Abstract
Neuroscience labs benefit from reliable, easily-monitored neural responses mediated by well-studied neural pathways. Xenopus laevis tadpoles have been used as a simple vertebrate model preparation in motor control studies. Most of the neuronal pathways underlying different aspects of tadpole swimming behavior have been revealed. These include the skin mechanosensory touch and pineal eye light-sensing pathways whose activation can initiate swimming, and the cement gland pressure-sensing pathway responsible for stopping swimming. A simple transection in the hindbrain can cut off the pineal eye and cement gland pathways from the swimming circuit in the spinal cord, resulting in losses of corresponding functions. Additionally, some pharmacological experiments targeting neurotransmission can be designed to affect swimming and, fluorescence-conjugated α–bungarotoxin can be used to label nicotinic receptors at neuromuscular junctions. These experiments can be readily adapted for undergraduate neuroscience teaching labs. Possible expansions of some experiments for more sophisticated pharmacological or neurophysiological labs are also discussed.
Keywords: Xenopus, tadpole, swimming, neuromuscular junction, behavior, pharmacology, physiology
Undergraduate neuroscience and biology practical sessions require animals or preparations that can be obtained in bulk at low cost, are easy to maintain during experiments and which provide easily recorded and interpretable results. Several invertebrate preparations, such as crayfish, snails, leeches, earthworms, cockroaches, locusts, and drosophila, fulfill these requirements (Johnson et al., 2002; Vilinsky and Johnson, 2012). In contrast, vertebrate preparations are generally more difficult to keep, and often a significant amount of dissection is needed to prepare animals before experimentation. The use of simpler and smaller vertebrates like zebrafish has been proposed recently (McKeown et al., 2009). Here we propose the use of another simple developing vertebrate, Xenopus laevis tadpoles, in undergraduate neuroscience teaching.
Xenopus embryo and tadpole movements develop from simple local twitching of a few swimming muscles to free forward-moving, upright, swimming between 24 to 44 hours after fertilization (Muntz, 1975). The function of the Xenopus nervous system is best understood in stage 37/38 tadpoles (approximately two days old; Nieuwkoop and Faber, 1956; Roberts et al., 2010). At stage 37/38, only some internal organs and parts of the nervous system involved in motor behavior have developed (Sive et al., 1998). Tadpole eyes are nonfunctional at this stage, and the mouth and digestive system have not developed. The heart has started to beat but blood cells are only present at later developmental stages.
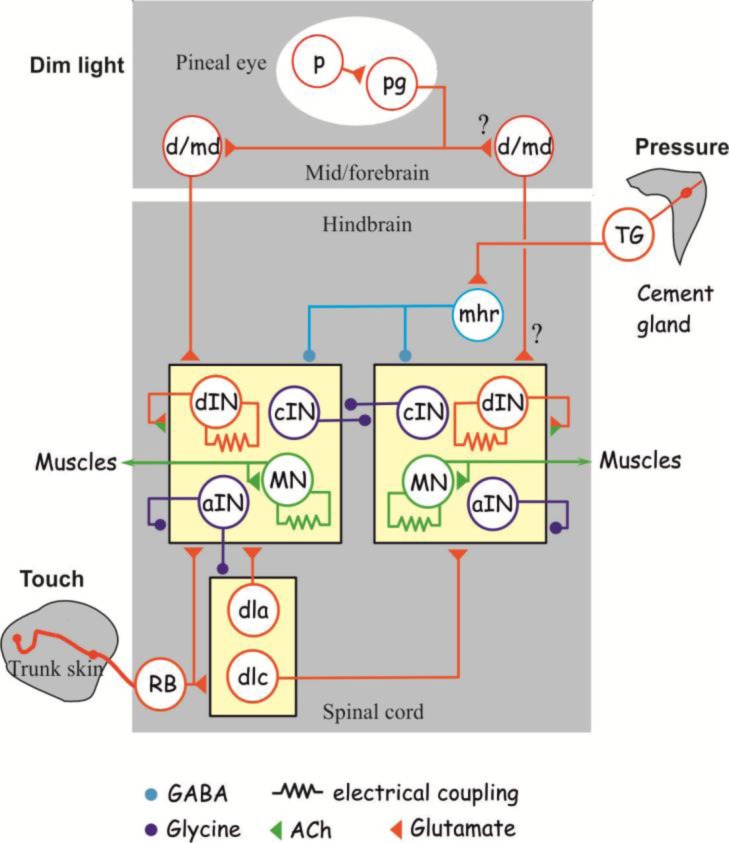
Vertebrate locomotor rhythms are controlled by the central pattern generator (CPG) circuits in the spinal cord. While the organization of CPG circuits remains unclear in mammals, deep insights into the workings of the swimming CPGs have been obtained in aquatic vertebrates like lamprey (Grillner, 2003), Xenopus tadpoles (Roberts et al., 2010) and zebrafish (Fetcho and McLean, 2010). In tadpoles, the caudal hindbrain is also an integral part of the longitudinally-distributed swimming CPG. The tadpole swimming CPG receives glutamatergic excitation from the mechanosensory (touch) pathway neurons in the spinal cord and pineal eye pathway. It is inhibited by the GABAergic inhibitory reticulospinal interneurons in the mid-hindbrain region. The CPG itself contains excitatory interneurons with ipsilateral descending axons, inhibitory commissural interneurons, inhibitory interneurons with ipsilateral axons and motoneurons (Fig.1).
