Abstract
Left ventricular remodeling post-myocardial infarction (MI) involves a multitude of mechanisms that regulate the repair response. Matrix metalloproteinases (MMPs) are a major family of proteolytic enzymes that coordinate extracellular matrix turnover. Both MMP-7 deletion and MMP-9 deletion reduce of the left ventricle post-MI, but the mechanisms have not been fully clarified. Both MMP-7 and MMP-9 have a large number of known in vitro substrates, but in vivo substrates for these two MMPs in the myocardial infarction setting are incompletely identified. Advances in proteomic techniques have enabled comprehensive profiling of protein expression in cells and tissue. In this chapter, we describe a protocol for the proteomic analysis of in vivo candidate MMP substrates in the post-MI left ventricle using two-dimensional electrophoresis, liquid chromatography coupled with tandem mass spectrometry, and immunoblotting.
Keywords: Proteomics, Cardiac remodeling, Mice, Extracellular matrix, Matrix metalloproteinase, Myocardial infarction, Substrates
1 Introduction
A disruption of blood supply in a coronary artery can lead to irreversible ischemia and the development of myocardial infarction (MI). Following MI, the left ventricle (LV) undergoes extensive remodeling, which results in reorganization and accumulation of new extracellular matrix (ECM) proteins that attempt to sustain cardiac integrity and function [1–3]. Matrix metalloproteinases (MMPs) are a family of zinc-dependent proteolytic enzymes capable of processing ECM proteins [4]. As such, MMPs orchestrate ECM turnover post-MI [5, 6]. The absence of several specific MMP family members, including MMP-7 and MMP-9, has positive effects on LV remodeling post-MI [7, 8]. However, the biological mechanisms have not been fully clarified. In vitro, MMP-7 is capable of cleaving a broad range of ECM proteins, including collagen IV, fibronectin, laminin, and tenascin-C [9–12], as well as non-ECM proteins, including tissue factor pathway inhibtor-2, E-cadherin, and MMP-2 [13–15]. Similar to MMP-7, MMP-9 in vitro cleaves many ECM proteins, including gelatin, collagen IV, and collagen V [16–18], and cleaves non-ECM proteins, including pro-TGFα, pro-TNFα, and other MMPs [19–21]. However, in vivo substrates for MMP-7 and MMP-9 post-MI have not been fully identified.
In this book chapter, we describe a protocol that uses two- dimensional electrophoresis (2-DE), along with high performance liquid chromatography coupled with electrospray tandem mass spectrometry (HPLC-ESI-MS/MS), to identify in vivo MMPs substrates in the infarcted myocardium [22, 23]. The discovery of new in vivo MMPs substrates would potentially identify novel therapeutic targets to reduce adverse LV remodeling.
2 Materials
2.1 Mouse Model of MI
C57BL/6 J WT, MMP-7 null, or MMP-9 null mice, 4–6 months of age were used. Both MMP-7 null mice and MMP-9 null mice were gifts from Dr. Lynn Matrisian from Vanderbilt University [24, 25].
Isoflurane.
Sterile polyamide monofilament 8-0 suture.
High-frequency ultrasound system (e.g., Vevo 770® High-Resolution Imaging System; VisualSonics, Toronto, Ontario, Canada).
Biofeedback surgery platform.
Anesthesia system.
Electrocardiogram recording system.
Buprenorphine.
2.2 Infarct Tissue Collection
Isoflurane.
2,3,5-Triphenyltertrazolium chloride (TTC).
2.3 Protein Extraction
Protein extraction reagent type 4: 7 M urea, 2 M thiourea, 40 mM trizma base and 1 % C7BzO (Sigma-Aldrich, St. Louis, MO, USA).
Complete protease inhibitor cocktail (Roche, Madison, WI, USA).
Bradford assay reagent.
Homogenizer.
2.4 Two-Dimensional Electrophoresis
Protein extraction reagent type 4 (Sigma-Aldrich).
Complete protease inhibitor cocktail (Roche).
Tributylphosphine.
Iodoacetamide.
Immobilized pH gradient (IPG) strip pH 3–10 (Bio-Rad Laboratories, Hercules, CA, USA).
Mineral oil.
CriterionXT4–12%bis–trisprecastgels(Bio-RadLaboratories).
Kaleidoscope prestained molecular weight standard (Bio-Rad Laboratories).
XT MOPS running buffer (Bio-Rad Laboratories).
XT MES running buffer (Bio-Rad Laboratories).
EZ Blue gel staining reagent (Sigma-Aldrich).
