Abstract
Background
It remains controversial whether dual antiplatelet therapy reduces stroke more than aspirin alone.
Aim
We aimed to assess the effects of adding clopidogrel to aspirin on the occurrence of stroke and major haemorrhage in patients with vascular disease.
Methods
Meta-analysis of published randomized trials comparing the combination of clopidogrel and aspirin vs. aspirin alone that reported stroke and major bleeding.
Results
Thirteen randomized trials were included with a total of 90 433 participants (mean age 63 years; 63% male) with a mean follow-up of 1·0 years and 2011 strokes. Stroke was reduced 19% by dual antiplatelet therapy (odds ratio = 0·81, 95% confidence interval 0·74–0·89) with no evidence of heterogeneity of effect across different trial populations (I2 index = 5%, P = 0·4 for heterogeneity). Dual antiplatelet therapy reduced ischemic stroke by 23% (odds ratio = 0·77; 95% confidence interval 0·70–0·85); there was a nonsignificant 12% increase in intracerebral haemorrhage (odds ratio = 1·12, 95% confidence interval 0·86–1·46). Among 1930 participants with recent (<30 days) brain ischemia from four trials, stroke was reduced by 33% (odds ratio = 0·67, 95% confidence interval 0·46–0·97) by dual antiplatelet therapy vs. aspirin alone. The risk of major bleeding was increased by 40% (odds ratio = 1·40, 95% confidence interval 1·26–1·55) by dual antiplatelet therapy.
Conclusions
This meta-analysis demonstrates a substantial relative risk reduction in stroke by clopidogrel plus aspirin vs. aspirin alone that is consistent across different trial cohorts. Major haemorrhage is increased by dual antiplatelet therapy.
Keywords: antiplatelet therapy, clinical trials, clopidogrel, stroke
Introduction
Based on a recent meta-analysis, we concluded that treatment with the combination of aspirin and clopidogrel has no effect on overall mortality compared with aspirin alone (1). An increased incidence of fatal bleeding was offset by a significant reduction in myocardial infarction. There was evidence of heterogeneity of the effect of dual antiplatelet therapy on death depending on the different trial populations analyzed, which ranged from asymptomatic patients with vascular risk factors to patients having acute coronary syndromes.
Here, we assess the effect of the combination of clopidogrel and aspirin vs. aspirin on stroke and major haemorrhage based on meta-analysis of all published randomized trials reporting stroke as an outcome. We hypothesized that the effect of dual antiplatelet therapy on stroke would be different depending on the trial cohort.
Methods
Search and selection process
Randomized trials involving adults were included in which clopidogrel in any dosage was added to aspirin in any dosage and in which stroke incidence was reported. Trials were excluded if stroke incidence was not provided or if published only in abstracts. We searched The Cochrane Central Register of Controlled Trials, the ClinicalTrials.gov website, and PubMed using the key words of clopidogrel and aspirin combined with clinical trial from 1966 to April 2012, not restricted by language.
Data extraction
Two physician reviewers (S. P., R. G. H.) independently extracted information about methodological features, the number of patients treated, total follow-up exposure, and the numbers of strokes and major haemorrhage. Trial quality was assessed by the Jadad score (2). In order to explore if the effect of the combination therapy was different according to the populations included in the individual trials, we classified the trials a priori into one of three categories: 1 – trials with cohorts having stable vascular disease (i.e. including vascular risk factors or a vascular event that occurred, on average, >30 days before entry); 2 – trials enrolling cohorts with a recent vascular event (i.e. ≤30 days) in any circulation; and 3 – trials enrolling cohorts undergoing a surgical or percutaneous intervention. Disagreements were resolved by joint review and consensus.
Outcomes
We accepted the criteria for diagnosis of stroke and major haemorrhage used in the individual trials. These were generally similar when provided, with outlying definitions described in footnotes to the tables. The primary meta-analysis focused on all strokes [ischemic and primary intracerebral haemorrhages (ICHs)]. We then analyzed separately, ischemic and unknown strokes vs. ICH, major haemorrhage, and all strokes in trials restricted to participants with recent brain ischemia (i.e. ≤30 days). In all trials, major haemorrhage included patients with ICH. Because the numbers of patients with other types of major haemorrhage (i.e. excluding ICH) could not be estimated reliably, ICHs are included both as strokes and as major haemorrhages in these analyses.