Figure 1.
Diagram showing neural pathways involved in the initiation and stopping of tadpole swimming. Synaptic connections are drawn with symbols representing electrical coupling and the use of different neurotransmitters. Question marks indicate uncertainty in connections. Abbreviations are: p - pineal photoreceptor; pg - pineal ganglion cell; d/md - diencephalic/mesencephalic descending interneuron; TG - trigeminal ganglion cell; mhr – mid-hindbrain reticulospinal neurons; dIN - descending interneurons, cIN - commissural interneurons; MN - motoneuron; aIN - ascending interneurons; dla - dorsolateral ascending interneurons; dlc - dorsolateral commissural interneurons; RB - Rohon-Beard cells. The tadpole swimming CPG includes dIN, cIN, aIN and MN (Figure courtesy of Alan Roberts and modified with permission).
Tadpole swimming is a very robust behavior. By observing tadpole swimming, students can learn about vertebrate sensory activation and behavior, motor systems and locomotor rhythm generation and aspects of neuropharmacology. A practical session considering these learning objectives requires only limited dissection skills and the use of dissection stereomicroscopes (and in some cases fluorescence microscopes). We give a brief review of tadpole neuroethology and outline some experiments that can be used or adapted for undergraduate neuroscience teaching.
MATERIALS AND METHODS
The African clawed frog, Xenopus laevis has been used widely in developmental research, and many universities already house colonies of them. Injections of human chorionic gonadotropin (HCG) can be used to induce mating throughout the year, providing a good supply of tadpoles. It is not necessary to keep a large colony of Xenopus if there is not already an existing one in the institution. Maintaining a few pairs of adult Xenopus for the period of labs is fairly easy and economical. Xenopus should be kept at around 19° C in static tanks filled with dechlorinated mains (tap) water. More husbandry information can be found in the laboratory manual book for Xenopus users (Sive et al., 1998). Poor husbandry conditions can severely affect egg fertility, so animals are better purchased one or two weeks before use to allow them to become accustomed to the new environment. HCG (Sigma-Aldrich, UK) is injected into the dorsal lymph sac in pairs of male and female frogs to induce mating. Procedures for the HCG injections comply with UK Home Office regulations and all experiments have been approved by the local ethical committee. Pre-feeding tadpoles (before stage 45, about 4 days old) are considered insentient, allowing observation of their swimming after dissections without anaesthetization. We prepare 1ml aliquots containing 1000 international HCG units in distilled water and keep them frozen at −20° C. One aliquot is sufficient for use between one pair of frogs depending on their size (females: ∼ 0.7 ml, males: ∼0.3 ml). Once eggs are fertilized, they rapidly begin to cleave and develop. Unfertilized eggs will turn grey after several hours and break down. The embryos can be collected in trays of dechlorinated water. Aerating water with fish tank filtration devices attached to a small air pump can help to improve the embryo survival rate but this is not essential. In our experience, leaving embryos in the injection tank normally provides better survival rates, while the adult frogs are kept in a separate post-injection tank. To increase the chance of getting enough tadpoles, it can be necessary to inject two pairs of adult Xenopus. Collected embryos can then be raised at different temperatures to stagger developmental rates. This can extend the supply of tadpoles at desired stages for up to three days. It takes embryos about 2 days to develop to stage 37/38 at 22° C, 3 days at 19 °C, and 4 days at 17 °C (Sive et al., 1998). Additional information on Xenopus laevis development rates at different temperatures can be found at http://www.xenbase.org. A small wine cooler, for example, with double chambers and separate temperature controls can be used for this purpose. Staging criteria for stage 37/38 tadpoles includes: darkened eye cups which haven’t closed; a darkened cement gland; a straight, obtuse posterior proctodeum outline (about 30 degree to longitudinal body axis); and an anus opening appears at about half body length.