Centrifugal vacuum concentrator.
Electrophoresis power supply.
Gel imaging system.
2-DE gel spot detection/analysis software (e.g., Progenesis PG240; Nonlinear Dynamics, Durham, NC, USA).
2.5 Identification of Proteins in 2-DE Gel Spots by Tandem Mass Spectrometry
Sequencing-grade trypsin.
Gel spot excision tool (e.g., One Touch™ 1.5 mm 2-DE spot picker; Gel Company, San Francisco, CA, USA).
Rotator.
Centrifugal vacuum concentrator.
Electrospray tandem mass spectrometer (e.g., LTQ, Thermo Fisher Scientific, Hudson, NH, USA).
Nanoflow HPLC system (e.g., NanoLC micro-HPLC; Eksigent/AB Sciex, Framingham, MA, USA).
Capillary HPLC column [e.g., self-packed PicoFrit (New Objective, Woburn, MA, USA; 75 μm i.d.) packed to 10 cm with C18 adsorbent (Grace Vydac, Hesperia, CA, USA; 5 μm, 300 Å)].
Mobile phases: A, 0.5 % acetic acid/0.005 % Trifluoroacetic acid (TFA); B, 90 % acetonitrile/0.5 % acetic acid/0.005 % TFA.
Database search software (e.g., Mascot; Matrix Science, Boston, MA, USA).
Post-processing software (e.g., Scaffold; Proteome Software, Portland, OR, USA).
2.6 Immunoblotting Analysis of In Vivo Candidate MMP Substrates
10× Tris/glycine buffer.
Criterion XT bis–tris gel, 18- or 26-well, 4–12 % (Bio-Rad Laboratories).
XT MOPS running buffer (Bio-Rad Laboratories).
XT MES running buffer (Bio-Rad Laboratories).
MemCode reversible protein stain kit (Pierce, Rockford, IL, USA).
Blotting grade blocker (e.g., nonfat dry milk).
Primary antibodies of the MMP substrates (e.g., anti-fibronectin).
Enhanced chemiluminescent (ECL) substrate for horseradish peroxidase (HRP).
Blotting paper.
Nitrocellulose membrane.
Criterion blotter (Bio-Rad Laboratories).
Imaging software.
2.7 In Vitro and Ex Vivo MMP Cleavage Assay
Recombinant human MMP-7 (Calibiochem, EMD Millipore, Darmstadt, Germany).
Recombinant human MMP-9 (Calibiochem).
Recombinant human proteins (e.g., fibronectin, R&D Systems, Minneapolis, MN, USA).
Zymogram developing buffer (Invitrogen, Grand Island, NY, USA).
XT sample buffer (Bio-Rad Laboratories).
Recombinant human MMP-7 (Calibiochem).
Recombinant human MMP-9 (Calibiochem).
XT sample buffer (Bio-Rad Laboratories).
Criterion Bis–Tris gel, 26-well, 4–12 % (Bio-Rad Laboratories).
XT MOPS running buffer (Bio-Rad Laboratories).
XT MES running buffer (Bio-Rad Laboratories).
10× Tris/glycine buffer.
Blotting grade blocker.
Primary antibodies of the MMP substrates (e.g., anti-fibronectin).
ECL substrate for HRP.
Nitrocellulose membrane.
Filter paper.
Criterion blotter (Bio-Rad Laboratories).
Imaging software.
3 Methods
3.1 Mouse Model of Myocardial Infarction
Induce MI by coronary artery ligation on wild-type and null mice, as previously described [8]. In brief, coronary ligation is made by using a sterile polyamide monofilament 8-0 suture to ligate the left coronary artery and disrupt the blood supply.
Confirm MI by looking for visible LV blanching and ST segment elevation by electrocardiography.
Inject 0.05 mg/kg of buprenorphine intraperitoneally into the mouse. Place the mouse in a 37 °C incubator with room air supplemented with oxygen for recovery.
Monitor continuously until the mouse is alert and ambulatory, at which point the mouse is returned to its cage and monitored daily.
3.2 Infarct Tissue Collection
Mice that survive through day 7 undergo a terminal echocardiographic analysis as previously described [8] and are then sacrificed for subsequent analyses.
Anesthetize each mouse with 2 % isoflurane (see Note 1), flush the coronary vasculature with phosphate buffered saline (PBS), and excise the heart.
Separate the LV from the right ventricle and weigh each individually.
To measure infarct size, section the LV into three slices, stain with 1 % TTC for 5 min at 37 °C, and photograph the slices.