Data synthesis
Intention-to-treat results were used when available (and foot-noted when not available). Meta-analyses of the trial results are presented as odds ratios (ORs) and 95% confidence intervals (CIs) comparing dual antiplatelet vs. aspirin (OR estimates may differ slightly from published hazard ratios for individual trials as hazard ratios use failure-time data, vs. event–no event data for OR). Each meta-analysis OR was computed assuming a random effects model. If the count in one or more of the cells for a trial was 0, then 0·5 was added to each of the four cells. Statistical heterogeneity across trials was evaluated using the I2 index (percentage of the total variability in a set of effect sizes due to between-studies variability) and the chi-square test for heterogeneity. I2 index and statistical significance for test of heterogeneity are reported for main results and for meta-analyses with I2 index >40% and/or P < 0·2 for test of heterogeneity. Relative risk reduction was calculated as 1 minus the OR. All tests and CIs are two sided. Software used for meta-analysis were MedCalc for Windows, version 12·1.4·0 (MedCalc Software, Mariakerke, Belgium) and RevMan 5.0 (3).
Results
Thirteen randomized trials were included with a total of 90 433 randomized participants with a mean age of 63 years and a mean follow-up of 1·0 years (4–16). All but three trials (7,9,10) were rated as having the highest Jadad score of five reflecting relative lack of potential bias (Table 1). More than half of the patients (51%) were from a Chinese trial involving patients with acute myocardial infarction with a mean follow-up for 15 days; this trial contributed 23% of stroke outcomes (11) (Table 2).
Table 1.
Randomized controlled trials grouped according to qualifying vascular event
| n | Population | Mean age |
Male (%) |
Mean follow-up |
Jadad score |
|
|---|---|---|---|---|---|---|
| Stable vascular disease trials | ||||||
| ACTIVE A 2009 (4) | 7 554 | Atrial fibrillation | 71 | 58 | 3·6 years | 5 |
| CHARISMA 2006 (5) | 15 603 | Cardiovascular disease or multiple risk factors | 68 | 70 | 2·3 years | 5 |
| PROCLAIM 2009 (6) | 181 | Metabolic syndrome | 56 | 43 | 6 weeks | 5 |
| REAL-LATE/ZEST-LATE 2010 (7) | 2 701 | Coronary artery stents >12 months before | 62 | 70 | 1·6 years | 3 |
| SPS3 2012 (8) | 3 020 | Recent lacunar stroke | 63 | 63 | 3·5 years | 5 |
| Combined | 29 059 | 64 | 61 | 2·7 years | – | |
| Recent vascular event (≤30 days) | ||||||
| CARESS 2005 (9) | 107 | Recently symptomatic carotid stenosis | 60 | 78 | 7 days | 4 |
| CLAIR 2010 (10) | 100 | Stroke/TIA within 7 days and intracranial stenosis | 57 | 38 | 7 days | 3 |
| COMMIT 2005 (11) | 45 852 | Acute myocardial infarct | 61 | 72 | 15 days | 5 |
| CURE 2001 (12) | 12 562 | Acute coronary syndromes within <24 h | 64 | 70 | 9 months | 5 |
| FASTER 2007 (13) | 392 | TIA within 24 h or minor ischemic stroke | 68 | 54 | 90 days | 5 |
| Combined | 59 013 | 62 | 62 | 71 days | – | |
| Perioperative/periprocedural | ||||||
| CASCADE 2010 (14) | 113 | Coronary artery bypass grafting | 67 | 89 | 1 years | 5 |
| Cassar et al. 2005 (15) | 132 | Lower extremity bypass surgery | 66 | 77 | 30 days | 5 |
| CREDO 2002 (16) | 2 116 | Coronary stenting | 62 | 71 | 1 years | 5 |
| Combined | 2 361 | 65 | 79 | 11·4 months | – | |
| Overall results | ||||||
| 13 trials above | 90 433 | 63 | 66 | 1·0 years | – |
TIA, transient ischemic attack.
Table 2.
