Abstract
Radical prostatectomy (RP) became a first choice of treatment for prostate cancer after the advance in nerve-sparing techniques. However, the difficult technical details still involved in nerve-sparing RP (nsRP) can invite unwanted complications. Therefore, learning to recognize key anatomical features of the prostate and its surrounding structures is crucial to further improve RP efficacy. Although the anatomical relation between the pelvic nerves and pelvic fascias is still under investigation, this paper characterizes the periprostatic fascias in order to define a novel fascial-sparing approach to RP (fsRP), which will help spare neurovascular bundles. In uroanatomic perspective, it can be stated that nsRP is a functional identification of the surgical technique while fsRP is an anatomic identification as well. The functional and oncological outcomes related to this novel fsRP are also reviewed.
Keywords: Cadaver, Urology, Prostate, Neurovascular bundle
INTRODUCTION
Radical prostatectomy (RP) is one of the main options for the treatment of localized and locally advanced prostate cancer in some cases [1]. The nerve-sparing technique remains the target anatomical approach to achieve better functional outcomes related to potency and continence. The technical details of this procedure make the operation difficult, however, and some authors have stressed the importance of anatomical landmarks in RP in their series [2–4]. RP has some associated morbidity, which can be decreased dramatically as a result of improved surgical technique [5,6]. Additionally, a positive impact of nerve-sparing RP (nsRP) on sexual function [7–9] and lower urinary tract function [7,10] has been shown in the literature. Various technological devices have been used to improve the technique of nsRP, such as laparoscopic and robotic devices. Furthermore, advances in the anatomical elucidation of the prostate and periprostatic structures have contributed excellent survival and functional results after RP [11–15].
Nielsen et al. [16] have reported perfecting the technique of nsRP with sequential modifications since 1982. In their report, Nielsen et al. [16] describe performing wide excision of the neurovascular bundle (NVB) in 1982 for 110 patients and high anterior release of the NVB in 2005 for 3,649 patients to ensure better functional outcome. However, the requirement for appreciation of the anatomy of the prostatic and periprostatic fascial layers to perform nsRP is widely acknowledged [17]. Many controversies exist in the literature regarding the description of these fascias [18]; moreover, the anatomical relation between the pelvic nerves and fascias is still under investigation. Cornu et al. [19] described the anatomy of the periprostatic fascias in order to spare the NVBs during RP. The aim of this article was define novel anatomical identification of nsRP and to review the functional and oncological outcomes related to fascial-sparing RP (fsRP).
ANATOMY OF PELVIC FASCIA AND FASCIAS OF PROSTATE
The anatomy of the prostate and fascias of the prostate is to a certain extent complicated by the close relations of the pelvic organs to each other and by the narrowness of the pelvis [20]. For this reason, performing surgery from an anatomical point of view will ensure better visualization and understanding of the pelvic anatomy and fascias. In the Skandalakis surgical anatomy atlas, the anatomical relation between the fascia (parietal layer of pelvic fascia), vessels (internal iliac vessels), and nerves (sacral plexus) is clearly shown from the skin to peritoneum scheme [21]. The atlas shows the close anatomical locations of the three major structures, which also have critical importance at the prostate level (Fig. 1). The main focus in nsRP is to protect the nerve that is completely adjacent to the fascias and vessels.
Fig. 1.
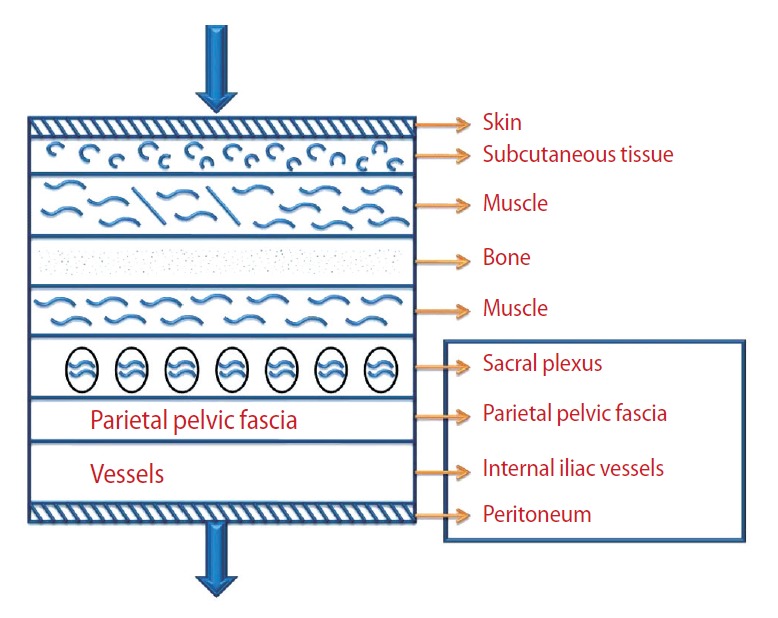
Anatomical relation between the fascia (parietal layer of pelvic fascia), vessels (internal iliac vessels), and nerve (sacral plexus) in the pelvic region.