Separate the infarct tissue from the remote tissue, then weigh, snap-freeze in liquid nitrogen, and store at −80 °C in individual tubes for further analysis.
3.3 Protein Extraction
To prepare the Protein Extraction Reagent Type 4 (Reagent 4), add 15 ml of deionized water, swirl and heat to 30 °C for 30–60 min until dissolved. Use immediately or aliquot (1 ml/tube) and store at −20 °C.
To prepare the 10× protease inhibitor, dissolve one tablet of Complete Protease Inhibitor Cocktail in 1 ml of deionized water and vortex until fully dissolved, then aliquot (0.5 ml/tube) and store at −20 °C.
Record the wet weights of the LV samples and place each in a 5 ml polypropylene round-bottom tube.
Add 800 μl of lysis buffer (720 μl of Reagent 4 and 80 μl of 10× protease inhibitor) per 50 mg of tissue and homogenize.
Transfer to a 1.5 ml polypropylene microcentrifuge tube and store at room temperature until all samples are homogenized.
Heat samples at 30 °C for 15 min and snap freeze.
Thaw samples at 30 °C for 30 min before determining the protein concentration of each sample by the Bradford assay following the manufacturer’s instructions (see Note 2).
3.4 Two-Dimensional Gel Electrophoresis
Mix 500 μg of each protein sample (~80 μl) with protease inhibitor cocktail (1× final), 1:40 (v/v) tributylphosphine solution, and water to make a total volume of 200 μl, and reduce proteins at room temperature for 1 h.
Add 1:10 (v/v) 10× protease inhibitor and 1:33 (v/v) iodo-acetamide solution (93.3 mg/ml) and alkylate in the dark at room temperature for 1 h (see Note 3).
Spin the sample at low speed (425 × g) for 5 min to pellet debris.
Transfer each supernatant into a new tube and discard the tubes with the pellets (see Note 4).
Add acetone to a final concentration of 80 % and place at room temperature for 30 min.
Centrifuge samples at 20,000 × g for 10 min and discard the supernatant.
Use a centrifugal vacuum concentrator to remove residual acetone for 5 min (or air-dry for 5–10 min) (see Note 5).
Resuspend samples in 200 μl of Reagent 4 and 1× protease inhibitor, vortex, and incubate at 30 °C for 30 min (see Note 6).
Add protein sample to the rehydration tray, then place an 11-cm IPG strip (pH 3–10) on top of the protein solution with the gel side facing down, and rehydrate overnight at room temperature (see Note 7).
On the next day, add mineral oil to wells on the isoelectric focusing (IEF) tray before inserting the strips.
Wet wicks (2/tray) with water and place at each end of the tray.
Place the IPG strip in the cell with gel side facing down (see Note 8).
Cover each IPG strip with mineral oil (see Note 9) and start the IEF voltage program. For an 11-cm IPG strip and an ElectrophoretIQ 2000, we use the following: 100 V, 1 h; 200 V, 1 h; 400 V, 2 h; 1,000 V, 2 h; 5,000 V, 12 h; 1,000 V, hold. The current is set to be constant at 2 mA.
After IEF is complete, blot the extra oil from the edges of the IPG strip using filter paper.
Put each strip in an equilibration tray slot, gel side down, and incubate in 5 ml of SDS equilibrating buffer (6 M urea, 0.05 M Tris-acetate, pH 7, 2 % SDS, and 0.0067 % bromophenol blue) with shaking for 10 min. Repeat (see Note 10).
Place an 11-cm precast gel (Criterion XT 4–12 % Bis–Tris) in an electrophoresis cell and add 1× XT MOPS running buffer up to the fill line.
Place the strip on the top of the precast gel and load 10 μl of Kaleidoscope Prestained Standard into the side well of the gel.
Run at 200 V (25–50 mA/gel) until the bromophenol blue reaches the bottom of the gel.
After the second dimension of electrophoresis, rinse each gel three times with deionized water for 5 min.
Fix each gel in 50 % methanol/10 % acetic acid at room temperature for 15 min.
Rinse each gel with deionized water for 15 min.
Add EZ Blue staining reagent to cover the gel and incubate for at least 45 min or overnight with shaking.
Destain each gel with deionized water until the background is clear, approximately 2 h.
Scan each gel and save images in the format compatible with the imaging software, using the exactly same parameters of the scanner and the exactly same parameters of the imaging software for each gel.
Analyze the 2-DE gel images with image analysis software (Fig. 1, see Notes 11 and 12).