Stroke incidence and stroke mortality in randomized trials testing clopidogrel added to aspirin
| n | Population | # Strokes (fatal) | All stroke OR (95% CI) |
||
|---|---|---|---|---|---|
| Combination | Aspirin alone | ||||
| Stable vascular disease trials | |||||
| ACTIVE A 2009 (4) | 7 554 | Atrial fibrillation | 296 (70) | 408 (93) | 0·70 (0·60–0·82) |
| CHARISMA 2006 (5) | 15 603 | Cardiovascular disease or multiple risk factors | 202* (NR) | 234* (NR) | 0·86 (0·71–1·04) |
| PROCLAIM 2009 (6) | 181 | Metabolic syndrome | 0 (0) | 0 (0) | 1·03 (0·02–53) |
| REAL-LATE/ZEST-LATE 2010 (7) | 2 701 | Coronary artery stents >12 months before | 9 (NR) | 4 (NR) | 2·24 (0·69–7·28) |
| SPS3 2012 (8) | 3 020 | Recent lacunar stroke | 125 (NR) | 138 (NR) | 0·89 (0·69–1·14) |
| Meta-analysis | 29 059 | 632 | 784 | 0·82 (0·69–0·97) | |
| Recent vascular event (≤30 days) | |||||
| CARESS 2005 (9) | 107 | Recently symptomatic carotid stenosis | 0 (0) | 4 (0) | 0·11 (0·006–2·16) |
| CLAIR 2010 (10) | 100 | Stroke/TIA within 7 days and intracranial stenosis | 0 (0) | 2 (0) | 0·22 (0·01–4·63) |
| COMMIT 2005 (11) | 45 852 | Acute myocardial infarct | 217 (39) | 250 (41) | 0·86 (0·72–1·04) |
| CURE 2001 (12) | 12 562 | Acute coronary syndromes within 24 h | 75 (NR) | 87 (NR) | 0·87 (0·64–1·18) |
| FASTER 2007 (13) | 392 | TIA or minor ischemic stroke within 24 h | 14† | 21† | 0·63 (0·31–1·27) |
| Meta-analysis | 59 013 | 306 | 364 | 0·84 (0·72–0·98) | |
| Perioperative/periprocedural | |||||
| CASCADE 2010 (14) | 113 | Coronary artery bypass grafting | 0 (0) | 2 (1) | 0·20 (0·009–4·19) |
| Cassar et al. 2005 (15) | 132 | Lower extremity bypass surgery | 2 (0) | 0 (0) | 5·00 (0·24–106) |
| CREDO 2002 (16) | 2 116 | Coronary stenting | 9 (NR) | 12 (NR) | 0·76 (0·32–1·80) |
| Meta-analysis | 2 361 | 11 | 14 | 0·80 (0·29–2·20) | |
| Overall results | |||||
| Meta-analysis (13 trials) | 90 433 | 949 | 1 162 | 0·81 (0·74–0·89) | |
A total of 441 strokes were reported, but 5 of them were not assigned to any of the therapies so they were excluded from the analysis.
One death from haemorrhagic stroke (unclear in which group).
OR, odds ratio; CI, confidence interval; NR, not reported; TIA, transient ischemic attack.
Trial categories
Stable vascular disease trials: five randomized trials were included with a total of 29 059 participants with a mean age of 64 years, 61% male, and a mean follow-up of 2·7 patient-years. Of the different trial categories, this group included a heterogeneous patient cohort, including patients with risk factors for vascular disease (5,6), coronary artery disease (7), lacunar stroke (8), and vascular risk factors (5).
Recent vascular events (≤30 days): five randomized trials were included with a total of 59 506 participants with a mean age of 62 years, 62% male, and a mean follow-up of 71 patient-days. This trial category was dominated by patients with acute coronary syndromes, as demonstrated by the 58 144 patients included in COMMIT (11) and CURE (12) trials.
Perioperative/periprocedural: Three randomized trials were included with a total of 2361 participants with a mean age of 65 years and an overrepresentation of males (79%) compared with the other trial categories (14–16). Mean follow-up was 11·4 patient-months. The vast majority of this cohort (n = 2229) underwent a cardiac intervention, either coronary artery bypass grafting or percutaneous angioplasty with stenting.