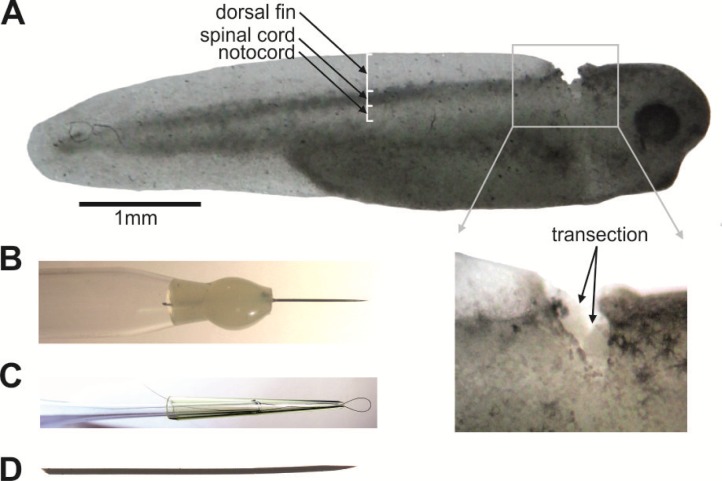
Normal tadpole swimming can be observed in dechlorinated water or in saline (concentrations in mM: NaCl 115, KCl 3, CaCl2 2, NaHCO3 2.4, MgCl2 1, HEPES 10, pH adjusted to 7.4 with NaOH). After observing natural tadpole swimming behavior, transections in the hindbrain or the spinal cord (spinalization, Fig. 2A) can be done in a Sylgard-coated (Dowcorning, Michigan, USA) petri-dish filled with saline using a small scalpel under a dissection microscope. Sylgard-coated dishes can be prepared in bulk and kept for use for many years. A normal scalpel blade may block the sight of the tadpole under the microscope due to their size. We use a tungsten wire (200 μm in diameter) with electrically-etched tip to achieve more precise cutting (Fig. 2B). The tadpoles must be briefly anesthetized with 0.1% MS222 (3-aminobenzoic acid ester, Sigma, UK) prior to dissections. This anaesthetization takes a couple of minutes, and its progression can be monitored by squirting the MS222 solution onto the tadpole and watching its responses. The anaesthetization can last for a few minutes, providing enough time for the transection. Recovery from anaesthetization can be monitored by flipping the tadpole occasionally using a hairloop until the tadpole swimming comes back (Fig. 2C). Common lab chemicals and N-methyl-D-aspartate (NMDA) are obtained from Sigma, while rhodamine-conjugated α-bungarotoxin for fluorescence microscopy can be obtained from Life Technologies (Paisley, UK).
Figure 2.
Hindbrain transection of a stage 37/38 tadpole and a dissection needle, a hairloop and a pin. A. A tadpole after hindbrain transection. The dorsal and side surface of the tadpole central nervous system is covered with black pigment cells. Therefore, the spinal cord and hindbrain form a dark stripe of tissue beneath the transparent dorsal fin and above the lighter notochord. The transection is made between the obex and otic capsule. B. A dissection needle is made by gluing a segment of tungsten wire (200 μm in diameter, tip etched electrically). C. A hairloop is made by trapping a thread of hair between a cut pipette tip and a fire-polished glass pipette. D. A pin etched from a short piece of tungsten wire (50 μm in diameter).
RESULTS
1. Motor behavior initiated by the mechanosensory pathway
At stage 37/38, tadpoles have limited motor behavior (Roberts et al., 2010), including flexion response, swimming, struggling, turning, and hanging from the water surface. Sensory pathways underlying some of these responses are shown in Fig. 1 and Fig. 3. The flexion response enables tadpoles to bend their body away from a stimulus (Li et al., 2003b); but it is very brief and difficult to observe with the naked eye. When sucked into a pipette at low speed, tadpoles can quickly turn around and try to swim out of it. This could be related to lateral line functions (Roberts et al., 2009). Forward swimming at 10–25 Hz can be initiated by briefly stroking tadpole skin or dimming ambient light. Tadpoles can show a more violent struggling behavior (5–10 Hz), which generates backward thrusts in response to a sustained press on the skin (Soffe, 1991; 1993; Green and Soffe, 1996; Li et al., 2007). Relatively speaking, swimming is much more easily and reliably evoked than struggling. We will focus on the pathways that are involved in the initiation, maintenance and termination of swimming behavior.
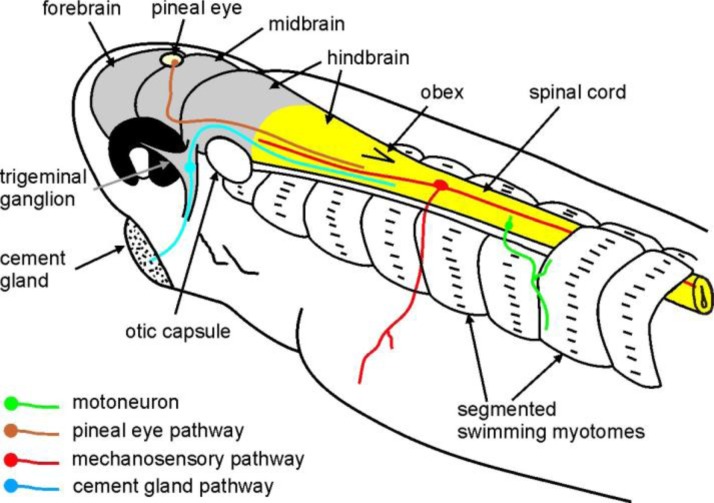
Figure 3.