Fig. 1.
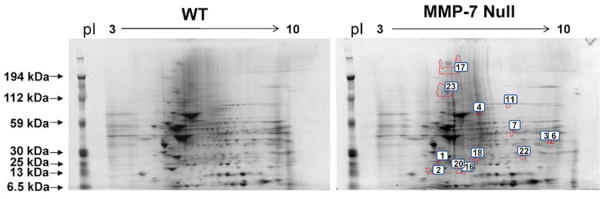
Representative 2-DE gels of protein extracts from the infarct area of WT mice (left) and MMP-7 null mice (right). Spot numbers are indicated in the MMP-7 null gel. Reprinted with permission from [23]. Copyright 2010 American Chemical Society
3.5 Identification of Proteins in 2-DE Gel Spots by Mass Spectrometry
Excise gel spots with significant differences in intensity between the two groups either manually (e.g., using a One Touch 1.5-mm 2-DE Spot Picker) or with a robotic system.
Destain and wash each gel spot with 200 μl of 40 mM NH4HCO3/50 % acetonitrile in a 0.5 ml polypropylene microcentrifuge tube, and then slowly invert on a rotator for 20 min.
Remove the liquid and repeat the destaining and washing step once again (see Note 13).
Remove the liquid, quickly spin down at 1,000 × g, and remove the residual liquid.
Add 100 μl of acetonitrile to each tube and shake up and down until the gel spot turns white.
Dry each gel spot in a centrifugal vacuum concentrator for 5–10 min.
Swell the dried gel spots in 5–20 μl of 20 ng/μl trypsin in 40 mM NH4HCO3/20 % acetonitrile on ice and incubate for 40 min.
Add 10 μl of 40 mM NH4HCO3/20 % acetonitrile and incubate at 37 °C for 4 h.
Add 0.1 % TFA to the digests to a final volume of 200 μl and incubate at 37 °C for up to 1 h.
Spin down at 14,000 × g for 5 min and transfer each supernatant to an autosampler vial and dry in a centrifugal vacuum concentrator.
Add 200 μl of 0.2 % TFA/50 % acetonitrile to each gel spot and incubate at 37 °C for up to 1 h as a second extraction.
Transfer each supernatant to the autosampler vial used for step 10 and dry in a centrifugal vacuum concentrator.
Add 100 μl of 100 % acetonitrile to the gel spots and dehydrate them for the third extraction.
Transfer each supernatant to the autosampler vial used for step 10 and dry in a centrifugal vacuum concentrator (see Note 14).
Resuspend the peptides in 12 μl of 0.5 % TFA just before HPLC-ESI-MS/MS analysis.
Analyze the digests by HPLC-ESI-MS/MS and separate the digests by reversed-phase capillary HPLC using a gradient of 2–42 % B in 30 min at a flow rate, 0.4 μl/min. (The method described here is for analysis using an Eksigent NanoLC micro-HPLC connected on-line to a Thermo Fisher LTQ linear ion trap mass spectrometer fitted with a New Objective PicoView 550 nanospray interface.)
Use a data-dependent scan strategy to collect precursor and tandem mass spectra. The settings used on a Thermo Fisher LTQ mass spectrometer are as follows: ESI voltage, 2.9 kV; isolation window for MS/MS, 3; relative collision energy, 35 %; scan strategy, survey scan followed by acquisition of data dependent collision-induced dissociation spectra of the seven most intense ions in the survey scan above a threshold of 3,000 intensity units.
(The parameters listed in steps 18–20 are for database searching with Mascot.)
Process the mass spectrometry raw data file and generate a peak list. The program extractmsn.exe (Thermo Fisher Scientific) can be used with default settings for LTQ data.
Use a database search program such as Mascot to search the processed data file against a protein database, using either a complete database or a species-specific subset. The following parameters are appropriate for Mascot: proteolytic enzyme, trypsin; allowed missed cleavages, two; precursor mass tolerance, ±1.5 Da; fragment ion mass tolerance, ±0.8 Da; variable modifications, methionine oxidation and cysteine carbamidomethylation.
A post-processing program like Scaffold can be used to search the processed data file with X!Tandem and for cross-correlation with the Mascot results and determination of the probabilities of the peptide assignments and protein identifications.
3.6 Validation Step: Immunoblotting Analysis of In Vivo Candidate MMP Substrates
Place the samples at 30 °C for at least 30 min (see Note 15). Vortex and spin briefly at low speed to bring down any debris.