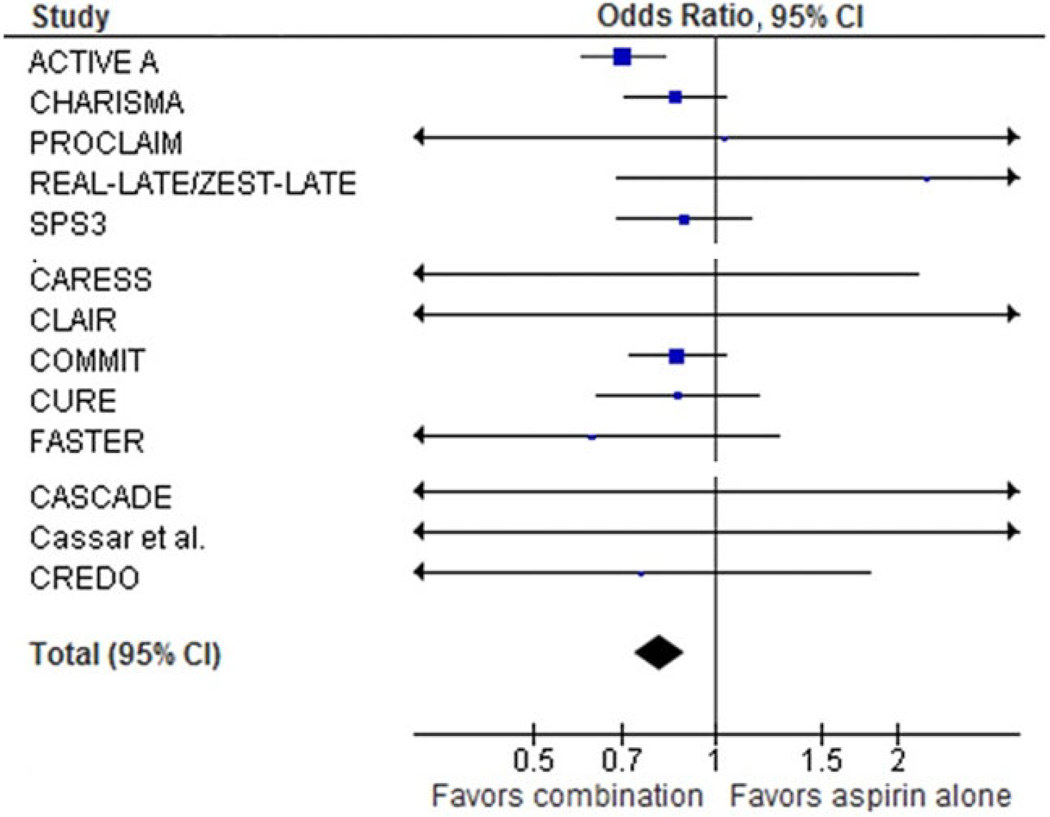
Effect of combination therapy on all strokes
The OR for all stroke by dual antiplatelet therapy vs. aspirin alone for all 13 trials was 0·81 (95% CI 0·74–0·89) (Table 2) with no evidence of heterogeneity of effect across the trials (I2 index = 5%, P = 0·4 for heterogeneity) (Fig. 1). Reduced stroke with dual antiplatelet therapy was similar for the three different trial categories: OR 0·82 (95% CI 0·69–0·97; I2 index 40%, P = 0·2 for heterogeneity) for patients with stable vascular disease; OR 0·84 (95% CI 0·72–0·98; I2 index 0%, P = 0·5 for heterogeneity) for patients with a recent vascular event; and OR 0·80 (95% CI 0·29–2·20; I2 index 9%, P = 0·4 for heterogeneity) for those with a recent surgery or procedure (Table 2). For fatal stroke, reported in seven trials, dual antiplatelet therapy was associated with an OR of 0·81 (95% CI 0·63–1·04) (Table 2).
Fig. 1.
Meta-analysis: all strokes. See table 1 for study references.
Ischemic stroke
The OR for ischemic strokes and strokes of unknown etiology combined by dual antiplatelet therapy vs. aspirin alone for the meta-analysis was 0·77 (95% CI 0·70–0·85) based on meta-analysis results of 10 trials with 1817 strokes [three trials (7,14,16) did not report strokes by etiology] (Table 3).
Table 3.