The surgico-anatomical layers are divided into five sections: pelvic peritoneum and its specialization, blood vessels of the pelvis, pelvic fascia, nerves of the pelvis, and the muscles. The pelvic organs are covered by the pelvic fascia. This fascia is referred to as the endopelvic fascia (EPF) by some authors [22,23]. The EPF has two major divisions: the parietal and the visceral parts. The parietal component, which is a strong, membranous layer, covers the medial aspects of the levator ani, obturator internus, and piriformis muscles. The visceral fascia is essentially the connective tissue that encapsulates the individual organs within the pelvis, such as the prostate, bladder, and rectum. Briefly, the EPF covers the pelvic organs and the pelvic side wall, and full access to the prostate can be obtained after incision of the EPF at the fusion between the parietal and visceral parts at the antero-lateral corner of the prostate [24–26].
The other important anatomical landmark is the tendinous arch of the pelvic fascia, at which point both layers of the prostatic fascia (PF) and the EPF are adherent and fused laterally. These structures join the puboprostatic ligaments (PPLs) that connect the prostate to the pubic bone [19] and are part of a larger urethral suspensor mechanism attaching the membranous urethra to the pubic bone and ensuring continence [27]. The PPLs and EPF have an indirect positive effect on the continence mechanism owing to the fascial continuum of EPF. After reopening the EPF on the prostatic side, the PF (periprostatic fascia, lateral pelvic fascia [LPF], paraprostatic fascia) can be clearly seen [19]. The PF covers the whole prostate surface in a dense fashion and anatomical dissection of the PF allows finding a plane front of cavernous nerves surrounded by fatty tissue. The LPF (PF, the part of prostate; rectal fascia, the part of rectum) extends in a posterior direction to also cover the NVB and consists not of a single layer of tissue but of collagen and connective tissue positioned in several layers over the prostate [22,25,28,29]. Thus, the close anatomical relation of the fascial structures and NVB over the prostate and at the posterior part of the prostate is evident.
The anterior surface of the prostate is located between the apex and the base. Multiple large veins called the dorsal venous complex separate the surface from the symphysis pubis (Fig. 2). The visceral part of the EPF also covers the vascular structures located at the anterior side of the prostate (Fig. 2).
Fig. 2.
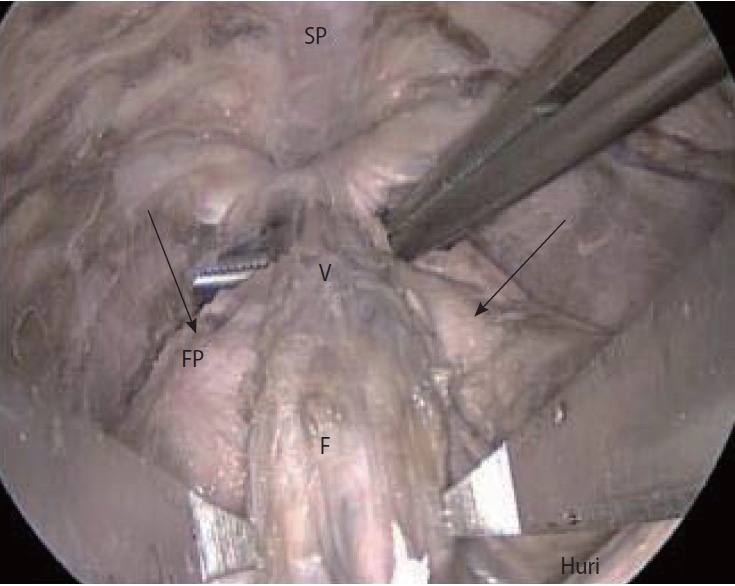
Dorsal vein complex (V) with visceral fascia of veins (F), periprostatic fascia (FP), and symphysis pubis (SP).