Prepare a positive control (see Note 16).
For each sample, load 10 μg of total protein in one lane of a 26-well 4–12 % Criterion Bis–Tris gel and run the gels in XT MES buffer or XT MOPS buffer at 200 V for 40 min (see Note 17).
After electrophoresis, remove the gels from the cassettes and rinse gently in deionized water.
Soak filter paper, sponges, and membrane in cold transfer buffer.
Place the layers in the following order, starting on the black side of the cassette: sponge, filter paper, gel, membrane, filter paper, sponge (see Note 18).
Transfer proteins at 65 V for 1 h (see Note 19).
After transfer, place the membrane in a clean container.
Stain the membrane with MemCode Reversible Protein Stain Kit as per the manufacturer’s instructions.
Scan and analyze the membrane with imaging software and record the densitometry value of total proteins in each lane for normalizing the densitometry of each immunoblot (see Notes 12 and 20).
Destain the membrane with MemCode Reversible Protein Stain Kit according to the manufacturer’s instructions.
Block nonspecific epitopes with 5 % nonfat dry milk in 1× PBS/0.1 % Tween-20 (blocking solution) at room temperature for 1 h with gentle shaking.
Wash the membrane with 1× PBS/0.1 % Tween-20 (PBST) for 10 min with gentle shaking, for a total of three wash steps.
Prepare the primary antibody by mixing the antibody at the concentration recommended by manufacturer (e.g., 1:2,000 for anti-fibronectin) in blocking solution. Incubate the membrane at room temperature for 2 h or at 4 °C overnight with gentle shaking.
Wash the membrane with 1× PBST for 10 min with gentle shaking and repeat, for a total of three washes.
Prepare the secondary antibody by mixing the antibody at the concentration recommended by the manufacturer in blocking solution. Incubate the membrane for 1 h at room temperature with gentle shaking.
Wash the membrane with 1× PBST for 10 min with gentle shaking and repeat, for a total of three washes.
Incubate the membrane with ECL substrate for 5 min and develop the film immediately in the dark room.
Scan and analyze the film with imaging software. Use the densitometry values to normalize the individual lanes for total protein (Fig. 2, see Notes 12 and 20).
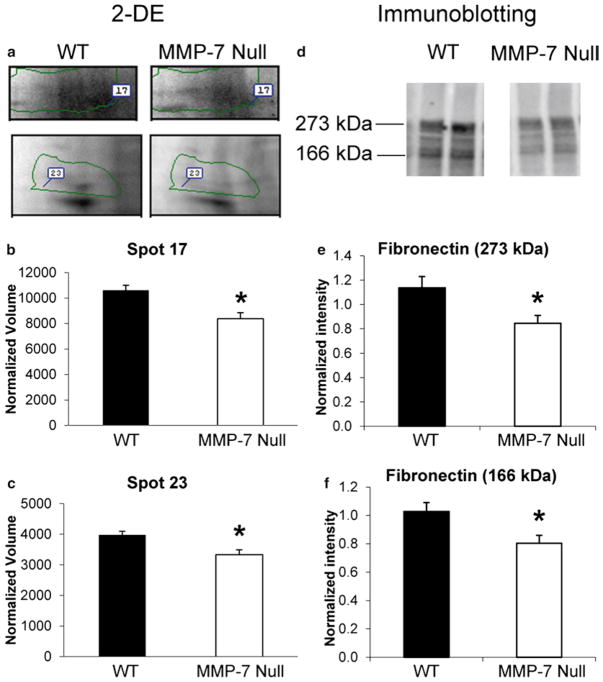
Fig. 2.
The infarct area of MMP-7 null mice showed lower levels of fibronectin by 2-DE and immunoblotting. (a) Fibronectin was identified in spot 17 and spot 23 of the 2-DE gels. Both spot 17 (b) and spot 23 (c) showed significant lower intensity in the LV infarct area from MMP-7 null mice compared to WT. (d) Immunoblotting of fibronectin in the LV infarct area of WT and MMP-7 null mice were performed. The densitometry of the 273 kDa full-length (e) and the 166 kDa fragments (f) of fibronectin indicated both bands showed significantly lower intensity in the LV infarct area of MMP-7 null mice when compared with the WT (p < 0.05). Reprinted with permission from [23]. Copyright 2010 American Chemical Society
3.7 In Vitro and Ex Vivo MMP-7 Cleavage Assay
For the in vitro MMP-7 cleavage assay, incubate 100 ng of the recombinant protein (e.g. recombinant fibronectin) with 10 pg, 100 pg, or 10 ng of recombinant human MMP-7 enzyme at 37 °C for 3 h in 1× zymogram developing buffer. Add 5 μl of SDS loading buffer to stop the reaction, followed by the steps 3 and 4 in this section for immunoblotting (see Note 21).
