Abstract
Over the last quarter century, researchers have peered into the living human brain to develop and refine mechanistic accounts of alcohol-induced behavior, as well as neurobiological mechanisms for development and maintenance of addiction. These in vivo neuroimaging studies generally show that acute alcohol administration affects brain structures implicated in motivation and behavior control, and that chronic intoxication is correlated with structural and functional abnormalities in these same structures, where some elements of these decrements normalize with extended sobriety. In this review, we will summarize recent findings about acute human brain responses to alcohol using neuroimaging techniques, and how they might explain behavioral effects of alcohol intoxication. We then briefly address how chronic alcohol intoxication (as inferred from cross-sectional differences between various drinking populations and controls) may yield individual brain differences between drinking subjects that may confound interpretation of acute alcohol administration effects.
Keywords: Alcohol, Neuroimaging, fMRI, Positron Emission Tomography, Addiction
1. Introduction: the search for brain-based accounts for alcohol-induced cognitive impairment
Alcohol abuse and dependence, including binge drinking, costs hundreds of billions of dollars a year in the United States alone, including lost productivity, health care costs, accidents and violence (Bouchery et al., 2011). Of critical public health importance is advancing understanding of the mechanisms by which ethyl alcohol (ethanol, or hereafter simply “alcohol”) intoxication results in severe behavioral disinhibition, disrupted socio-emotional processing, impaired psychomotor performance, and loss of control over alcohol use itself. Using neuroimaging to elucidate how acute or chronic alcohol causes structural or functional changes in brain regions implicated in cognitive processes holds potential to foster brain-based interventions to ultimately mitigate public health burden. In this review, we will summarize recent findings about acute human brain responses to alcohol that were derived from neuroimaging modalities. We discuss how these findings might explain behavioral effects of alcohol, and how they may underpin theories of development of alcoholism. We will then highlight how chronic alcohol intoxication (as inferred from cross-sectional differences between various drinking populations and controls) may yield individual structural and baseline-functional differences between drinking subjects that may confound interpretation of acute alcohol administration effects. Finally, we will address future directions to advance this research.
Imaging the intoxicated brain holds potential to provide a mechanistic account for observed alcohol effects on behavior. To this end, we suggest that the most reliable mechanistic associations between brain signal and behavior would be found with respect to laboratory behavior (as opposed to anecdotal behavior) in that alcohol-induced behavior change is critically sensitive to individual differences in the expectancies of alcohol effects. For example, expectancies of increased aggression while intoxicated (Lindman and Lang, 1994) or for certain elements of positive mood (Lindman et al., 2000) vary between cultures. Laboratory performance findings, while still vulnerable to some expectancy effects, provide a better reference for imaging findings in that they more objectively indicate how acute alcohol selectively disrupts (or spares) narrowly-defined elements of cognitive performance (e.g (Reynolds et al., 2006)).
A comprehensive review of acute alcohol effects on mood, motor performance, or cognitive performance in the laboratory is beyond the scope of this review. Zoethout et al. (2011), however, reviewed 342 alcohol-vs-placebo comparisons in healthy controls reported in 218 papers, and concluded that the most reliable and potent effects of alcohol were on divided attention, focused attention, visuomotor control, and subjective ratings of “high”, where additional impairments in reaction time, working memory, and response inhibition were consistently observed at alcohol doses above 0.7 g/l). Neuroimaging experiments have predominantly assessed these effects in two ways; (1) by examining unconditioned alcohol effects on the brain’s “reward” neurocircuitry (to detect a signature of the “high”) or (2) examining in real-time alcohol effects on brain activations while a subject is performing a task that requires attention, memory, or self-control. Other experiments have assessed brain signatures of emotion-processing, which may advance understanding alcohol effects on social behaviors like aggression. Understanding brain mechanisms of alcohol’s disinhibitory effects may be useful in understanding transition from social drinking to addiction, especially in vulnerable individuals.
2. Alcohol and potential brain targets of alcohol effects
2.1. Alcohol as a psychopharmacological agent: methodological and interpretive considerations
Alcohol presents both advantages and challenges for acute pharmacology imaging studies. A key advantage is that alcohol pervades the brain quickly (the diffusion of alcohol throughout the body resembles that of water) and has a short duration of effect. Compared to other drugs of abuse, alcohol is not a typical lipophilic drug with a small set of receptor ligands. Rather, it is a highly-soluble small molecule that is passively diffused in and out of cells, and requires significant concentrations to induce intoxication (reviewed in Feldman et al., 1997). Alcohol has both a polar and a lipophilic moiety, enabling weak interaction with a wide variety of molecules. Compared to other psychotropic agents, alcohol effects are shorter in duration, where this has been attributed to its high dissociation rate with cellular targets, and its low lipid solubility (reviewed in Feldman et al., 1997).
Combining imaging and acute alcohol administration presents a number of challenges. First, because the pharmacokinetics of alcohol can vary greatly as a function of situational factors such as drinking rate, concentration of alcohol in the beverage, or ingestion with food, well-controlled laboratory administration is essential for mechanistic studies. Moreover, great variation in the timing of oral alcohol action in the brain remains between laboratory participants due to sex differences, age, genetics, or due to adaptations to chronic alcohol in first-pass metabolism in the digestive system. Fortunately, titrated intravenous (IV) administration techniques (Ramchandani et al., 1999) can avoid much of these confounds, while still enabling study of the most commonly used and abused psychoactive substance.
A second challenge to using acute alcohol for fMRI studies is that alcohol itself has biphasic vasoactive properties (Kawano, 2010) that may disrupt the normal coupling between the hemodynamic response (what is typically measured in fMRI) and the underlying local neural firing activity. Event-related fMRI typically involves detection of brain regions where hemodynamic activity is temporally correlated with an idealized (canonical) impulse response/hemodynamic function that is time locked to experimental events. If an identical, inflexible model is applied to the time-series image data collected under both intoxicated and sober conditions, one could spuriously infer a directional dose-dependent difference in neural activity from what may really be a vascular effect. Luchtmann et al. (2010) examined the effects of oral alcohol on brain fMRI hemodynamic responses to basic visual stimulation and finger-tapping tasks, and reported that acute alcohol caused statistically-significant and region-specific changes in the contours of hemodynamic responses. However, fitting an identical canonical model (provided by a popular image analysis software package) to the data under both alcohol and placebo conditions did not reveal an appreciable difference in actual task activations. Furthermore, large individual differences in vasoactive effects of alcohol introduce an additional layer of noise in group-wise fMRI analyses.