The anatomy of the tadpole sensory pathways, swimming myotomes and central nervous system. Yellow coloring shows the caudal hindbrain and spinal cord, where the swimming circuit is located. Grey shows the forebrain, midbrain, trigeminal ganglion, rostral and middle hindbrain. The spinal cord is normally embedded in the segmented myotomes used for swimming. The dorsal parts of a few rostral myotomes have been drawn removed. Different sensory pathways and a motoneuron are schematically shown in a simplified form and color-coded.
The mechanosensory system is comprised of free sensory nerve endings embedded in the tadpole skin, which contains only two layers of cells (Roberts and Hayes, 1977; Clarke et al., 1984). These nerve endings arise from sensory Rohon-Beard cell somata located in the dorsal spinal cord. Any local distortion in the skin (touch) can activate up to a few sensory Rohon-Beard cells, whose central axons run longitudinally in the dorsal tract making en passant glutamatergic synapses onto sensory interneurons in the cord (Fig. 1, Fig. 3, Sillar and Roberts, 1988; Li et al., 2003b, 2004a). We found that the most effective way to activate the mechanosensory pathway is by flipping the tadpole using a hairloop.
2. Swimming termination by the cement gland pathway
Swimming stops immediately when the tadpole head makes contact with a solid surface or the water surface. In both cases, the inhibitory cement gland pathway is activated. This pathway is comprised of bilaterally located glutamatergic sensory neurons in the trigeminal ganglia with neurites in the cement gland (Fig. 1, Fig. 3). The ganglion cells then activate GABAergic mid-hindbrain reticulospinal neurons, which synapse onto the spinal neurons controlling swimming by activating postsynaptic GABAA receptors (Boothby and Roberts, 1992; Perrins et al., 2002; Li et al., 2003a). While the tadpole is hanging from the water surface, some midhindbrain reticulospinal neurons will fire tonically, reducing tadpoles’ responses to other sensory stimuli (Lambert et al., 2004a; Lambert et al., 2004b).
Observation of swimming behavior can be done with or without a dissection microscope. Caution should be taken when a dissection microscope is used because intense light can heat up the water/saline rapidly. Tadpoles show some drastic responses to overheating, such as big flexion and markedly shortened spontaneous swimming bouts at temperatures up to 38°C (Robertson and Sillar, 2009). If not addressed swiftly, overheating can kill tadpoles within minutes.
3. Light dimming responses
Different from the endocrine pineal gland in mammals, the pineal eye (parietal eye) in amphibians is photoreceptive. In stage 37/38 tadpoles, the pineal eye is located on top of the forebrain (Fig. 3). Pineal photoreceptors can sense light intensity changes and initiate swimming (Roberts, 1978). Dimming light activates diencephalic-mesencephalic interneurons which project to the hindbrain region where swimming is initiated (Jamieson and Roberts, 1999). Dimming light during ongoing swimming can increase swimming frequency and make tadpoles turn upwards (Jamieson and Roberts, 2000). This behavior could presumably guide tadpoles broadly toward more shaded areas of water and help their survival in the wild. Light dimming can be achieved by quickly casting a shadow over the tadpole or dimming lighting on the dissection microscope.
4. Responses after hindbrain transection or spinalization
Tadpole hindbrain transection or spinalization needs to be performed in a Sylgard-lined petri dish in saline. This dissection cuts off the cement gland and pineal eye pathways from the rest of swimming circuit, so related responses will disappear. Fine pins etched from 50–100 μm tungsten wire (Fig. 2D, or similar insect pins) can be used to pin through the tadpole head end rostral to the transection line after MS222 anesthetization. This will ensure that the mechanosensory pathways caudal to spinalization remain intact. Extra pins can be put through the yolk belly block if needed. The trigeminal nerve containing the afferent axons of trigeminal ganglion cells enters the hindbrain just rostral to the otic capsule. The transection can be done in the caudal hindbrain between the otic capsule and obex. However, many excitatory interneurons important for swimming maintenance are located in this region (Li et al., 2006; Soffe et al., 2009). The more caudal the transection is performed, the more likely that swimming rhythm generation mechanisms remaining in the spinal cord may be disrupted. Transections at the caudal edge of the otic capsule leave tadpole swimming mostly unaffected. Transections more caudal to the obex tend to shorten swimming episodes significantly. Minimal cutting during transection can help reserve the streamlined shape of the tadpole body and stabilize its swimming through water, although removal of the head completely does not affect the generation of swimming rhythm itself. After transection, flipping the tadpole using a hairloop can still start tadpole swimming. Dimming light, on the other hand, should fail to do so. In addition, tadpoles should carry on swimming forward when they hit the petri dish or water surface.