For the ex vivo MMP-7 cleavage assay, incubate 10 μg of protein extract from the infarct area of an MMP-7 null mouse with 10 pg, 100 pg, or 10 ng of recombinant human MMP-7 enzyme at 37 °C for 3 h in 1× zymogram developing buffer (Fig. 3, see Notes 12, 21, and 22). Add 5 μl of SDS loading buffer to stop the reaction, followed by steps 3 and 4 for immunoblotting.
Load each reaction mixture in a lane of a 26-well 4–12 % Criterion Bis–Tris gel, and run the gels in XT MES buffer or XT MOPS buffer at 200 V for 40 min (see Note 17).
Immunoblot the proteins of interest as described above in Subheading 3.6 to evaluate the action of exogenous MMP-7 enzyme on degradation of the substrate, comparing extracts from the control mouse to those from the MMP-7 null mouse (Fig. 3, see Notes 21 and 22).
Fig. 3.
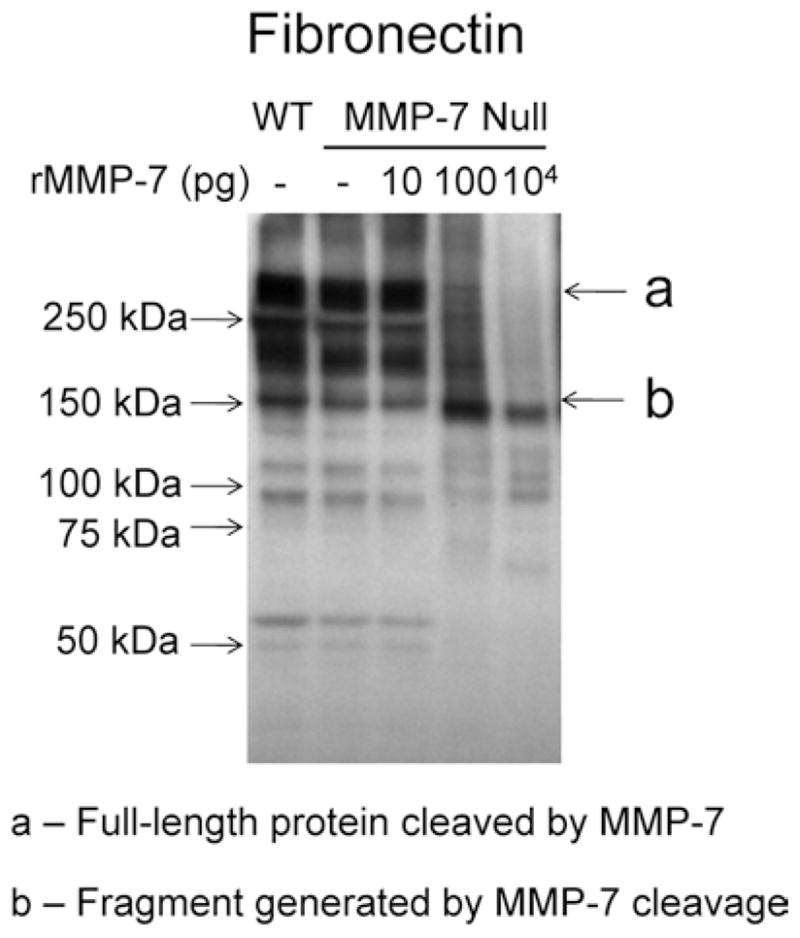
The addition of exogenous MMP-7 generated fibronectin fragments in protein extract from the infarct area of an MMP-7 null mouse. (a) Protein extract from the LV infarct area of an MMP-7 null mouse treated with 100 pg or 10 ng of recombinant MMP-7 showed reduced levels of full-length fibronectin (273 kDa) and increased levels of the 166 kDa fibronectin fragment compared to the untreated control. Reprinted with permission from [23]. Copyright 2010 American Chemical Society
Acknowledgments
We acknowledge support from NIH/NHLBI HHSN 268201000036C (N01-HV-00244) for the San Antonio Cardiovascular Proteomics Center and R01 HL075360, the Max and Minnie Tomerlin Voelcker Fund, and the Veteran’s Administration (Merit) to M.L.L. Mass spectrometry analyses were conducted in the UTHSCSA Institutional Mass Spectrometry Laboratory.