A third challenge is the tendency of alcohol to affect multiple neurotransmitter systems (Eckardt et al., 1998), which limits our ability to interpret alcohol’s effect on any one system. Alcohol affects gamma-aminobutyric acid (GABA), glutamate, serotonin, dopamine (DA), and acetylcholine. At high concentrations, alcohol binds to a variety of cell membrane sites and can alter the phospholipid constituents of cell membranes. At physiologically-relevant (intoxication) concentrations, alcohol is thought to work primarily by way of allosteric modulation or direct interaction with cell-surface receptors for N-methyl-d-aspartate (NMDA), GABA-A, and other proteins (Fadda and Rossetti, 1998) to alter ion transport across the cell membrane. Therefore, it can be difficult to attribute alcohol-elicited brain activation or metabolism (energyutilization) changes to specific neurotransmitter systems, or to pre- versus post-synaptic effects of enhancing concentrations of a specific neurotransmitter.
2.2. Brain regions of interest as mediators of alcohol effects on behavior
Alcohol pharmacological fMRI experiments typically assess either the effects of alcohol on the brain in a resting state (in the absence of experimenter-prescribed task demands), or its effects on the brain as it is performing a cognitive task. Where in the brain do we look for acute alcohol effects? While it is well-understood that the brain operates as a set of interconnected networks (Laird et al., 2011), certain structures are of particular interest. Data from invasive preclinical intervention studies, behavioral studies of human lesion patients, and dynamic meta-analytic maps derived from human functional magnetic resonance imaging (fMRI) studies of cognitive tasks (e.g. www.brainmap.org and www.neurosynth.org) can be used to form hypotheses about where alcohol might act in the brain. These findings converge to indicate, for example, that: 1) the ventral striatum (VS), including nucleus accumbens (NAcc) is critically important for the learning and motivational aspects of instrumental (rewarded) behavior (Haber and Knutson, 2010; Salamone et al., 2003), 2) dorsolateral prefrontal and mesial prefrontal cortex (e.g. anterior cingulate cortex; ACC) are critical for self-control (Bechara et al., 1994; Bechara and Van Der Linden, 2005; Hare et al., 2009) and error monitoring (Ridderinkhof et al., 2004), and for integrating representations of reward/incentive value (Hare et al., 2009; Kable and Glimcher, 2007), 3) the amygdala is a critical node in processing of emotional stimuli (like faces) such as threat (Kim et al., 2011), and 4), portions of dorsolateral and inferior prefrontal and parietal cortices are recruited by attentional demand under different contexts (Corbetta and Shulman, 2002). Therefore, alcohol-induced changes in the recruitment of these structures may support mechanistic accounts of alcohol action.
3. Effects of acute alcohol administration on the “resting” brain
3.1. Positron Emission Tomography (PET) studies of energy utilization and dopaminergic activity
Positron Emission Tomography (PET) studies have provided the foundation of for our understanding of acute alcohol effects on the brain when the subject is not actively engaged in a task. These studies have proved crucial in understanding how a bolus of alcohol to the brain causes regional changes in measures of energy utilization, and in displacement of labeled, receptor-specific ligands. Region-specific changes may differ in directionality, with important functional effects depending on which circuits may be up- or down-regulated. One compelling application of PET is the examination of acute alcohol effects on DA levels in structures of the mesolimbic incentive-motivational system. As with other drugs of abuse, alcohol causes an increase in endogenous DA (displacement of radiolabeled raclopride) in the VS in adult controls (Boileau et al., 2003; Yoder et al., 2007). Individual differences in magnitude of this release have correlated with self-reported intoxication (Yoder et al., 2007).
Another fundamental question is what acute alcohol does to metabolism (energy utilization) across the brain, as a proxy for localized neuronal activity. Many studies have shown that low to moderate doses of alcohol (0.25–0.75 g/kg) significantly reduce glucose metabolism in the brain, from 10 to 30% (Volkow et al., 1990, 2006; Wang et al., 2003; Wang et al., 2000). This decrease is dose-dependent, and is most pronounced in the occipital cortex and cerebellum (Volkow et al., 2008). Reduced glucose metabolism was also more pronounced in men, though women reported higher levels of subjective intoxication despite similar blood-alcohol concentration time course between male and female subjects (Wang et al., 2003). More recently, Volkow et al. (2008) proposed that alcohol also affects patterns of brain functional organization. Indeed, using PET, they found that a moderate dose of alcohol (0.75 g/kg) significantly decreased absolute whole brain and regional metabolism, but increased metabolism in the striatum (including nucleus accumbens), the amygdala, insula and mesencephalon. Since converging evidence implicates these limbic regions in incentive-motivational processes in drug reward (Breiter et al., 1997; Gilman et al., 2008; Stein et al., 1998), it is tempting to speculate that acute alcohol primes limbic incentive neurocircuitry while suppressing top-down cognitive control circuits, resulting in reward-seeking behavior despite the risk of deferred aversive outcomes, where cortically-mediated behavior-monitoring during the drinking bout may be compromised.
Recent research suggests that under intoxication, the brain may use acetate as an alternative brain energy source to glucose (Volkow et al., 2013). During an alcohol challenge, the brain areas showing the largest decreases in glucose metabolism measured with FDG (i.e. the cerebellum and occipital cortex) showed the largest increases in [1-11C]acetate brain uptake. Furthermore, [1-11C]acetate brain uptake was higher in heavy drinkers than in occasional drinkers, and increases in [1-11C]acetate were positively associated with the reported amount of alcohol consumed. The behavioral and other functional effects of this shift in energy source await discovery.
3.2. PET and MRI-based studies of alcohol effects on brain blood flow
Another signal inferred as localized changes in neural activity is regional brain blood flow-either indexed by a radiotracer in PET studies, or by arterial spin labeling (ASL) as an alternative, functional MRI-based method for obtaining quantitative measures of regional CBF that does not require administration of a radiotracer or ligand (reviewed in Liu and Brown, 2007). PET and ASL studies also indicate that alcohol effects on brain blood flow are not global. Seminal PET studies have demonstrated CBF increases in cortical gray matter after low (Sano et al., 1993), moderate (Mathew and Wilson, 1986; Newlin et al., 1982) and high (Sano et al., 1993) doses of alcohol. Volkow et al. (1988) showed increases in blood flow in prefrontal and temporal regions, and decreases in the cerebellum in social drinkers who had consumed 1.0 g/kg of alcohol. Ingvar et al. (1998) reported that acute alcohol increased activation of mesial temporal lobe and the brainstem reticular activating system, and reduced blood flow in cerebellum and occipital cortex. In a study using ASL in 88 young adult social drinkers, CBF increased up to 17% after alcohol when compared to placebo in several frontal brain regions (Tolentino et al., 2011).