Stroke incidence by sub-type in randomized trials testing clopidogrel added to aspirin
| Stroke of ischemic + unknown etiology | Intracerebral haemorrhages* | ||||||
|---|---|---|---|---|---|---|---|
| Trial | n | Combination | Aspirin alone | OR (95% CI) | Combination | Aspirin alone | OR (95% CI) |
| Stable vascular disease trials | |||||||
| ACTIVE A 2009 (4) | 7 554 | 276 | 394 | 0·68 (0·58– 0·80) | 30 | 22 | 1·37 (0·79– 2·38) |
| CHARISMA 2006 (5) | 15 603 | 171 | 198 | 0·86 (0·70– 1·06) | 11 | 15 | 0·73 (0·34– 1·60) |
| PROCLAIM 2009 (6) | 181 | 0 | 0 | 1·03 (0·02– 53) | 0 | 0 | 1·03 (0·02– 53) |
| REAL-LATE/ZEST-LATE 2010 (7) | 2 701 | NR | NR | – | NR | NR | – |
| SPS3 2012 (8) | 3 020 | 104 | 125 | 0·81 (0·62– 1·06) | 15 | 8 | 1·87 (0·79– 4·42) |
| Meta-analysis | 26 358 | 551 | 717 | 0·76 (0·67– 0·87) | 56 | 45 | 1·24 (0·83– 1·84) |
| Recent vascular event (≤30 days) | |||||||
| CARESS 2005 (9) | 107 | 0 | 4 | 0·11 (0·006– 2·16) | 0 | 0 | 1·10 (0·02– 56) |
| CLAIR 2010 (10) | 100 | 0 | 2 | 0·22 (0·01– 4·63) | 0 | 0 | 1·13 (0·02– 58) |
| COMMIT 2005 (11) | 45 852 | 164 | 194 | 0·84 (0·68– 1·04) | 55 | 56 | 0·98 (0·68– 1·42) |
| CURE 2001 (12) | 12 562 | 68 | 82 | 0·83 (0·60– 1·15) | 7 | 5 | 1·41 (0·45– 4·45) |
| FASTER 2007 (13) | 392 | 12 | 21 | 0·53 (0·25– 1·11) | 2* | 0 | 4·95 (0·24– 104) |
| Meta-analysis | 59 013 | 244 | 303 | 0·81 (0·68– 0·96) | 64 | 61 | 1·04 (0·73– 1·47) |
| Surgical/periprocedural | |||||||
| CASCADE 2010 (14) | 113 | NR | NR | – | NR | NR | – |
| Cassar et al. 2005 (15) | 132 | 2 | 0 | 5·00 (0·24– 106) | 0 | 0 | 0·97 (0·02– 50) |
| CREDO 2002 (16) | 2 116 | NR | NR | – | NR | NR | – |
| Overall results | |||||||
| Meta-analysis (10 trials) | 85 503 | 797 | 1 020 | 0·77 (0·70– 0·85) | 120 | 106 | 1·12 (0·86– 1·46) |
Two trials (SPS3 and Active A) reported unknown strokes separately. Unknowns totaled 45 in the combination vs. 52 with aspirin alone. Excluding unknowns, the meta-analysis OR for ischemic stroke was 0·77 (0·70–0·86).
Only one trial (SPS3) reported sub-dural haematomas: combination 7/1517 vs. with aspirin alone 6/1503.
Primary intracerebral.
OR, odds ratio; CI, confidence interval; NR, not reported.
Intracerebral haemorrhage (ICH)
Intracerebral haemorrhage (ICH) was not significantly increased by combination therapy vs. aspirin alone (OR 1·12, 95% CI 0·86–1·46) based on meta-analysis analysis of 10 trials with 226 ICHs [three trials (7,14,16) did not report strokes by etiology] (Table 3).
Major haemorrhage
Major haemorrhage was significantly increased by combination therapy vs. aspirin alone (OR 1·40, 95% CI 1·26–1·55) based on meta-analysis of 13 trials (Table 4).
Table 4.
Major haemorrhage incidence by sub-type in randomized trials testing clopidogrel added to aspirin
| Major haemorrhage | ||||
|---|---|---|---|---|
| Trial | n | Combination | Aspirin alone | OR (95% CI) |
| Stable vascular disease trials | ||||
| ACTIVE A 2009 (4) | 7 554 | 251 | 162 | 1·59 (1·30–1·95) |
| CHARISMA 2006 (5) | 15 603 | 130 | 104 | 1·25 (0·97–1·63) |
| PROCLAIM 2009 (6) | 181 | 0 | 0 | 1·03 (0·02–53) |
| REAL-LATE/ZEST-LATE 2010 (7) | 2 701 | 3 | 1 | 2·98 (0·31–29) |
| SPS3 2012 (8) | 3 020 | 105 | 56 | 1·92 (1·38–2·68) |
| Meta-analysis | 29 059 | 489 | 323 | 1·54 (1·30–1·82) |
| Recent vascular event (≤30 days) | ||||
| CARESS 2005 (9) | 107 | 0 | 0 | 1·10 (0·02–56) |
| CLAIR 2010 (10) | 100 | 0 | 0 | 1·13 (0·02–58) |
| COMMIT 2005 (11) | 45 852 | 134 | 125 | 1·07 (0·84–1·37) |
| CURE 2001 (12) | 12 562 | 231* | 169* | 1·39 (1·14–1·70) |
| FASTER 2007 (13) | 392 | 3 | 0 | 6·96 (0·36–136) |
| Meta-analysis | 59 013 | 368 | 294 | 1·26 (1·08–1·47) |
| Surgical/periprocedural | ||||
| CASCADE 2010 (14) | 113 | 1 | 0 | 3·11 (0·12–78) |
| Cassar et al. 2005 (15) | 132 | 1 | 0 | 2·96 (0·12–74) |
| CREDO 2002 (16) | 2 116 | 93* | 71* | 1·35 (0·98–1·87) |
| Meta-analysis | 2 361 | 95 | 71 | 1·38 (1·00–1·89) |
| Overall results | ||||
| Meta-analysis (13 trials) | 90 433 | 952 | 688 | 1·40 (1·26–1·55) |
Include procedural bleeds (arterial puncture and surgical site bleeding).