5. NMDA-induced swimming
The tadpole swimming circuit contains excitatory interneurons activating AMPA, nicotinic and NMDA receptors (Li et al., 2004b). Activation of NMDA receptors in the spinal circuits of tadpoles and various other vertebrates has been shown in vitro to induce locomotor-like rhythms, including that for swimming (Brodin et al., 1985; Cazalets et al., 1992; Hochman et al., 1994; Guertin and Hounsgaard, 1998; Alford et al., 2003; Li et al., 2010; Li, 2011). NMDA at concentrations ranging from 20–100 μM can induce long-lasting swimming in tadpoles transected in the caudal hindbrain. The transected tadpole must be moved from control saline to the NMDA solution. Using a pipette with a small opening of ∼2 mm in diameter to transfer the animal can minimize the dilution of NMDA. The transection cut alone should allow drug access, but further opening of the dorsal fin can increase drug access rate. This can be achieved by slitting the dorsal fin using a dissection needle at a ∼ 45° angle to the rostral-caudal axis when the tadpole is anaesthetized for the transection. Due to drug access issues, it may take tens of seconds for the swimming to start. Once it has started, swimming can potentially carry on for many minutes though the tail flapping amplitude will drop with time. This differs from the self-sustaining swimming evoked in spinalized tadpoles following activation of mechanosensory touch pathways, which runs down and stops naturally within a couple of minutes after initiation (Dale and Gilday, 1996).
6. Tadpole immobilization and labeling nicotinic receptors
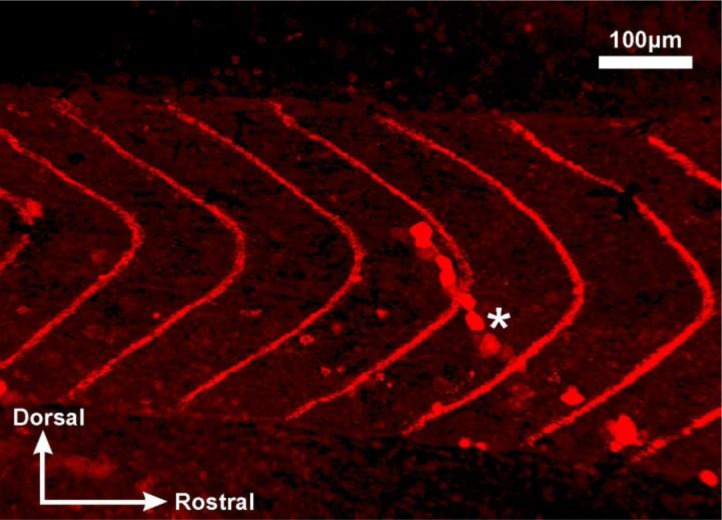
As in all vertebrates, the postsynaptic receptors at the tadpole NMJ are nicotinic receptors. At stage 37/38, most of the motor nerve innervation is restricted to the clefts between chevron-shaped myotomes. Using Rhodamine-conjugated α-bungarotoxin, we can visualize the location of NMJs conveniently (Zhang et al., 2011). Stock α-bungarotoxin solution prepared in distilled water (100 μl at 100 μM) can be kept frozen in 1.5 ml Eppendorf tubes and stay effective for many months. Each tube is diluted with saline to 1 ml (10 μM) before final use and should be kept at 4°C when not in use. Each tube is sufficient for immobilizing 20–30 tadpoles. The blockade of nicotinic receptors at NMJs by α-bungarotoxin will immobilize tadpoles. However, swimming rhythms can still be started and maintained in the spinal cord as usual by stimulating the skin. Such rhythmic activity (fictive swimming) can be recorded and monitored using an extracellular recording setup (see example recordings in Li and Moult, 2012). The immobilization/binding processes, again, can be monitored by flipping the tadpole periodically using the hairloop. The whole process normally takes about 20–30 minutes at stage 37/38, after which the tadpoles can be washed in normal saline for 5–10 minutes before observation under a fluorescence microscope (Fig. 4). It is not necessary to peel away the trunk skin over the myotome blocks but skin removal can reduce background fluorescence levels. Interestingly, there is little fluorescence binding in the central nervous system, where nicotinic receptors have been found (Li et al., 2004b). This is because muscle type nicotinic receptors have a distinctly different subunit composition to those in the central nervous system (Rang et al., 2011).
Figure 4.
Labeling of tadpole neuromuscular nicotinic receptors at the myotomal clefts using rhodamine-conjugated α-bungarotoxin. Fluorescent chevron-like stripes represent myotome clefts. An asterisk marks an incidental slash in the myotomes during dissection. The image was taken on a Zeiss confocal-like AxioImager M2 microscope. The image was produced by Monica Wagner when she was an undergraduate.