It remains unclear why increased CBF has been reported in the same regions (e.g. frontal cortex) that also show reduced glucose metabolism. Neuronal activity may be differentially coupled with CBF versus metabolic markers. PET measures of blood flow versus energetics may be incomparable due to the large differences in both modeling and sampling time windows between the two techniques. Measures of CBF from labeled O11 are often captured in brief (e.g. 2-min) time windows, and like the fMRI BOLD signal, provide a relative measure (not precisely quantified and modeled). A brief sampling may not capture a reliable “resting-state” due to the non-stationarity observed in functional brain networks (Smith et al., 2012). In contrast, measures of glucose metabolism are extensively quantitatively modeled, and are captured across a larger time-window (e.g. 20–30 min), across which alcohol may exert different vascular and other pharmacodynamic properties. Notably, alcohol effects on cerebral vasculature (vasoconstriction vs dilation) are dependent on dose and time course (e.g. acute withdrawal), and the rate and mode of alcohol intake tends to vary across studies.
Other sources of discrepancies may relate to an interpretive issue that pervades functional neuroimaging – the meaning of the directionality of activation differences, where sometimes “less (activation) is more.” A key example for this interpretation would be increased frontocortical recruitment in schizophrenia, which is typically interpreted as evidence of the struggles of an inefficient brain. Tolentino et al. (2011) also found that subjects with a lower subjective response to alcohol demonstrated less of an increase in CBF compared to subjects with a higher response to alcohol. The authors interpreted this directionality as a neurobiological account for longitudinal findings that persons with low sensitivity to alcohol at baseline are at greater risk for progression to alcoholism (Schuckit et al., 2007). The pattern of individual differences suggested that high concentrations of alcohol are necessary for the frontal cortex of low-responders to engender or reflect subjective intoxication.
3.3. fMRI studies of alcohol effects on resting-state connectivity
More recently, there has been an explosion in the understanding of innate functional connectivity between brain regions as inferred from inter-regional activation synchrony detected using continuous fMRI in the absence of a task (Fox and Raichle, 2007). Notably, portions of the midline of the brain, including precuneus, show synchronized activity when the subject is not focusing on a cognitive demand, and this is termed the “resting-state” network. Independent component analysis of resting-state fMRI time series have revealed eight coherent brain networks when the subject is at rest (Beckmann et al., 2005), and these spatially reflect patterns of co-activated structures in task fMRI (Cole et al., 2010). Critically, individual differences in inter-regional resting connectivity correlated with task-elicited activity in those regions (Mennes et al., 2010) suggesting that inherent resting-state connectivity is predictive of how well-coordinated the brain regions will be when confronted with a cognitive demand that invokes them.
Acute intravenous (IV) alcohol effects on these eight a priori networks was recently assessed while controlling for alcohol’s physiological effects such as blood flow (Khalili-Mahani et al., 2012). Alcohol increased intrinsic connectivity in a network composed of auditory (temporal lobe) and anterior cingulate cortex, and also in brainstem. Acute alcohol has also been shown to strengthen measures of the intrinsic connectivity of visual cortex, in correlation with blood-alcohol levels (Esposito et al., 2010). We note that the development of alcoholism and other substance dependence has been described as “stimulus-driven,” where the incentive salience hypothesis of addiction (Berridge and Robinson, 2003) posits that environmental cues that are predictive of the abused substance themselves acquire motivational properties. If acute alcohol increases the functional connectivity (communication) within sensory regions and between sensory regions and cortex, this may augment alcohol cue/stimulus-reward learning during intoxication. Increased connectivity with the anterior cingulate cortex may relate to its recruitment by risky rewards and by monitoring action-outcome results of instrumental behavior (Ridderinkhof et al., 2004).
3.4. Diffusion imaging and magnetic resonance spectroscopy studies of alcohol effects
Finally, diffusion tensor imaging (DTI) and magnetic resonance spectroscopy (MRS) have been used to examine the effects of acute alcohol on water diffusion and chemical composition in brain tissues. Diffusion Tensor Imaging (DTI) is a MRI-based measurement of Brownian motion of water molecules, where metrics of water diffusion could provide indirect evidence of cytotoxicity or transient edema (accumulation of fluid within the brain parenchyma that locally enlarges the tissue). In the only DTI study of acute alcohol in humans to date, acute alcohol consumption significantly reduced apparent diffusion coefficient (ADC) values of the frontal lobe, thalamus, and middle cerebellar peduncle in social drinkers (Kong et al., 2012). In addition, fractional anisotropy (FA) values (indicating an increase in diffusion along a specific axis or trajectory) of the frontal lobe were significantly increased. The authors concluded that these brain regions may be the most sensitive to the effects of acute alcohol consumption.
MRS probes the magnetic responses of atomic nuclei to determine physical and chemical properties of atoms, and can identify molecules in (a focal volume of) brain tissue. This provides a dynamic measure of changes in concentrations of neurotransmitter metabolites, or other markers (e.g. N-acetylaspartate; NAA) that have been interpreted as indicators of general neuronal health or localized activity (Baslow, 2010; Moore et al., 1999). Recently, Gomez et al. (2012) reported that acute IV alcohol administration reduced cortical GABA and NAA levels in occipital cortex in humans, which they interpreted as evidence of facilitation of GABA receptor function by ethanol and concomitant inhibition of neuronal activity in the brain. Moreover, across alcohol detoxification in a clinical setting, increased levels of glutamate metabolites in anterior cingulate cortex were detected early in detoxification, but not after two weeks of abstinence (Hermann et al., 2012), providing evidence for theories of excitatory neurotransmitter-based theories of alcohol withdrawal.
4. Effects of acute alcohol pre-treatment on task-elicited brain activity
4.1. Alcohol effects on brain activation during simple perception and during cognitive tasks
Alcohol is implicated in a substantial portion of motor vehicle accidents and falls (www.niaaa.nih.gov), and causes impaired memory and other cognitive decrements (Oscar-Berman and Marinkovic, 2007; Zoethout et al., 2011). The first fMRI “tasks” administered to alcohol-intoxicated subjects featured simple exposure to visual/photic (Levin et al., 1998) and auditory (Seifritz et al., 2000) stimulation, where alcohol reduced BOLD responsiveness in visual and auditory cortices. This is consistent with the understanding that alcohol is generally a CNS depressant. Subsequently, many studies have administered alcohol, either orally or intravenously, to subjects while they performed more complex cognitive or emotional tasks in the fMRI scanner. These experiments generally indicate that acute alcohol reduces activation in cortical regions that govern cognitive control and error monitoring.