Footnotes
Isoflurane is harmful to humans. Therefore, surgery should be performed in a well-ventilated room with a scavenging system, and the operator should turn off the anesthesia system when not in use.
Due to the high urea concentration in the extraction buffer, the protein extracts need to be diluted 1:40 with water prior to assaying for protein content to avoid interference with the colorimetric readings.
To make the iodoacetamide solution, add 600 μl of deionized water to dissolve the powder in a glass vial (56 mg/vial). Iodoacetamide should be prepared and used fresh. Discard any leftover solution.
This step must be completed prior to acetone precipitation.
The experimental process can be stopped here if needed and continued later.
Use 120 μl of Reagent 4 and 1× proteinase inhibitor for a 7 cm strip; 200 μl for an 11 cm strip; 300 μl for an 18 cm strip; and 440 μl for a 24 cm strip.
Make sure the rehydration tray is clean and dry, with no bubbles under the strips; and keep the tray in a horizontal position. Put the tray in a sealable plastic bag and place a wet paper towel in the bag to keep the strips moist (avoid drying out the strips). The strips can be frozen after rehydration and before equilibration.
Make sure the positive and negative poles are appropriately placed, with no bubbles at the end of the strip to prevent burning.
Make sure there is sufficient mineral oil to cover the strip and that the strip is well covered by the wicks.
Make sure that the equilibration buffer covers the strips, and remove the solution by aspiration.
When using the Progenesis PG240 software, image quality control can be performed to optimize image capture and image alignment, and the prefilter can be used to remove artifacts (e.g., gel areas with noise). The volume of each spot should be normalized to the total spot volume in the gel. Spots that exhibit significant differences between the two groups (p <0.05 by Students t-test) are selected for protein identification by mass spectrometry.
All protein extracts should be analyzed individually and not pooled at any step. Analyze normalized spot volumes of 2-DE gels and normalized densitometries of the immunoblots between WT and null groups. A p < 0.05 is considered significant.
Steps 1–3 in Subheading 3.5 may be repeated if necessary.
The dried peptides can be stored at room temperature in the dark.
Because of the high concentration of urea in Reagent 4, this step is needed to allow the samples to fully thaw in solution.
Spleen or tumor protein homogenates are often used as positive controls. If available, recombinant proteins are the most specific and best controls.
XT MES buffer is used for resolving proteins up to 100 kDa and XT MOPS buffer for proteins >100 kDa. Run times and voltages may differ, depending on the molecular weight of the protein of interests, the buffer, the electrophoresis apparatus, and the type of gel.
Make sure to roll out any bubbles while laying down the gel, the membrane, and the filter paper.
If the molecular weight of the target protein is more than 100 kDa, the transfer process will need to be modified to 65 V for 2 h or 40 V overnight.
Make sure that the blot is not overexposed and that the signal is in the dynamic range by the imaging software (e.g., Molecular Imaging Software version 4).
In vitro and ex vivo MMP-9 cleavage assays can be conducted using recombinant MMP-9, protein extract from LV infarct area of MMP-7 null mouse, and protein extract from LV infarct area of WT mouse.
Use a protein extract from the infarct area of an MMP-7 null mouse without MMP-7 treatment as the negative control, and use protein extract from the infarct area of a WT mouse without MMP-7 treatment as the positive control (Fig. 3).