The posterior surface of the prostate is in direct contact with Denonvilliers’ fascia (DF; rectoprostatic fascia) (Fig. 3). It lies at the posterior and lateral angle of the prostate and also covers the posterior aspect of the seminal vesicle (Fig. 3) [30]. At the posterior aspect of the prostate, the anatomical locations of the fascial layers from the anterior to the posterior side are the anterior layer of DF, space of Proust, posterior layer of DF, and rectal fascia, consecutively [21]. The DF also covers the plexus vesicoprostaticus and the ampoules of the ductus deferens [2]. Laterally, it is interwoven with the fascia pelvis. Van Ophoven and Roth [31] reported that the DF consists of a single layer that is formed from the fusion of two walls of embryological peritoneal cul-de-sac. A double layer fashion exists histologically, but is not distinguishable intraoperatively. As in the pelvis, the nerves and fascial layers on the posterior side of the prostate show a similar anatomical distribution.
Fig. 3.
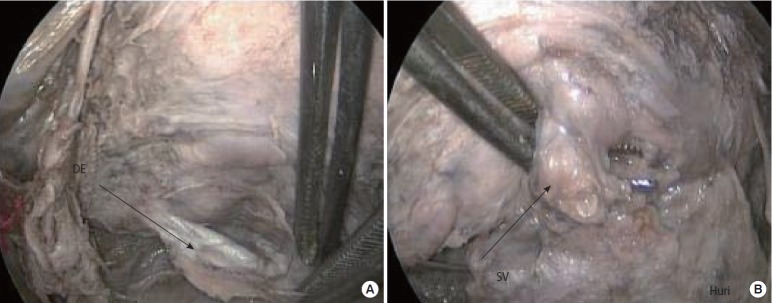
(A) Posterior part of the prostate, anterior layer of Denonvilliers’ fascia (DE). (B) Seminal vesicles (SVs) at the posterior view of the prostate.
The fascial parts of the prostate and periprostatic structures have various topographic relations. At the center of the posterior prostate surface, in almost all cases a fusion of the DF with the prostatic capsule is shown. Conversely, the DF shows no adherence to the prostatic capsule on the lateral aspect [29]. However, it was confirmed that the space between the DF and the prostatic capsule is filled by adipose tissue and the NVB [32]. As a result, the anatomical spaces between the fascial layers are completely related to the NVBs, which are of primary importance in nsRP.
HISTORY OF nsRP AND TECHNICAL ADVANCES IN FASCIAL SURGERY
The discovery of the cavernous nerves was a milestone for identifying a purposeful nsRP technique for the treatment of localized prostate cancer. Walsh stressed that anatomy texts are not helpful for determining the exact anatomical configuration of the autonomic innervations to the cavernous body and reported that an infant cadaver is the best model for understanding more about the location of the branches [33]. The vascular part of the NVBs provides the scaffolding for the nerves and can be used as a macroscopic landmark to identify the nerves during surgery [34]. However, it was reported that during the nerve-sparing technique, one part of the LPF, the PF, must remain on the prostate [34], and then the levator fascia or periprostatic fascia must be spared with the nerves.
The first nsRP was performed by Patrick Walsh in a 52-year-old patient [33]. The development of a database that included anatomical observations, changes in technique, cancer control, and quality of life was reported as the key technique for perfecting nsRP [35] As we know, the most important advantage of nsRP is related to functional outcomes during the follow-up after the surgery. Improvements in the surgical technique have had a significant positive impact on sexual [7–9] and lower urinary tract function [7–10]. The nerve-sparing technique mainly focuses on the preservation of the autonomic nerve fibers from the pelvic plexus (including afferent and efferent fibers) that form the nervi erigentes, which are responsible for penile erection and also innervate the sphincteric mechanism [36]. One report confirmed that in sexually active men with organ-confined disease, the bilateral nerve-sparing technique preserved erectile function in 32% to 86% and unilateral nerve-sparing surgery preserved function in 13% to 56% [8,37,38].