Using a simple continuous performance task (Anderson et al., 2011), oral alcohol increased reaction times, commission and omission errors, and caused a concomitant dose-dependent reduction in BOLD response to commission errors in ACC, insula, lateral prefrontal cortex, and parietal lobe. Notably, the ACC is consistently implicated in processing and behavioral adjustment in response to errors in fMRI experiments (Ridderinkhof et al., 2004), where blunted error-elicited activity in ACC has also been detected with electrophysiological measures (Ridderinkhof et al., 2002). This suggests that alcohol intoxication may result in impaired monitoring of one’s behavior and errors by way of suppressing the ACC. In contrast, when subjects performed a visual discrimination/mental rotation task, alcohol had only a modest effect on visual perception performance, yet caused dose-dependent activation increases in the insula, dorsolateral prefrontal, and precentral cortices, and dose-dependent decreases in anterior and posterior cingulate, precuneus, middle frontal, and superior frontal areas (Calhoun et al., 2004). In a working memory task, alcohol also did not affect task performance (errors or response latency), but reduced memory load-dependent activation in the dorsolateral prefrontal cortex (Paulus et al., 2006). In another study of acute alcohol and working memory (Gundersen et al., 2008), subjects performed an n-back task after drinking an alcoholic beverage that resulted in a BAC of 0.02% in one session and 0.08% in a second session. Activation was decreased in the dorsal anterior cingulate cortex (dACC) and in the cerebellum in the alcohol group at the BAC of 0.08% when the participants performed the most demanding task, when subjects showed a trend toward poorer performance. These studies indicate that it is possible for behavior to stay intact during either simple tasks or at low alcohol doses, even while local changes in activation are observed in regions of the brain important in cognition, attention, and error monitoring.
Sometimes acute alcohol clearly blunts both activation and performance. In a simulated driving experiment in which subjects received single-blind doses of alcohol to produce BACs of 0.10% (high), 0.05% (moderate), or 0% (placebo), Meda et al. (2009) found dose-related decreased activation in driving-associated regions, including the superior, middle and orbitofrontal gyri, anterior cingulate, primary/supplementary motor areas, basal ganglia, and cerebellum. Notably, activation in identified functional networks correlated with individual differences in driving decrement while intoxicated. These studies suggest that alcohol, especially at high doses, can cause significant alterations of brain regions involved in memory, perception, and motor planning and control.
These findings on the whole suggest that alcohol reduces functional activation of cortical regions implicated in cognitive control (in non-pharmacological studies), yet they vary in terms of whether the alcohol dose actually affected behavior. Brain changes induced by alcohol amid normative performance may call into question the functional significance of the brain changes. One possibility is that reduced activation reflects ambivalence about performance (where activations while sober are affect-related, but not essential for performance). Another possibility is that the reduced activity indicates sub-optimal functioning that would have become problematic if the task demands had been more intense. In any case, these findings illustrate the brain’s ability to accomplish integrative and complex cognitive tasks despite significant alcohol present—at least for a short time in an artificial laboratory setting. In many instances, the brain signal might be a more sensitive indicator than overt behavior (Wilkinson and Halligan, 2004). Since behavior remained intact in these studies, altered brain activations could not be attributed to subject perception/experience of greater trial errors or experience of frustration (or amusement). For example, normative performance is crucial for interpreting dACC activation differences in that this region is also activated by notification of errors (Ridderinkhof et al., 2004).
4.2. Alcohol effects on brain activation during emotion-processing tasks
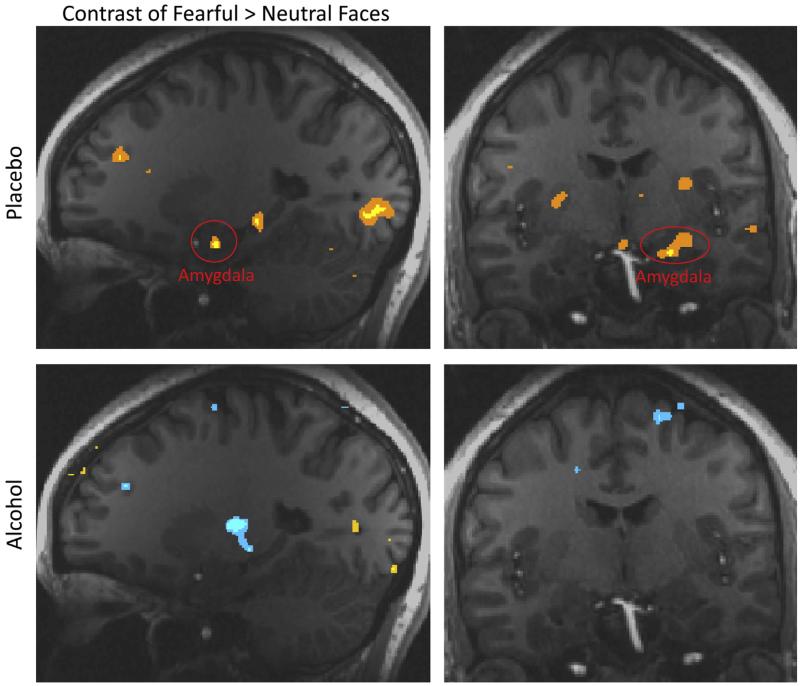
Of additional interest are the brain effects of acute alcohol on emotional processing. For example, among all substances of abuse, alcohol is linked most strongly to aggression as a direct psychopharmacological effect (Hoaken and Stewart, 2003). We administered intravenous alcohol resulting in a BAC of 0.08% using a “patch clamp” titration procedure (Ramchandani et al., 1999), and showed images of neutral and fearful faces (Gilman et al., 2008). Alcohol had a significant unconditioned effect on brain regions involved in emotional processing, including the amygdala, insula, and parahippocampal gyrus, and visual processing regions. In the placebo condition, these areas showed greater activation to fearful than to neutral faces. Conversely, in the alcohol condition, we observed no significant differences in activation between fearful and neutral faces (Fig. 1), indicating that alcohol blunted emotional processing in these regions.
Fig. 1.
Linear contrast between fearful vs. neutral faces under placebo and alcohol conditions. Increased activation to fearful faces is shown in yellow/orange (p < 0.01), while increased activation to neutral faces is shown in blue (p < 0.01). In the placebo condition (top panels), there was greater activation to fearful than to neutral faces in the amygdala (circled in red), parahippocampal gyrus, and visual cortex. These activations were not significant in the alcohol condition (bottom panels). Images copyright 2008, Journal of Neuroscience.