DISCUSSION
We have reported some potential use of tadpole swimming observation for undergraduate neuroscience teaching practical sessions. These simple experiments can be adapted or combined to accommodate different lab needs depending on the learning objectives. For example, combining experiments 1–4 will be suitable for a sensory activation and behavior lab (touch and light dimming initiates swimming; pressure at the cement gland stops swimming); experiments 1, 4 and 5 can be put together for a motor control practical (role of spinal cord and hindbrain in locomotor rhythm generation; importance of NMDA receptors); experiments 1, 4, 5 and 6 may be used for a neuropharmacology lab (excitation by NMDA receptors; nicotinic receptors at NMJ). Some questions can be set for students to answer in these practical sessions. For example (key points/responses in brackets):
How many different types of motor behavior can you categorize in control tadpoles (swimming, swimming stopping, hanging from water surface, flexion, struggling, turning)?
Is there any observable difference between swimming evoked by light-dimming and that by skin stimulation (no)?
What differences have you noticed in the tadpole’s swimming behavior after transection (light-dimming fails to initiate swimming; bumping into petri dish or water surface fails to stop swimming)?
If you have noticed some changes in swimming episode length before and after transection, explain what may underlie the changes (longer after transection because swimming episodes in control are cut short by the cement gland pathway activation).
How is the head required for responses to dimming (pineal eye pathway)?
Is there any difference between NMDA-induced swimming and swimming initiated by skin stimulation in spinalized tadpoles, and why (NMDA-induced swimming lasts much longer because the skin stimulation-evoked swimming has run-down mechanisms)?
Why does blocking nicotinic receptors immobilize tadpoles (specific binding of α-bungarotoxin to a subtype of nicotinic receptor)?
It should be noted that NMDA-induced swimming and immobilization can be done in tadpoles without transection as long as some cuts are made in the tadpole skin (e.g., slits in the dorsal fin) to facilitate drug access.
Some experiments can be expanded for further behavioral, pharmacological, anatomical or physiological analyses. One such experiment would be to carry out more detailed swimming behavior analysis using a compact digital camera with high-speed video functions. We captured some video clips of tadpole swimming using a Casio EX-FC100 camera at 420 fps. The camera was mounted onto a trinocular microscope (BMSZ, Brunel microscopes Ltd, Chippenham, UK). For tadpole swimming at 10–25 Hz, this gave 17–42 frames for one swimming cycle. Such videos can then be edited easily using software such as Windows Movie Maker, e.g., by cutting out periods without movement. Replaying the video at reduced speed allows analysis of swimming frequency, the observation of rostral-to-caudal propagation of muscle contractions (the speed of which can be calculated) and average swimming speed. We have tried to break some videos into a series of JPG images using a “video to JPG converter” (freely downloadable from http://www.dvdvideosoft.com/). This enables us to examine swimming in a frame-by-frame manner using Windows photo viewer, giving a time resolution of ∼2.5 ms in calculating swimming frequency or speed. Occasionally, a flexion response to skin stimulation can also be seen before swimming is initiated in these videos.
A further set of experiments involves testing the pharmacology of the tadpole swimming circuit or basic synaptic transmission in general. For example, omitting calcium from the saline should stop swimming; 100–500 μM cadmium chloride can be used to block calcium channels and stop swimming (Dale, 1993). The tadpole swim stopping pathway relies on activation of GABAA receptors, so the swim stopping response via the cement gland pathway should disappear (without hindbrain transections) if tadpoles are immersed in 20–50 μM GABAzine (Abcam, Cambridge, UK). Tetrodotoxin or N-(2,6-Dimethylphenylcarbamoylmethyl) triethylammonium bromide (QX314, Tocris, Bristol, UK) can be used to block voltage-dependent Na+ currents and block all motor responses including swimming.
A third set of experiments can be designed to observe the developmental change of NMJ by using α-bungarotoxin conjugated with two different fluorophores. The tadpole motor neuron innervation pattern changes during early development: at stage 37/38, most NMJs are confined within the muscle clefts, while at stage 42, the motoneuron axon innervation of swimming myotomes becomes more refined (Zhang et al., 2011), and some NMJs are formed outside the muscle clefts. Sequential labeling of nicotinic receptors using bungarotoxin conjugated with different fluorophores can reveal these changes during development. We have been successfully running this set of experiments as an undergraduate lab for the last four years and have found them to be a valuable neuroethology teaching aid.
Footnotes
This work was supported by a Royal Society University Research Fellowship to Li.