Thus, it has been shown that the key anatomical point during nsRP is the nerves. However, the extension and location of the periprostatic nerves are still controversial. As such, the location of the nerves according to the topographic prostate anatomy has been studied by some authors. The variability in recovery of erectile function can be attributed to the fact that there is no definite or exact anatomy of the periprostatic nerve fibers, especially the cavernosal nerves. Several urologists have reported surgical techniques according to the anatomy of the NVB and periprostatic fascia related to postoperative functional outcomes. For example, Lunacek et al. [39] reported a modified technique of nerve sparing called “curtain dissection,” which involves periprostatic fascial incision and dissection of the NVB far more anteriorly than previously described. The modifications of periprostatic fascial surgery continued with the report of Graefen et al. [40], who stressed incision of the parapelvic fascia of the prostate at the lateroventral aspect of the prostate at 10 o’clock and 2 o’clock. Whereas Kiyoshima et al. [29] reported a cord-like pattern in the NVB, some authors [40] have confirmed a scattered pattern of the NVB at the periprostatic region. Menon et al. [41] and Graefen et al. [40] both noted the importance of starting the incision high up on the ventral aspect of the prostate to preserve the maximum number of nerve fibers, because substantial numbers of nerve fibers are located ventrally. The preservation of periprostatic fascia (veil of Aphrodite) was described by Menon et al. [41], who reported that the veil-nerve-sparing procedure offers superior erectile function compared with the traditional nerve-sparing technique [42].
In another histologic study [43], the anatomical location of the periprostatic nerve bundles that run along the surface of the anterolateral zones was identified within the lateral PF (periprostatic fascia). It was found that the nerve bundle counts of the anterolateral zones differ between the two techniques (veil of Aphrodite technique and standard technique) with statistical significance [43]. However, Sung et al. [44] confirmed that the mean percentage of nerves in the ventral part of the prostate was 6.7%, with 33.3% in the dorsal, 29.6% in the right lateral, and 30.1% in the left lateral parts. The distance between the prostatic capsule and nerve fibers and the thickness of the periprostatic nerve fibers were reported as important anatomical features related to nsRP in cadavers [44]. Ganzer et al. [45] reported that the periprostatic nerve density decreases from the base towards the apex. Additionally, they stressed the variability of periprostatic nerve distribution [45]. Clarebrough et al. [46] reported a similar periprostatic nerve distribution pattern that showed that the most periprostatic neural tissue was located in the posterolateral region with a smaller proportion on the anterior surface of the prostate.
fsRP: NOVEL ANATOMICAL IDENTIFICATION
Since the description of nsRP, many technical advances have been identified for perfecting nsRP. Nevertheless, the relationship between the PF and the NVB is still controversial and under investigation [31,47,48]. However, Hong et al. [32] stressed that periprostatic adipose tissue is present on 48% of all prostatic surfaces and that this may cause the difficulty in making an exact anatomical determination of the NVB and fascial compartments. The location of the NVB is identified between the prostate capsule and either the levator ani fascia or the posterior PF. No nerve fibers are found lateral to the levator ani fascia or dorsal to the posterior PF [4,28,32,49,50]. Cornu et al. [19] reported that when performing RP, it is mandatory to locate the fascia surrounding the prostate, the EPF, the PF, and the posterior PF to respect the NVBs. This report also confirms the preservation of the fascial layers located at the periprostatic region during nsRP.
In the technology era, surgical advances regarding the nerve-sparing technique are still being made with awareness of the periprostatic fascial anatomy. Depending on the dissection plane chosen during the procedure, intra- and interfascial technical variations have been identified [18]. Both techniques can be identified as fascial-sparing surgery. The main goal in intrafascial dissection is to remove the prostate without the fascial layers on the prostate capsule [2,51,52]; however, the dissection is considered outside or lateral to the PF at the anterolateral and posterolateral aspects of the prostate [18]. The NVB might be more prone to partial resection with interfascial dissection because this dissection will not allow preserving more fascial layers at the anterolateral surface of the prostate, presumably resulting in an oncologically safer approach [2,25,52,53].