This lack of amygdala recruitment by threat-indicating faces during intoxication was replicated in a study using oral alcohol administration and an emotional face assessment task, which also showed that the alcohol significantly reduced amygdala reactivity threatening (fearful and angry) faces (Sripada et al., 2011). An additional study (Padula et al., 2011) found that anterior insula response to emotional faces was significantly attenuated following consumption of alcohol when compared with placebo. These studies indicate that alcohol blunts the recruitment of limbic structures that would normally be engaged by threatening or uncertain outcomes in social contexts, and may be a source of the anecdotal “beer muscles” in communal drinking settings.
A recent experiment delineated effects of IV alcohol administration on emotion processing between light and heavy drinkers, and also captured unconditioned responses to the alcohol infusion itself (Gilman et al., 2012a). Heavy drinkers reported lower subjective alcohol effects than social drinkers despite similar blood-alcohol concentration to light drinkers, and alcohol significantly activated the NAcc in light drinkers, but not in heavy drinkers, where self-reported intoxication correlated with striatal activation. In the task, fearful faces significantly activated the amygdala in the light drinkers only, and this activation was attenuated by alcohol. Reflecting other research on alcoholics (Volkow et al., 2007), the heavy drinkers not only experienced reduced subjective effects of alcohol, but also demonstrate a blunted response to alcohol in the brain’s reward system. Due to the cross-sectional design, however, it is unclear whether these group differences arose from simple pharmacological tolerance to alcohol, from some prodromal allostatic process (en route to alcohol dependence) (Koob and Le Moal, 2008), or from pre-existing decreased sensitivity to alcohol’s effects (Schuckit et al., 2007).
4.3. Alcohol effects on brain activation during decision-making tasks
Alcohol intoxication has a rich anecdotal connection to poor decision-making and risk-taking, and has also increased risky behavior in the laboratory (Lane et al., 2004). To our knowledge, there has only been one imaging study on the effects of acute alcohol on risky decision-making. In this study, social drinkers were given either intravenous alcohol (BAC of 0.08) or placebo, and performed a risk-taking task in which they could either choose safe or risky options, and could win or lose money as a result of those choices (Gilman et al., 2012b). When subjects were given alcohol, they made more risky choices, and showed increased activation in the striatum to risky compared with safe choices. Alcohol compared to placebo also dampened the neural response to notification of both wins and loses throughout the NAcc, caudate, thalamus and insula. This study suggests that alcohol may increase risk-taking behavior by both activating brain regions involved in reward when a decision is made, and dampening the response to negative and positive feedback.
In sum, these task-fMRI experiments clearly indicate that alcohol intoxication alters (and typically blunts) the brain’s dynamic response to task demands. Notably, activation differences from placebo conditions were frequently found in brain regions governing motor and inhibitory control. One caveat to these studies is the difficulty in maintaining a true “placebo” condition for alcohol, whether administered orally, or IV. Subjects can readily discriminate moderate to high doses of alcohol. Individual differences in personality traits relate to both alcohol expectancies (Leonard and Blane, 1988) and to placebo effects in brain (Pecina et al., 2013), where both sober and intoxicated behavior relate to individual differences in expectancies (Fillmore and Blackburn, 2002). Therefore, it cannot be ruled out that some differences attributed to the pharmacodynamic properties of alcohol in task-fMRI could be manifestations of psychological expectancy effects. In experiments involving emotion or impulse control tasks, this could be critical in that subjects who exhibit high levels of impulsive behavior show exaggerated estimates of both alcohol content received and of intoxication by placebo (Bjork and Dougherty, 1998). Debriefing questionnaires that probe elements of expectancy may provide interesting new variables or clarifying covariates.
5. Chronic alcohol use effects on the brain: confounds in acute administration studies
For ethical and practical reasons, laboratory alcohol administration studies typically recruit subjects with appreciable recent drinking histories. Findings that even subclinical chronic alcohol use causes gross morphological change are of critical importance in that individual differences in either drinking histories or in brain sensitivity to chronic alcohol (Srivastava et al., 2010), may result in individual differences in gray matter density or volume, to in turn add variance to fMRI and other modalities that center on transient changes in brain blood flow. This is because gray matter is more heavily vascularized than brain white matter, where the majority of fMRI BOLD signal is thought to be derived from capillary beds (Kim and Ogawa, 2012). Increased activation in one group may result in part from differences in vascularization not related to (differentially coupled with) neural activity or maintenance of membrane local field potentials (Logothetis, 2003). The effects of chronic alcohol on human brain morphology in vivo has largely been inferred from cross-sectional comparisons between controls and alcoholics or other heavy drinkers, and has been reviewed in significant depth elsewhere (e.g. Sullivan and Pfefferbaum, 2009; Zahr et al., 2011). We briefly address this literature to illustrate alcohol’s extensive linkage to pronounced morphological change, and to discuss how macrostructural effects of chronic alcohol may complicate or aid interpretation of acute-infusion imaging.
5.1. Morphometric signatures of chronic alcohol neurotoxicity
Chronic alcohol intoxication results in a complex chain of compensatory and other neuroadaptations in the brain (Fadda and Rossetti, 1998), including pharmacological tolerance. Intensifying alcohol bouts can result in neuronal death attributable to excitatory chemical cascades and neuroinflammatory responses (reviewed in Alfonso-Loeches and Guerri, 2011). These changes are thought to underpin cognitive decrements frequently found in patients with alcohol dependence (Sullivan et al., 2000). The first powerful in vivo evidence of brain abnormalities in chronic alcohol abuse came from seminal cross-sectional studies published decades ago by Pfefferbaum and colleagues, first using computerized tomographic scanning (Jernigan et al., 1982; Pfefferbaum et al., 1988), then structural MRI (Pfefferbaum et al., 1992). These studies have repeatedly shown that alcoholics (Fein et al., 2002, 2006; Pfefferbaum et al., 1992, 1995) and non-dependent heavy drinkers (Kubota et al., 2001) are characterized by enlarged lateral ventricles and global brain volume reduction (especially in gray matter) relative to age-matched controls. In some studies, gray matter loss has correlated directly with years of alcohol dependence (Fein et al., 2002) as well as estimates of total lifetime alcohol quantity after controlling for age (Bjork et al., 2003), and is also affected by comorbid smoking (Gazdzinski et al., 2005). These differences are reminiscent of within-subject changes with normal aging (Gur et al., 2002), such that chronic alcohol effects have been framed as an acceleration of aging-related effects (Giorgio et al., 2010; Kubota et al., 2001; Pfefferbaum et al., 1992).