REFERENCES
- Alford S, Schwartz E, Viana di Prisco G. The pharmacology of vertebrate spinal central pattern generators. Neuroscientist. 2003;9:217–228. doi: 10.1177/1073858403009003014. [DOI] [PubMed] [Google Scholar]
- Boothby KM, Roberts A. The stopping response of Xenopus laevis embryos: behaviour, development and physiology. J Comp Physiol A. 1992;170:171–180. doi: 10.1007/BF00196899. [DOI] [PubMed] [Google Scholar]
- Brodin L, Grillner S, Rovainen CM. N-Methyl-D-aspartate (NMDA), kainate and quisqualate receptors and the generation of fictive locomotion in the lamprey spinal cord. Brain Res. 1985;325:302–306. doi: 10.1016/0006-8993(85)90328-2. [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Sqalli-Houssaini Y, Clarac F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J Physiol. 1992;455:187–204. doi: 10.1113/jphysiol.1992.sp019296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JD, Hayes BP, Hunt SP, Roberts A. Sensory physiology, anatomy and immunohistochemistry of Rohon-Beard neurones in embryos of Xenopus laevis. J Physiol. 1984;348:511–525. doi: 10.1113/jphysiol.1984.sp015122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N. A large, sustained Na(+)− and voltage-dependent K+ current in spinal neurons of the frog embryo. J Physiol. 1993;462:349–372. doi: 10.1113/jphysiol.1993.sp019559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N, Gilday D. Regulation of rhythmic movements by purinergic neurotransmitters in frog embryos. Nature. 1996;383:259–263. doi: 10.1038/383259a0. [DOI] [PubMed] [Google Scholar]
- Fetcho JR, McLean DL. Some principles of organization of spinal neurons underlying locomotion in zebrafish and their implications. Ann N Y Acad Sci. 2010;1198:94–104. doi: 10.1111/j.1749-6632.2010.05539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CS, Soffe SR. Transitions between two different motor patterns in Xenopus embryos. J Comp Physiol A. 1996;178:279–291. doi: 10.1007/BF00188169. [DOI] [PubMed] [Google Scholar]
- Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat Rev Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- Guertin PA, Hounsgaard J. Chemical and electrical stimulation induce rhythmic motor activity in an in vitro preparation of the spinal cord from adult turtles. Neurosci Lett. 1998;245:5–8. doi: 10.1016/s0304-3940(98)00164-5. [DOI] [PubMed] [Google Scholar]
- Hochman S, Jordan LM, MacDonald JF. N-methyl-D-aspartate receptor-mediated voltage oscillations in neurons surrounding the central canal in slices of rat spinal cord. J Neurophysiol. 1994;72:565–577. doi: 10.1152/jn.1994.72.2.565. [DOI] [PubMed] [Google Scholar]
- Jamieson D, Roberts A. A possible pathway connecting the photosensitive pineal eye to the swimming central pattern generator in young Xenopus laevis tadpoles. Brain Behav Evol. 1999;54:323–337. doi: 10.1159/000006632. [DOI] [PubMed] [Google Scholar]
- Jamieson D, Roberts A. Responses of young Xenopus laevis tadpoles to light dimming: possible roles for the pineal eye. J Exp Biol. 2000;203(Pt 12):1857–1867. doi: 10.1242/jeb.203.12.1857. [DOI] [PubMed] [Google Scholar]
- Johnson BR, Wyttenbach RA, Hoy RR. In: The crawdad project: crustaceans as model systems for teaching principles of neuroscience In Frontiers in crustacean neurobiology. Wiese, Schmidt, editors. Berlin: Springer Verlag; 2002. [Google Scholar]
- Lambert TD, Howard J, Plant A, Soffe S, Roberts A. Mechanisms and significance of reduced activity and responsiveness in resting frog tadpoles. J Exp Biol. 2004a;207:1113–1125. doi: 10.1242/jeb.00866. [DOI] [PubMed] [Google Scholar]
- Lambert TD, Li WC, Soffe SR, Roberts A. Brainstem control of activity and responsiveness in resting frog tadpoles: tonic inhibition. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004b;190:331–342. doi: 10.1007/s00359-004-0505-8. [DOI] [PubMed] [Google Scholar]
- Li WC. Generation of locomotion rhythms without inhibition in vertebrates: the search for pacemaker neurons. Integr Comp Biol. 2011;51:879–889. doi: 10.1093/icb/icr021. [DOI] [PubMed] [Google Scholar]
- Li WC, Moult PR. The control of locomotor frequency by excitation and inhibition. J Neurosci. 2012;32:6220–6230. doi: 10.1523/JNEUROSCI.6289-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WC, Perrins R, Walford A, Roberts A. The neuronal targets for GABAergic reticulospinal inhibition that stops swimming in hatchling frog tadpoles. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2003a;189:29–37. doi: 10.1007/s00359-002-0372-0. [DOI] [PubMed] [Google Scholar]