OR, odds ratio; CI, confidence interval.
Patients with recent brain ischemia (≤30 days)
Four trials (9,10,13,17) were included in this meta-analysis (Table 5). The OR for all stroke with combination therapy was 0·67 (95% CI 0·46–0·97), and the OR for ischemic and strokes of unknown etiology combined was 0·64 (95% CI 0·43–0·94) (Table 5). Major haemorrhage (n = 21) was not increased by dual antiplatelet therapy in these trials (OR 0·91, 95% CI 0·40–2·07).
Table 5.
Effect of adding clopidogrel to aspirin in patients with recent brain ischemia (≤ 30 days)
| All stroke | Ischemic + unknown etiology | Major haemorrhage | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trial | n | CPD + ASA | ASA | OR (95% CI) | CPD + ASA | ASA | OR (95% CI) | CPD + ASA | ASA | OR (95% CI) |
| CARESS 2005 (9) | 107 | 0 | 4 | 0·11 (0·006–2·16) | 0 | 4 | 0·11 (0·006–2·16) | 0 | 0 | 1·10 (0·02–56) |
| CHARISMA sub-group 2011 (17) | 1331 | 34 | 46 | 0·73 (0·46–1·15) | 32 | 43 | 0·74 (0·46–1·18) | 9 | 11 | 0·82 (0·34–1·99) |
| CLAIR 2010 (10) | 100 | 0 | 2 | 0·22 (0·01–4·63) | 0 | 2 | 0·22 (0·01–4·63) | 0 | 0 | 1·13 (0·02–58) |
| FASTER 2007 (13) | 392 | 14 | 21 | 0·63 (0·31–1·27) | 12 | 21 | 0·53 (0·25–1·11) | 1 | 0 | 2·95 (0·12–73) |
| Meta-analysis | 1930 | 48 | 73 | 0·67 (0·46–0·97) | 44 | 70 | 0·64 (0·43–0·94) | 10 | 11 | 0·91 (0·40–2·07) |
A total of seven intracerebral haemorrhages were reported in the four trials: four assigned to combination (two in CHARISMA sub-study and two in FASTER) and three assigned to aspirin alone (all in CHARISMA sub-group); meta-analysis OR 1·12 (0·29–4·32).
CPD, clopidogrel; ASA, aspirin; OR, odds ratio; CI, confidence interval.
Discussion
This meta-analysis demonstrates a consistent 19% (95% CI 11–26) reduction in the occurrence of stroke by the use of aspirin and clopidogrel vs. aspirin alone. This reduction was evident in prespecified sub-groups of patients with stable vascular disease, individuals undergoing procedures, and those with recent (mostly cardiological) vascular events. For individuals with recent cerebral ischemia, we noted a benefit of 33% (95% CI 3–54). The relative reduction in stroke was similar to that seen for myocardial infarction (risk reduction 18% by dual antiplatelet therapy) in our previous meta-analysis (1). Ischemic stroke was reduced by 23% (95% CI 15–30), and there was a nonsignificant increase (12%) in ICH.
Major haemorrhage was increased 40% (95% CI 26–55) by dual antiplatelet therapy. From indirect comparison (data not shown), there was no apparent relationship between aspirin dosage and bleeding risk. In support of this observation, the large CURRENT OASIS 7 trial reported no difference in major haemorrhage when clopidogrel was randomly assigned to be given with aspirin 300–325 mg daily vs. 75–100 mg daily (18).