An interesting controversy is whether male or female drinkers are more susceptible to brain morphological effects of alcohol, which could in turn lead to spurious inference of sex differences in dynamic fMRI signals. Pfefferbaum et al. (2001) reported that men are disproportionately susceptible to alcohol-induced brain damage compared to women, whereas the Hommer group reported that that women showed more severe alcoholism-induced global reductions in gray and white matter (Hommer et al., 2001) and corpus callosum (Hommer et al., 1996) volume compared to men. Similarly, the negative correlation between alcohol use and total cerebral brain volume (corrected for total intracranial volume) was slightly stronger in women than in men of the Framingham longitudinal cohort (Paul et al., 2008). More recently, however (Demirakca et al., 2011) reported no sex differences in either brain volumetric reductions with alcoholism or in volumetric recovery at three-month abstinence (albeit in a small sample).
Advances in MRI spatial resolution and image processing, as well as larger sample sizes enabled by the continued expansion of imaging capacity (and expanded data-sharing between laboratories) hold the potential to better detect and control for sex differences and other individual differences in effects of alcohol on the human brain. Selecting subjects within a narrow band of recent and long-term patterns of alcohol use could minimize potential within-group differences in cognitive disruption and in gray matter effects. When comparing gray matter activation between groups defined by very different drinking histories, another approach is to sample BOLD signal data from a probabilistic mask for VOI analysis that would only include voxels that exceed a certain proportion of gray matter in both subject groups (Bjork et al., 2008).
5.2. Chronic alcohol effects on task performance and striatal responsiveness
In task fMRI experiment designs where the intent is to normalize or equalize task success between alcohol recipients (or subcategories of drinkers) to improve interpretation (e.g. Bjork et al., 2012), it is important to accommodate large individual differences in performance due to individual differences in (even clinically-insignificant) alcohol exposure. While evidence for cognitive and motor decrements in alcoholism is extensive (Oscar-Berman and Marinkovic, 2007), there is some evidence that moderate drinking may confer cognitive advantages, including resistance to dementia (Neafsey and Collins, 2011). For example, not only were fMRI NAcc responses to alcohol infusion itself blunted in heavy but not lighter non-dependent drinkers (Gilman et al., 2012a), PET studies have also uncovered reduced dynamic responsiveness of the striatal incentive neurocircuitry after chronic heavy drinking. Alcoholics showed blunted endogenous DA release (displacement of radiolabeled raclopride) by administration of the psychostimulants methylphenidate (Volkow et al., 2007) and dextroamphetamine (Martinez et al., 2005). Individual differences in core motivational function could affect downstream metrics of cognitive task activation in cases where the subject focus and performance in a task are rewarded. In this instance, substantial incentives for optimum performance might be required to normalize motivation across subjects to reveal cognitive ability/capacity differences of interest.
5.3. Chronic alcohol effects on task performance and striatal responsiveness
Individual differences in drinking histories and effects on white matter fiber tracts present another source of variance in restingstate fMRI studies, psychophysiological interaction (PPI) analyses in task fMRI, or other studies of dynamic connectivity patterns elicited or affected by acute alcohol. DTI is typically utilized to infer the microstructural integrity and path structure of brain white matter (Ciccarelli et al., 2008) in that water diffuses more readily along the orientation of axonal fibers than across the fibers due to hindrance from structural elements such as the axolemma and the myelin sheath. Critically, chronic alcoholism disrupts the integrity of while matter microarchitecture (Giorgio et al., 2010; Pfefferbaum et al., 2010; Schulte et al., 2010; Yeh et al., 2009). As such, individual differences in co-activation between brain regions (Camchong et al., 2013) may be function of differences in white matter structural integrity. Instead of being a confound, this structure–function relationship can be directly assessed in future multi-modal imaging projects where diffusion images and task-elicited images and resting-state images are collected from the same subject, and also related to off-line neurocognitive performance (Van Essen et al., 2012).
5.4. Brain morphometric and behavioral recovery from chronic alcohol effects
Fortunately, alcoholism recovery frequently results in improvement of cognitive decrements, and neuroimaging findings have provided a neuromorphological account for functional recovery. Notably, longitudinal studies have revealed diverging trajectories in repeatedly-scanned alcoholics: progressive gray matter volume reduction in subjects who relapse, and evidence of recovery of morphological decrements in abstainers (Alhassoon et al., 2012; Crews et al., 2005; Pfefferbaum et al., 1995, 1998). The reversibility of brain tissue loss with repeated alcohol intoxication may operate on rapid time scales. Recently, Zahr et al. (2013) reported that ventricle size in the rats was normalized seven days after cessation of repeated “binge” alcohol administration in rats, suggesting that a significant component of ventricular enlargement in alcoholism may simply be transient changes in brain tissue water regulation (fluid distribution).
6. Future directions in acute alcohol clinical neuroimaging
To conclude, evidence from neuroimaging studies are providing powerful signatures of acute alcohol intoxication in the brain – not only of the intoxication or “high” itself when subjects may not be doing anything, but also alcohol-intoxication effects on the brain when it is trying to sustain attention, evaluate stimuli, or perform other cognitive tasks. Notably, acute alcohol effects on the brain at rest reveal activation of subcortical and cortical structures or networks linked to reward valuation, instrumental motivation, and executive control over behavior. Emerging findings are therefore providing further mechanistic connections for what have largely been abstract theories of alcohol effects and addiction.
There are several understudied areas of research that would enhance our understanding of acute effects of alcohol. One area of interest is exploration of sex differences in alcohol response. For example, alcohol-induced reductions in whole-brain glucose utilization was more pronounced in men, though women reported higher levels of subjective intoxication, despite similar blood-alcohol concentration time course between male and female subjects (Wang et al., 2003). A more recent ASL study indicated that increased frontal perfusion from acute alcohol administration was specific to men (Rickenbacher et al., 2011). These kinds of sex differences remain largely unstudied. Might sexual dimorphisms in brain development result in different mechanistic pathways to intoxication? Also virtually unknown are genotypic differences in acute alcohol responses in human brain. Ramchandani et al. (2011) reported that alcohol-induced striatal DA release in the ventral striatum of healthy men was specific to carriers of the 118G allele of the mu-opioid receptor OPRM1, but was minimal in AA homozygotes. Genetic polymorphisms may not only account for individual-difference variance in alcohol response, but may also yield clues about mechanisms of alcohol effects.