- Li WC, Roberts A, Soffe SR. Specific brainstem neurons switch each other into pacemaker mode to drive movement by activating NMDA receptors. J Neurosci. 2010;30:16609–16620. doi: 10.1523/JNEUROSCI.3695-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WC, Sautois B, Roberts A, Soffe SR. Reconfiguration of a vertebrate motor network: specific neuron recruitment and context-dependent synaptic plasticity. J Neurosci. 2007;27:12267–12276. doi: 10.1523/JNEUROSCI.3694-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WC, Soffe SR, Roberts A. The spinal interneurons and properties of glutamatergic synapses in a primitive vertebrate cutaneous flexion reflex. J Neurosci. 2003b;23:9068–9077. doi: 10.1523/JNEUROSCI.23-27-09068.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WC, Soffe SR, Roberts A. Dorsal spinal interneurons forming a primitive, cutaneous sensory pathway. J Neurophysiol. 2004a;92:895–904. doi: 10.1152/jn.00024.2004. [DOI] [PubMed] [Google Scholar]
- Li WC, Soffe SR, Roberts A. Glutamate and acetylcholine corelease at developing synapses. Proc Natl Acad Sci U S A. 2004b;101:15488–15493. doi: 10.1073/pnas.0404864101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WC, Soffe SR, Wolf E, Roberts A. Persistent responses to brief stimuli: feedback excitation among brainstem neurons. J Neurosci. 2006;26:4026–4035. doi: 10.1523/JNEUROSCI.4727-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown KA, Downes GB, Hutson LD. Modular laboratory exercises to analyze the development of zebrafish motor behavior. Zebrafish. 2009;6:179–185. doi: 10.1089/zeb.2008.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntz L. Myogenesis in the trunk and leg during development of the tadpole of Xenopus laevis (Daudin 1802) J Embryol Exp Morphol. 1975;33:757–774. [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal tables of Xenopus laevis (Daudin) Amsterdam: North Holland; 1956. [Google Scholar]
- Perrins R, Walford A, Roberts A. Sensory activation and role of inhibitory reticulospinal neurons that stop swimming in hatchling frog tadpoles. J Neurosci. 2002;22:4229–4240. doi: 10.1523/JNEUROSCI.22-10-04229.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang HP, Dale MM, Ritter JM, Flower R, Henderson G. Rang & Dale’s Pharmacology: with STUDENT CONSULT Online Access. Edinburgh: Churchill Livingstone; 2011. [Google Scholar]
- Roberts A. Pineal eye and behaviour in Xenopus tadpoles. Nature. 1978;273:774–775. doi: 10.1038/273774a0. [DOI] [PubMed] [Google Scholar]
- Roberts A, Feetham B, Pajak M, Teare T. Responses of hatchling Xenopus tadpoles to water currents: first function of lateral line receptors without cupulae. J Exp Biol. 2009;212:914–921. doi: 10.1242/jeb.027250. [DOI] [PubMed] [Google Scholar]
- Roberts A, Hayes BP. The anatomy and function of ‘free’ nerve endings in an amphibian skin sensory system. Proc R Soc Lond B Biol Sci. 1977;196:415–429. doi: 10.1098/rspb.1977.0048. [DOI] [PubMed] [Google Scholar]
- Roberts A, Li WC, Soffe SR. How neurons generate behavior in a hatchling amphibian tadpole: an outline. Front Behav Neurosci. 2010;4:16. doi: 10.3389/fnbeh.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson RM, Sillar KT. The nitric oxide/cGMP pathway tunes the thermosensitivity of swimming motor patterns in Xenopus laevis tadpoles. J Neurosci. 2009;29:13945–13951. doi: 10.1523/JNEUROSCI.3841-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillar KT, Roberts A. Unmyelinated cutaneous afferent neurons activate two types of excitatory amino acid receptor in the spinal cord of Xenopus laevis embryos. J Neurosci. 1988;8:1350–60. doi: 10.1523/JNEUROSCI.08-04-01350.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early development of Xenopus laevis - a laboratory manual. New York, NY: Cold Spring Harbor Laboratory Press; 1998. [Google Scholar]
- Soffe SR. Triggering and gating of motor responses by sensory stimulation: behavioural selection in Xenopus embryos. Proc Biol Sci. 1991;246:197–203. doi: 10.1098/rspb.1991.0145. [DOI] [PubMed] [Google Scholar]
- Soffe SR. Two distinct rhythmic motor patterns are driven by common premotor and motor neurons in a simple vertebrate spinal cord. J Neurosci. 1993;13:4456–4469. doi: 10.1523/JNEUROSCI.13-10-04456.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffe SR, Roberts A, Li WC. Defining the excitatory neurons that drive the locomotor rhythm in a simple vertebrate: insights into the origin of reticulospinal control. J Physiol. 2009;587:4829–4844. doi: 10.1113/jphysiol.2009.175208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilinsky I, Johnson KG. Electroretinograms in Drosophila: a robust and genetically accessible electrophysiological system for the undergraduate laboratory. J Undergrad Neurosci Educ. 2012;11:A149–157. [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Issberner J, Sillar KT. Development of a spinal locomotor rheostat. Proc Natl Acad Sci U S A. 2011;108:11674–11679. doi: 10.1073/pnas.1018512108. [DOI] [PMC free article] [PubMed] [Google Scholar]