The robotic and laparoscopic approaches have brought innovation and better anatomical perspectives to RP. All technical advances have been made with the use of technologic tools. However, the experience of surgeons who prefer the open approach has increased with the anatomical technical improvements in robotic RP [54]. Recently, the high anterior release technique [16] and the veil of Aphrodite technique [43] of robot-assisted nsRP were identified. With these techniques, the PF is incised very anteriorly to spare the anterior accessory nerves, which can be important anatomical structures for potency. It is clear that high anterior release of the levator fascia (or periprostatic fascia) refers to the preservation of the periprostatic fascia during nsRP and can be called fascial-sparing RP, or fsRP (Fig. 4). Park et al. [55] stressed the importance of the neuroanatomy of the prostate in relation to functional outcomes in their article. Nielsen et al. [16] reported excellent oncological results and improved postoperative sexual function after this technique. I suggest that working collaboratively with a clinical anatomist is crucial to update the anatomical terminology of the prostate, which contiguous structures are clinically useful, and the surgical procedure [56].
Fig. 4.
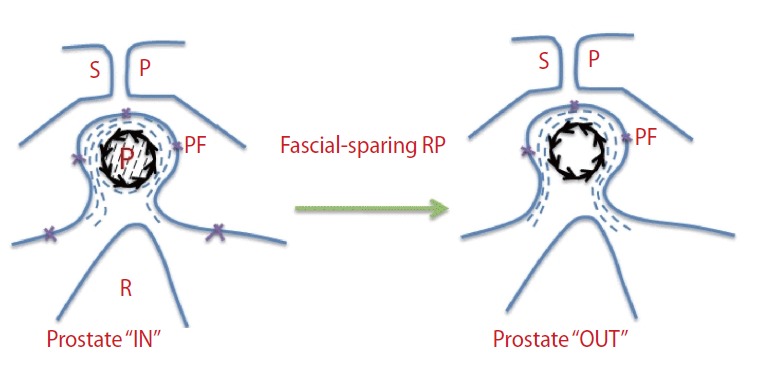
Anatomical description of fascial-sparing radical prostatectomy (RP). SP, symphysis pubis; P, prostate; PF, periprostatic fascia; R, rectum; →→→, prostatic fascia; ---, periprostatic nerve fibers.
CONCLUSION
For urologists performing RP and uroanatomists performing anatomical cadaveric prostate dissection, the gross anatomy of the prostate and periprostatic fascial layers, the microscopic anatomy of the prostate, the location of the NVBs, and the relation of the periprostatic fascial layers on the anterior, lateral, and posterior sides of the prostate should be of great interest. A better understanding of the relation between nerve fibers and pelvic fascial layers is crucial for performing anatomical RP. The novel anatomical identification of nsRP as fsRP may be useful for future reports related to anatomical RP.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Heidenreich A, Aus G, Bolla M, Joniau S, Matveev VB, Schmid HP, et al. EAU guidelines on prostate cancer. Eur Urol. 2008;53:68–80. doi: 10.1016/j.eururo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Stolzenburg JU, Schwalenberg T, Horn LC, Neuhaus J, Constantinides C, Liatsikos EN. Anatomical landmarks of radical prostatecomy. Eur Urol. 2007;51:629–39. doi: 10.1016/j.eururo.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Abbou CC, Hoznek A, Salomon L, Lobontiu A, Saint F, Cicco A, et al. Remote laparoscopic radical prostatectomy carried out with a robot. Report of a case. Prog Urol. 2000;10:520–3. [PubMed] [Google Scholar]
- 4.Ayala AG, Ro JY, Babaian R, Troncoso P, Grignon DJ. The prostatic capsule: does it exist? Its importance in the staging and treatment of prostatic carcinoma. Am J Surg Pathol. 1989;13:21–7. [PubMed] [Google Scholar]
- 5.Myers RP. Improving the exposure of the prostate in radical retropubic prostatectomy: longitudinal bunching of the deep venous plexus. J Urol. 1989;142:1282–4. doi: 10.1016/s0022-5347(17)39057-2. [DOI] [PubMed] [Google Scholar]
- 6.Walsh PC. Anatomic radical prostatectomy: evolution of the surgical technique. J Urol. 1998;160(6 Pt 2):2418–24. doi: 10.1097/00005392-199812020-00010. [DOI] [PubMed] [Google Scholar]
- 7.Kübler HR, Tseng TY, Sun L, Vieweg J, Harris MJ, Dahm P. Impact of nerve sparing technique on patient self-assessed outcomes after radical perineal prostatectomy. J Urol. 2007;178:488–92. doi: 10.1016/j.juro.2007.03.100. [DOI] [PubMed] [Google Scholar]