Other individual difference effects on acute alcohol response remain unexplored, such as alcohol expectancies, temperament, and family histories of alcohol use disorder. Critically, alcohol-induced decrements may interact with individual differences to increase violence and addiction. Various opponent-process theories of addiction (e.g. Bickel et al., 2007) or of behavior disorders like conduct disorder (CD) (e.g. Newman and Wallace, 1993) that predispose to addiction, posit that addiction or addiction-risk is characterized by overactive or oversensitive motivational neurocircuitry that is relatively unconstrained by self-control neurocircuitry (Crews and Boettiger, 2009). Individuals showing this motivational imbalance are theoretically more likely to binge-drink, more likely to transition from recreational to problematic use, and less likely to be able to stop themselves from continued problematic use. Might pharmacological fMRI studies find more mechanistic underpinnings of this purported imbalance?
Application of alcohol to an under-regulated brain (by neurobiological trait) will be a critical combination to study. For example, acute alcohol intoxication disproportionately increases laboratory aggression in subjects with low baseline executive cognitive function (Lau et al., 1995). Since alcohol acutely reduces behavior control (e.g. Dougherty et al., 1999; Reynolds et al., 2006), alcohol intoxication may exacerbate already sub-optimal executive control neurocircuitry to increase risk for aggression or addiction. For example, both acute alcohol intoxication (Dougherty et al., 1999) as well as a childhood history of CD (as a between-subject factor) (Dougherty et al., 2003) increase commission errors (failures to withhold a pre-potent motor response) in a continuous performance task. Over time, youth with CD are more likely to develop alcohol use disorder (AUD) (Pardini et al., 2007). Boileau et al. (2003) reported that self-reported impulsivity on a personality questionnaire accounted for significant variance in radiolabeled raclopride displacement (by endogenous DA release) following alcohol ingestion. Future studies could build into the design and consent procedures the potential to follow-up subjects over time, to see whether brain responses at baseline are especially predictive of future transition from recreational alcohol use to dependence.
Future fMRI alcohol research would also benefit from greater precision afforded by better control of cardiovascular (hemodynamic) confounds. For example, investigators could add a basic stimulus task to the protocol from which modeling adjustments could be derived for statistically modeling the cognitive task of interest. Alternatively, investigators could perform an additional ASL scan under each dose condition to get a marker for general brain blood flow. More simply, investigators could model the time series under both dose conditions with a flexible hemodynamic model that would not penalize the precise contour of one hemodynamic response over another, yet would still capture large hemodynamic response differences.
7. Conclusion
To conclude, while there are some discrepancies in specific regional effects of acute alcohol on the resting brain and on the brain at work, the preponderance of evidence indicates that acute alcohol exerts region-specific suppression (e.g. cerebellum) or enhancement (e.g. ventral striatum) of brain metabolic or hemodynamic activity, and by inference, neuronal activity. Critically, these effects are seen in regions thought to govern motor control, motivation, and executive control, such as working memory and attention. Pharmacological fMRI in particular holds considerable promise to further our understanding of alcohol effects on the brain, through advances in cognitive task design, scanner signal detection, exploration of individual differences such as genotype and temperament, and longitudinal study designs.
Acknowledgments
We are indebted to the training, fellowship, and inspiration of our former mentor, Dr. Daniel W. Hommer. Cited original research performed by the authors was funded by intramural funding of the National Institute on Alcohol Abuse and Alcoholism.
References
- Alfonso-Loeches S, Guerri C. Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain. Crit. Rev. Clin. Lab. Sci. 2011;48:19–47. doi: 10.3109/10408363.2011.580567. [DOI] [PubMed] [Google Scholar]
- Alhassoon OM, Sorg SF, Taylor MJ, Stephan RA, Schweinsburg BC, Stricker NH, Gongvatana A, Grant I. Callosal white matter microstructural recovery in abstinent alcoholics: a longitudinal diffusion tensor imaging study. Alcohol. Clin. Exp. Res. 2012;36:1922–1931. doi: 10.1111/j.1530-0277.2012.01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BM, Stevens MC, Meda SA, Jordan K, Calhoun VD, Pearlson GD. Functional imaging of cognitive control during acute alcohol intoxication. Alcohol. Clin. Exp. Res. 2011;35:156–165. doi: 10.1111/j.1530-0277.2010.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baslow MH. Evidence that the tri-cellular metabolism of N-acetylaspartate functions as the brain’s “operating system”: how NAA metabolism supports meaningful intercellular frequency-encoded communications. Amino Acids. 2010;39:1139–1145. doi: 10.1007/s00726-010-0656-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Van Der Linden M. Decision-making and impulse control after frontal lobe injuries. Curr. Opin. Neurol. 2005;18:734–739. doi: 10.1097/01.wco.0000194141.56429.3c. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007;90(Suppl. 1):S85–S91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Dougherty DM. Differences in alcohol expectancy between aggressive and nonaggressive social drinkers. Alcohol. Clin. Exp. Res. 1998;22:1943–1950. [PubMed] [Google Scholar]
- Bjork JM, Grant SJ, Hommer DW. Cross-sectional volumetric analysis of brain atrophy in alcohol dependence: effects of drinking history and comorbid substance use disorder. Am. J. Psychiatry. 2003;160:2038–2045. doi: 10.1176/appi.ajp.160.11.2038. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Momenan R, Smith AR, Hommer DW. Reduced posterior mesofrontal cortex activation by risky rewards in substance-dependent patients. Drug Alcohol Depend. 2008;95:115–128. doi: 10.1016/j.drugalcdep.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Mesolimbic recruitment by nondrug rewards in detoxified alcoholics: effort anticipation, reward anticipation, and reward delivery. Hum. Brain Mapp. 2012;33:2174–2188. doi: 10.1002/hbm.21351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am. J. Prev. Med. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Altschul D, McGinty V, Shih R, Scott D, Sears E, Pearlson GD. Alcohol intoxication effects on visual perception: an fMRI study. Hum. Brain Mapp. 2004;21:15–26. doi: 10.1002/hbm.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Stenger A, Fein G. Resting-state synchrony in long-term abstinent alcoholics. Alcohol. Clin. Exp. Res. 2013;37:75–85. doi: 10.1111/j.1530-0277.2012.01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli O, Catani M, Johansen-Berg H, Clark C, Thompson A. Diffusion-based tractography in neurological disorders: concepts, applications, and future developments. Lancet Neurol. 2008;7:715–727. doi: 10.1016/S1474-4422(08)70163-7. [DOI] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front. Syst. Neurosci. 2010;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol. Biochem. Behav. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Buckley T, Dodd PR, Ende G, Foley N, Harper C, He J, Innes D, Lohel W, Pfefferbaum A, Zou J, Sullivan EV. Alcoholic neurobiology: changes in dependence and recovery. Alcohol. Clin. Exp. Res. 2005;29:1504–1513. doi: 10.1097/01.alc.0000175013.50644.61. [DOI] [PubMed] [Google Scholar]