In the four randomized trials that included patients with recent brain ischemia, meta-analysis showed a 33% relative risk reduction (95% CI 3–54) in stroke by dual antiplatelet therapy. The dose of aspirin ranged from 75 to 160 mg. The three smaller trials (9,10,13) used a loading dose of 300 mg of clopidogrel at randomization followed by a maintenance dose of 75 mg daily whereas in the largest trial (17) the dose of aspirin was between 75 and 162 mg daily. The period immediately after transient ischemic attack (TIA) and nondisabling stroke carries a high risk of recurrent events and provides an attractive target for a strategy of intensive antithrombotic therapy. A reduction in stroke by combination of clopidogrel plus aspirin over aspirin alone in patients with recent TIA/stroke is currently being tested in ongoing randomized trials (19,20).
The MATCH trial compared aspirin plus clopidogrel with clopidogrelmonotherapy in 7599 individuals with stroke or TIA and found a nonsignificant reduction in stroke with an increase in life-threatening bleeding (21). Results of MATCH influenced current guideline recommendations to avoid the use of clopidogrel combined with aspirin for long-term secondary prevention of stroke (22). Our results suggest that aspirin plus clopidogrel is more effective than aspirin alone for stroke prevention. This result does not appear consistent with indirect comparisons with the results of MATCH (21) and CAPRIE trials (23). A recent meta-analysis of randomized trials that compared the efficacy and safety of two antiplatelet combinations (clopidogrel and aspirin and dipyridamole and aspirin) vs. monotherapy (aspirin, clopidogrel, or dipyridamole) in acute stroke or TIA showed that dual antiplatelet therapy was associated with a significant reduction in early stroke recurrence along with a nonsignificant increase in major bleeding (24).
There are a number of limitations to this study. We abstracted data from summary published results rather than a synthesis from patient-level data. We are therefore limited in exploring the effects of important variables such as time since event, blood pressure control, time on treatment, and stroke etiology. The overall benefit of dual antiplatelet therapy needs to balance stroke reduction against the increased risk of haemorrhage. Both benefit and risk may relate to these variables.
We acknowledge that we included different cohorts in this analysis, but our hypothesis was that there would be a differential effect of the therapy across the different predefined groups. This was not the case. In our view, the reduction in stroke by the combination of clopidogrel plus aspirin vs. aspirin in a wide spectrum of vascular disease is a key finding of this systematic review and meta-analysis. This has interesting and provocative pathophysiological implications.
This meta-analysis suggests that there is clear reduction in stroke by adding clopidogrel to aspirin that is offset by increased major haemorrhage. Individual randomized stroke prevention trials with longer exposure to the combination of aspirin and clopidogrel have consistently shown a marginal efficacy that was offset by a significant increase in major bleeding. It would certainly be premature that guideline recommendations be modified on the basis of this meta-analysis.
Footnotes
Conflict of interest:
Dr Santiago Palacio declares no conflict of interest.
Dr Robert G. Hart was co-principal investigator of the NIH sponsored SPS 3 trial.
Lesly A. Pearce declares no conflict of interest.
David C. Anderson served as site principal investigator of the NIH sponsored SPS 3 trial.
Dr Mukul Sharma has received honoraria as speaker from BMS/Pfizer and has participated as consultant for BMS/Pfizer.
Lee A. Birnbaum declares no conflict of interest.
Dr Oscar R. Benavente was the principal investigator of the NIH sponsored SPS 3 trial. He has received research support, honoraria and has participated as consultant for Sanofi/BMS.
Funding: None declared.
Disclosures: The author declares no potential conflict of interest.