- 8.Michl UH, Friedrich MG, Graefen M, Haese A, Heinzer H, Huland H. Prediction of postoperative sexual function after nerve sparing radical retropubic prostatectomy. J Urol. 2006;176:227–31. doi: 10.1016/S0022-5347(06)00632-X. [DOI] [PubMed] [Google Scholar]
- 9.Dubbelman YD, Dohle GR, Schroder FH. Sexual function before and after radical retropubic prostatectomy: a systematic review of prognostic indicators for a successful outcome. Eur Urol. 2006;50:711–8. doi: 10.1016/j.eururo.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Burkhard FC, Kessler TM, Fleischmann A, Thalmann GN, Schumacher M, Studer UE. Nerve sparing open radical retropubic prostatectomy--does it have an impact on urinary continence? J Urol. 2006;176:189–95. doi: 10.1016/S0022-5347(06)00574-X. [DOI] [PubMed] [Google Scholar]
- 11.Catalona WJ, Carvalhal GF, Mager DE, Smith DS. Potency, continence and complication rates in 1,870 consecutive radical retropubic prostatectomies. J Urol. 1999;162:433–8. [PubMed] [Google Scholar]
- 12.Rabbani F, Stapleton AM, Kattan MW, Wheeler TM, Scardino PT. Factors predicting recovery of erections after radical prostatectomy. J Urol. 2000;164:1929–34. [PubMed] [Google Scholar]
- 13.D’Amico AV, Whittington R, Malkowicz SB, Cote K, Loffredo M, Schultz D, et al. Biochemical outcome after radical prostatectomy or external beam radiation therapy for patients with clinically localized prostate carcinoma in the prostate specific antigen era. Cancer. 2002;95:281–6. doi: 10.1002/cncr.10657. [DOI] [PubMed] [Google Scholar]
- 14.Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, Scardino PT. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol. 2002;167(2 Pt 1):528–34. doi: 10.1016/S0022-5347(01)69079-7. [DOI] [PubMed] [Google Scholar]
- 15.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910–4. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen ME, Schaeffer EM, Marschke P, Walsh PC. High anterior release of the levator fascia improves sexual function following open radical retropubic prostatectomy. J Urol. 2008;180:2557–64. doi: 10.1016/j.juro.2008.08.047. [DOI] [PubMed] [Google Scholar]
- 17.Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492–7. doi: 10.1016/s0022-5347(17)53012-8. [DOI] [PubMed] [Google Scholar]
- 18.Walz J, Burnett AL, Costello AJ, Eastham JA, Graefen M, Guillonneau B, et al. A critical analysis of the current knowledge of surgical anatomy related to optimization of cancer control and preservation of continence and erection in candidates for radical prostatectomy. Eur Urol. 2010;57:179–92. doi: 10.1016/j.eururo.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Cornu JN, Phe V, Fournier G, Delmas V, Sebe P. Fascia surrounding the prostate: clinical and anatomical basis of the nerve-sparing radical prostatectomy. Surg Radiol Anat. 2010;32:663–7. doi: 10.1007/s00276-010-0668-7. [DOI] [PubMed] [Google Scholar]
- 20.Baader B, Herrmann M. Topography of the pelvic autonomic nervous system and its potential impact on surgical intervention in the pelvis. Clin Anat. 2003;16:119–30. doi: 10.1002/ca.10105. [DOI] [PubMed] [Google Scholar]
- 21.Skandalakis JE, Colborn GL, Weidman TA, Foster RS, Jr, King-snorth AN, Skandalakis LJ, et al. Skandalakis surgical anatomy: the embryologic and anatomic basis of modern surgery. Athens: PMP; 2004. [Google Scholar]
- 22.Takenaka A, Hara R, Soga H, Murakami G, Fujisawa M. A novel technique for approaching the endopelvic fascia in retropubic radical prostatectomy, based on an anatomical study of fixed and fresh cadavers. BJU Int. 2005;95:766–71. doi: 10.1111/j.1464-410X.2005.05397.x. [DOI] [PubMed] [Google Scholar]
- 23.Steiner MS. Continence-preserving anatomic radical retropubic prostatectomy. Urology. 2000;55:427–35. doi: 10.1016/s0090-4295(99)00462-8. [DOI] [PubMed] [Google Scholar]
