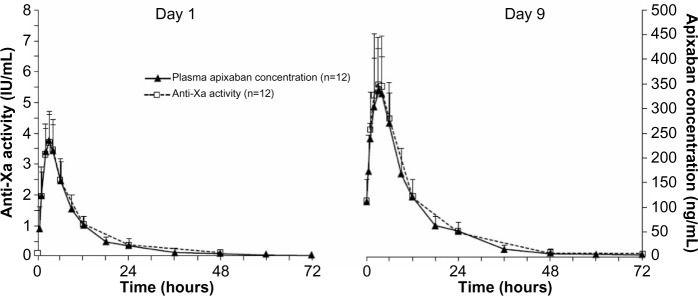
Mean anti-Xa activity and plasma apixaban concentration versus time following single-dose administration (day 1) and at steady state (day 9). Error bars show +1 standard deviation from the mean.