References
- 1.Dixon JA, Spinale FG. Myocardial remodeling: cellular and extracellular events and targets. Annu Rev Physiol. 2011;73:47–68. doi: 10.1146/annurev-physiol-012110-142230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindsey ML, Weintraub ST, Lange RA. Using extracellular matrix proteomics to understand left ventricular remodeling. Circ Cardiovasc Genet. 2012;5:o1–7. doi: 10.1161/CIRCGENETICS.110.957803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gajarsa JJ, Kloner RA. Left ventricular remodeling in the post-infarction heart: a review of cellular, molecular mechanisms, and therapeutic modalities. Heart Fail Rev. 2011;16:13–21. doi: 10.1007/s10741-010-9181-7. [DOI] [PubMed] [Google Scholar]
- 4.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kandalam V, Basu R, Abraham T, et al. Early activation of matrix metalloproteinases underlies the exacerbated systolic and diastolic dysfunction in mice lacking TIMP3 following myocardial infarction. Am J Physiol Heart Circ Physiol. 2010;299:H1012–1023. doi: 10.1152/ajpheart.00246.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindsey ML, Zamilpa R. Temporal and spatial expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases following myocardial infarction. Cardiovasc Ther. 2012;30:31–41. doi: 10.1111/j.1755-5922.2010.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindsey ML, Escobar GP, Dobrucki LW, et al. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;290:H232–239. doi: 10.1152/ajpheart.00457.2005. [DOI] [PubMed] [Google Scholar]
- 8.Lindsey ML, Escobar GP, Mukherjee R, et al. Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation. 2006;113:2919–2928. doi: 10.1161/CIRCULATIONAHA.106.612960. [DOI] [PubMed] [Google Scholar]
- 9.Agnihotri R, Crawford HC, Haro H, et al. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin) J Biol Chem. 2001;276:28261–28267. doi: 10.1074/jbc.M103608200. [DOI] [PubMed] [Google Scholar]
- 10.Siri A, Knauper V, Veirana N, et al. Different susceptibility of small and large human tenascin-C isoforms to degradation by matrix metalloproteinases. J Biol Chem. 1995;270:8650–8654. doi: 10.1074/jbc.270.15.8650. [DOI] [PubMed] [Google Scholar]
- 11.von Bredow DC, Nagle RB, Bowden GT, et al. Degradation of fibronectin fibrils by matrilysin and characterization of the degradation products. Exp Cell Res. 1995;221:83–91. doi: 10.1006/excr.1995.1355. [DOI] [PubMed] [Google Scholar]
- 12.Imai K, Yokohama Y, Nakanishi I, et al. Matrix metalloproteinase 7 (matrilysin) from human rectal carcinoma cells. Activation of the precursor, interaction with other matrix metalloproteinases and enzymic properties. J Biol Chem. 1995;270:6691–6697. doi: 10.1074/jbc.270.12.6691. [DOI] [PubMed] [Google Scholar]
- 13.Lee KH, Choi EY, Hyun MS, et al. Association of extracellular cleavage of E-cadherin mediated by MMP-7 with HGF-induced in vitro invasion in human stomach cancer cells. Eur Surg Res. 2007;39:208–215. doi: 10.1159/000101452. [DOI] [PubMed] [Google Scholar]
- 14.Belaaouaj AA, Li A, Wun TC, et al. Matrix metalloproteinases cleave tissue factor pathway inhibitor. Effects on coagulation. J Biol Chem. 2000;275:27123–27128. doi: 10.1074/jbc.M004218200. [DOI] [PubMed] [Google Scholar]
- 15.Crabbe T, Smith B, O’Connell J, et al. Human progelatinase A can be activated by matrilysin. FEBS Lett. 1994;345:14–16. doi: 10.1016/0014-5793(94)00412-9. [DOI] [PubMed] [Google Scholar]
- 16.Trocme C, Gaudin P, Berthier S, et al. Human B lymphocytes synthesize the 92-kDa gelatinase, matrix metalloproteinase-9. J Biol Chem. 1998;273:20677–20684. doi: 10.1074/jbc.273.32.20677. [DOI] [PubMed] [Google Scholar]
- 17.Birkedal-Hansen H. Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol. 1995;7:728–735. doi: 10.1016/0955-0674(95)80116-2. [DOI] [PubMed] [Google Scholar]
- 18.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 19.Tan RJ, Liu Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am J Physiol Ren Physiol. 2012;302:F1351–1361. doi: 10.1152/ajprenal.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteo-lytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 21.McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 22.Zamilpa R, Lopez EF, Chiao YA, et al. Proteomic analysis identifies in vivo candidate matrix metalloproteinase-9 substrates in the left ventricle post-myocardial infarction. Proteomics. 2010;10:2214–2223. doi: 10.1002/pmic.200900587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiao YA, Zamilpa R, Lopez EF, et al. In vivo matrix metalloproteinase-7 substrates identified in the left ventricle post-myocardial infarction using proteomics. J Proteome Res. 2010;9:2649–2657. doi: 10.1021/pr100147r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haro H, Crawford HC, Fingleton B, et al. Matrix metalloproteinase-7-dependent release of tumor necrosis factor-alpha in a model of herniated disc resorption. J Clin Invest. 2000;105:143–150. doi: 10.1172/JCI7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acuff HB, Carter KJ, Fingleton B, et al. Matrix metalloproteinase-9 from bone marrow-derived cells contributes to survival but not growth of tumor cells in the lung microenvironment. Cancer Res. 2006;66:259–266. doi: 10.1158/0008-5472.CAN-05-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]