- 24.Walsh PC. Anatomic radical retropubic prostatectomy. In: Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Campell’s urology. 8th ed. Philadelphia: Saunders; 2002. pp. 3107–29. [Google Scholar]
- 25.Kriby RS, Partin AW, Feneley M, Parsons JK, editors. Prostate cancer: principles and practice. London: Taylor & Francis; 2006. [Google Scholar]
- 26.Myers RP. Practical surgical anatomy for radical prostatectomy. Urol Clin North Am. 2001;28:473–90. doi: 10.1016/s0094-0143(05)70156-7. [DOI] [PubMed] [Google Scholar]
- 27.Presti JC, Jr, Schmidt RA, Narayan PA, Carroll PR, Tanagho EA. Pathophysiology of urinary incontinence after radical prostatectomy. J Urol. 1990;143:975–8. doi: 10.1016/s0022-5347(17)40155-8. [DOI] [PubMed] [Google Scholar]
- 28.Costello AJ, Brooks M, Cole OJ. Anatomical studies of the neurovascular bundle and cavernosal nerves. BJU Int. 2004;94:1071–6. doi: 10.1111/j.1464-410X.2004.05106.x. [DOI] [PubMed] [Google Scholar]
- 29.Kiyoshima K, Yokomizo A, Yoshida T, Tomita K, Yonemasu H, Nakamura M, et al. Anatomical features of periprostatic tissue and its surroundings: a histological analysis of 79 radical retropubic prostatectomy specimens. Jpn J Clin Oncol. 2004;34:463–8. doi: 10.1093/jjco/hyh078. [DOI] [PubMed] [Google Scholar]
- 30.Walz J, Graefen M, Huland H. Basic principles of anatomy for optimal surgical treatment of prostate cancer. World J Urol. 2007;25:31–8. doi: 10.1007/s00345-007-0159-6. [DOI] [PubMed] [Google Scholar]
- 31.van Ophoven A, Roth S. The anatomy and embryological origins of the fascia of Denonvilliers: a medico-historical debate. J Urol. 1997;157:3–9. doi: 10.1097/00005392-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Hong H, Koch MO, Foster RS, Bihrle R, Gardner TA, Fyffe J, et al. Anatomic distribution of periprostatic adipose tissue: a mapping study of 100 radical prostatectomy specimens. Cancer. 2003;97:1639–43. doi: 10.1002/cncr.11231. [DOI] [PubMed] [Google Scholar]
- 33.Walsh PC. The discovery of the cavernous nerves and development of nerve sparing radical retropubic prostatectomy. J Urol. 2007;177:1632–5. doi: 10.1016/j.juro.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Walsh PC, Lepor H, Eggleston JC. Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations. Prostate. 1983;4:473–85. doi: 10.1002/pros.2990040506. [DOI] [PubMed] [Google Scholar]
- 35.Walsh PC. Perfecting nerve-sparing radical prostatectomy: sailing in uncharted waters. Can J Urol. 2008;15:4230–2. [PubMed] [Google Scholar]
- 36.Kessler TM, Burkhard FC, Studer UE. Nerve-sparing open radical retropubic prostatectomy. Eur Urol. 2007;51:90–7. doi: 10.1016/j.eururo.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Geary ES, Dendinger TE, Freiha FS, Stamey TA. Nerve sparing radical prostatectomy: a different view. J Urol. 1995;154:145–9. [PubMed] [Google Scholar]
- 38.Kundu SD, Roehl KA, Eggener SE, Antenor JA, Han M, Catalona WJ. Potency, continence and complications in 3,477 consecutive radical retropubic prostatectomies. J Urol. 2004;172(6 Pt 1):2227–31. doi: 10.1097/01.ju.0000145222.94455.73. [DOI] [PubMed] [Google Scholar]
- 39.Lunacek A, Schwentner C, Fritsch H, Bartsch G, Strasser H. Anatomical radical retropubic prostatectomy: ‘curtain dissection’ of the neurovascular bundle. BJU Int. 2005;95:1226–31. doi: 10.1111/j.1464-410X.2005.05510.x. [DOI] [PubMed] [Google Scholar]
- 40.Graefen M, Walz J, Huland H. Open retropubic nerve-sparing radical prostatectomy. Eur Urol. 2006;49:38–48. doi: 10.1016/j.eururo.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Menon M, Tewari A, Peabody J, VIP Team Vattikuti Institute prostatectomy: technique. J Urol. 2003;169:2289–92. doi: 10.1097/01.ju.0000067464.53313.dd. [DOI] [PubMed] [Google Scholar]