References
- 1.Palacio S, Hart RG, Pearce LA, Benavente O. Effect of addition of clopidogrel to aspirin on mortality: systematic review of randomized trials. Stroke. 2012;43:2157–2162. doi: 10.1161/STROKEAHA.112.656173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jadad A, Moore R, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 3.Reviewer Manager (RevMan) (Computer Program). Version 5.0. Copenhagen: The Nordic Cochrane Center, the Cochrane Collaboration; 2008. [Google Scholar]
- 4.Connolly SJ, Pogue J, Hart RG, et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009;360:2066–2078. doi: 10.1056/NEJMoa0901301. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt DL, Fox KA, Hacke E, et al. Clopidogrel plus aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 6.Willerson T, Cable G, Yeh E on behalf of the PROCLAIM Investigators. Pilot study to examine the effects of clopidogrel on inflammatory markers in patients with metabolic syndrome receiving low-dose aspirin (PROCLAIM) Tex Heart Inst J. 2009;36:530–539. [PMC free article] [PubMed] [Google Scholar]
- 7.Park S-J, Park D-W, Kim Y-H, et al. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N Engl J Med. 2010;362:1374–1382. doi: 10.1056/NEJMoa1001266. [DOI] [PubMed] [Google Scholar]
- 8.The SPS3 Investigators. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–825. doi: 10.1056/NEJMoa1204133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markus HS, Droste DW, Kaps M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using Doppler embolic signal detection. The Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation. 2005;111:2233–2240. doi: 10.1161/01.CIR.0000163561.90680.1C. [DOI] [PubMed] [Google Scholar]
- 10.Wong KS, Chen C, Fu J, et al. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:489–497. doi: 10.1016/S1474-4422(10)70060-0. [DOI] [PubMed] [Google Scholar]
- 11.Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45852 patients with acute myocardial infarction: randomized placebo-controlled trial (COMMIT) Lancet. 2005;366:1607–1621. doi: 10.1016/S0140-6736(05)67660-X. [DOI] [PubMed] [Google Scholar]
- 12.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognomi G, Fox KK Clopidogrel in Unstable angina to prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes with ST-segment elevations. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy J, Hill MD, Ryckborst KJ, Eliasziw M, Demchuck AM, Buchan AM for the FASTER Investigators. Fast assessment of stroke and transient ischemic attack to prevent early recurrence (FASTER): a randomized controlled pilot trial. Lancet Neurol. 2007;6:961–969. doi: 10.1016/S1474-4422(07)70250-8. [DOI] [PubMed] [Google Scholar]
- 14.Kulik A, Le May MR, Voisine P, et al. Aspirin plus clopidogrel versus aspirin alone after coronary artery bypass grafting. Clopidogrel after Surgery for Coronary Artery Disease (CASCADE) Trial. Circulation. 2010;122:2680–2687. doi: 10.1161/CIRCULATIONAHA.110.978007. [DOI] [PubMed] [Google Scholar]
- 15.Cassar K, Ford I, Greaves M, Bachoo P, Brittenden J. Randomized clinical trial of the antiplatelet effects of aspirin-clopidogrel combination versus aspirin alone after lower limb angioplasty. Br J Surg. 2005;92:159–165. doi: 10.1002/bjs.4810. [DOI] [PubMed] [Google Scholar]
- 16.Steinhubl SR, Berger PB, Mann JT, III, et al. for the CREDO Investigators. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 17.Hankey GJ, Johnston C, Easton D, et al. Effect of clopidogrel plus ASA. vs. ASA early after TIA and ischaemic stroke: a substudy of the CHA-RISMA trial. Int J Stroke. 2011;6:3–9. doi: 10.1111/j.1747-4949.2010.00535.x. [DOI] [PubMed] [Google Scholar]
- 18.Mehta S, Tanguay J, Eikelboom J, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233–1243. doi: 10.1016/S0140-6736(10)61088-4. [DOI] [PubMed] [Google Scholar]
- 19.Johnston C. Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) Trial. NCT00991029. Last updated on 23 January 2012. [accessed 12 March 2012]; Available at http://www.clinicaltrials.gov.
- 20.Wang Y, Johnston C for CHANCE Investigators. Rationale and design of a randomized, double-blind trial comparing the effects of a 3 month clopidogrel-aspirin regimen versus aspirin alone for the treatment of high-risk patients with acute nondisabling cerebrovascular event. Am Heart J. 2010;160:380–386. doi: 10.1016/j.ahj.2010.05.017. e.1. [DOI] [PubMed] [Google Scholar]
- 21.Diener HC, Bogousslavsky J, Brass L, et al. Aspirin and clopidogrel compared to clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomized, double blind, placebo-controlled trial. Lancet. 2004;364:331–337. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 22.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for health professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 23.CAPRIE Steering Committee. A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events. Lancet. 1996;9038:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 24.Geeganage CM, Diener HC, Algra A, et al. Dual or mono antiplatelet therapy for patients with acute ischemic stroke or transient ischemic attack. Systematic review and meta-analysis of randomized controlled trials. Stroke. 2012;43:1058–1066. doi: 10.1161/STROKEAHA.111.637686. [DOI] [PubMed] [Google Scholar]