- 42.Menon M, Shrivastava A, Kaul S, Badani KK, Fumo M, Bhandari M, et al. Vattikuti Institute prostatectomy: contemporary technique and analysis of results. Eur Urol. 2007;51:648–57. doi: 10.1016/j.eururo.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 43.Savera AT, Kaul S, Badani K, Stark AT, Shah NL, Menon M. Robotic radical prostatectomy with the “Veil of Aphrodite” technique: histologic evidence of enhanced nerve sparing. Eur Urol. 2006;49:1065–73. doi: 10.1016/j.eururo.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 44.Sung W, Lee S, Park YK, Chang SG. Neuroanatomical study of periprostatic nerve distributions using human cadaver prostate. J Korean Med Sci. 2010;25:608–12. doi: 10.3346/jkms.2010.25.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ganzer R, Blana A, Gaumann A, Stolzenburg JU, Rabenalt R, Bach T, et al. Topographical anatomy of periprostatic and capsular nerves: quantification and computerised planimetry. Eur Urol. 2008;54:353–60. doi: 10.1016/j.eururo.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 46.Clarebrough EE, Challacombe BJ, Briggs C, Namdarian B, Weston R, Murphy DG, et al. Cadaveric analysis of periprostatic nerve distribution: an anatomical basis for high anterior release during radical prostatectomy? J Urol. 2011;185:1519–25. doi: 10.1016/j.juro.2010.11.046. [DOI] [PubMed] [Google Scholar]
- 47.Kourambas J, Angus DG, Hosking P, Chou ST. A histological study of Denonvilliers’ fascia and its relationship to the neurovascular bundle. Br J Urol. 1998;82:408–10. doi: 10.1046/j.1464-410x.1998.00749.x. [DOI] [PubMed] [Google Scholar]
- 48.Lindsey I, Guy RJ, Warren BF, Mortensen NJ. Anatomy of Denonvilliers’ fascia and pelvic nerves, impotence, and implications for the colorectal surgeon. Br J Surg. 2000;87:1288–99. doi: 10.1046/j.1365-2168.2000.01542.x. [DOI] [PubMed] [Google Scholar]
- 49.Lepor H, Gregerman M, Crosby R, Mostofi FK, Walsh PC. Precise localization of the autonomic nerves from the pelvic plexus to the corpora cavernosa: a detailed anatomical study of the adult male pelvis. J Urol. 1985;133:207–12. doi: 10.1016/s0022-5347(17)48885-9. [DOI] [PubMed] [Google Scholar]
- 50.McNeal JE. Normal histology of the prostate. Am J Surg Pathol. 1988;12:619–33. doi: 10.1097/00000478-198808000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Menon M, Kaul S, Bhandari A, Shrivastava A, Tewari A, Hemal A. Potency following robotic radical prostatectomy: a questionnaire based analysis of outcomes after conventional nerve sparing and prostatic fascia sparing techniques. J Urol. 2005;174:2291–6. doi: 10.1097/01.ju.0000181825.54480.eb. [DOI] [PubMed] [Google Scholar]
- 52.Secin FP, Serio A, Bianco FJ, Jr, Karanikolas NT, Kuroiwa K, Vickers A, et al. Preoperative and intraoperative risk factors for side-specific positive surgical margins in laparoscopic radical prostatectomy for prostate cancer. Eur Urol. 2007;51:764–71. doi: 10.1016/j.eururo.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 53.Zorn KC, Gofrit ON, Orvieto MA, Mikhail AA, Zagaja GP, Shalhav AL. Robotic-assisted laparoscopic prostatectomy: functional and pathologic outcomes with interfascial nerve preservation. Eur Urol. 2007;51:755–62. doi: 10.1016/j.eururo.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 54.Lee DI. Robotic prostatectomy: what we have learned and where we are going. Yonsei Med J. 2009;50:177–81. doi: 10.3349/ymj.2009.50.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park YH, Jeong CW, Lee SE. A comprehensive review of neuroanatomy of the prostate. Prostate Int. 2013;1:139–45. doi: 10.12954/PI.13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huri E, Tatar I, Sargon MF, Germiyanoglu C, Basar R. Revisiting anatomical landmarks of cadaveric nerve-sparing radical prostatectomy in accordance to Terminologia Anatomica. Anatomy. 2011;5:18–22. [Google Scholar]