- Demirakca T, Ende G, Kammerer N, Welzel-Marquez H, Hermann D, Heinz A, Mann K. Effects of alcoholism and continued abstinence on brain volumes in both genders. Alcohol. Clin. Exp. Res. 2011;35:1678–1685. doi: 10.1111/j.1530-0277.2011.01514.x. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Bjork JM, Harper RA, Mathias CW, Moeller FG, Marsh DM. Validation of the immediate and delayed memory tasks in hospitalized adolescents with disruptive behavior disorders. Psychol. Rec. 2003;53:509–532. doi: 10.1111/1469-7610.00197. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Moeller FG, Steinberg JL, Marsh DM, Hines SE, Bjork JM. Alcohol increases commission error rates for a continuous performance test. Alcohol. Clin. Exp. Res. 1999;23:1342–1351. [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcohol. Clin. Exp. Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Esposito F, Pignataro G, Di Renzo G, Spinali A, Paccone A, Tedeschi G, Annunziato L. Alcohol increases spontaneous BOLD signal fluctuations in the visual network. Neuroimage. 2010;53:534–543. doi: 10.1016/j.neuroimage.2010.06.061. [DOI] [PubMed] [Google Scholar]
- Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog. Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol. Clin. Exp. Res. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Landman B, Tran H, McGillivray S, Finn P, Barakos J, Moon K. Brain atrophy in long-term abstinent alcoholics who demonstrate impairment on a simulated gambling task. Neuroimage. 2006;32:1465–1471. doi: 10.1016/j.neuroimage.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman RS, Meyer JS, Quenzer LF. Principles of Neuropsychopharmacology. Sinauer Associates, Inc.; Sunderland, MA: 1997. [Google Scholar]
- Fillmore MT, Blackburn J. Compensating for alcohol-induced impairment: alcohol expectancies and behavioral disinhibition. J. Stud. Alcohol. 2002;63:237–246. doi: 10.15288/jsa.2002.63.237. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Studholme C, Song E, Banys P, Meyerhoff DJ. Quantitative brain MRI in alcohol dependence: preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volumes. Alcohol. Clin. Exp. Res. 2005;29:1484–1495. doi: 10.1097/01.alc.0000175018.72488.61. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Crouss T, Hommer DW. Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacology. 2012a;37:467–477. doi: 10.1038/npp.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Smith AR, Ramchandani VA, Momenan R, Hommer DW. The effect of intravenous alcohol on the neural correlates of risky decision making in healthy social drinkers. Addict. Biol. 2012b;17:465–478. doi: 10.1111/j.1369-1600.2011.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J. Neurosci. 2008;28:4583–4591. doi: 10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, Johansen-Berg H. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 2010;51:943–951. doi: 10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R, Behar KL, Watzl J, Weinzimer SA, Gulanski B, Sanacora G, Koretski J, Guidone E, Jiang L, Petrakis IL, Pittman B, Krystal JH, Mason GF. Intravenous ethanol infusion decreases human cortical gamma-aminobutyric acid and N-acetylaspartate as measured with proton magnetic resonance spectroscopy at 4 tesla. Biol. Psychiatry. 2012;71:239–246. doi: 10.1016/j.biopsych.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen H, Gruner R, Specht K, Hugdahl K. The effects of alcohol intoxication on neuronal activation at different levels of cognitive load. Open Neuroimag. J. 2008;2:65–72. doi: 10.2174/1874440000802010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Gunning-Dixon FM, Turetsky BI, Bilker WB, Gur RE. Brain region and sex differences in age association with brain volume: a quantitative MRI study of healthy young adults. Am. J. Geriatr. Psychiatry. 2002;10:72–80. [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hermann D, Weber-Fahr W, Sartorius A, Hoerst M, Frischknecht U, Tunc-Skarka N, Perreau-Lenz S, Hansson AC, Krumm B, Kiefer F, Spanagel R, Mann K, Ende G, Sommer WH. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol. Psychiatry. 2012;71:1015–1021. doi: 10.1016/j.biopsych.2011.07.034. [DOI] [PubMed] [Google Scholar]
- Hoaken PN, Stewart SH. Drugs of abuse and the elicitation of human aggressive behavior. Addict. Behav. 2003;28:1533–1554. doi: 10.1016/j.addbeh.2003.08.033. [DOI] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Kaiser E, Rawlings R. Evidence for a gender-related effect of alcoholism on brain volumes. Am. J. Psychiatry. 2001;158:198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Rawlings R, Ragan P, Williams W, Rio D, Eckardt M. Decreased corpus callosum size among alcoholic women. Arch. Neurol. 1996;53:359–363. doi: 10.1001/archneur.1996.00550040099019. [DOI] [PubMed] [Google Scholar]
- Ingvar M, Ghatan PH, Wirsen-Meurling A, Risberg J, Von Heijne G, Stone-Elander S, Ingvar DH. Alcohol activates the cerebral reward system in man. J. Stud. Alcohol. 1998;59:258–269. doi: 10.15288/jsa.1998.59.258. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Zatz LM, Ahumada AJ, Jr., Pfefferbaum A, Tinklenberg JR, Moses JA., Jr. CT measures of cerebrospinal fluid volume in alcoholics and normal volunteers. Psychiatry Res. 1982;7:9–17. doi: 10.1016/0165-1781(82)90048-8. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat. Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y. Physio-pathological effects of alcohol on the cardiovascular system: its role in hypertension and cardiovascular disease. Hypertens. Res. 2010;33:181–191. doi: 10.1038/hr.2009.226. [DOI] [PubMed] [Google Scholar]
- Khalili-Mahani N, Zoethout RM, Beckmann CF, Baerends E, de Kam ML, Soeter RP, Dahan A, van Buchem MA, van Gerven JM, Rombouts SA. Effects of morphine and alcohol on functional brain connectivity during “resting state”: a placebo-controlled crossover study in healthy young men. Hum. Brain Mapp. 2012;33:1003–1018. doi: 10.1002/hbm.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav. Brain Res. 2011;223:403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SG, Ogawa S. Biophysical and physiological origins of blood oxygenation level-dependent fMRI signals. J. Cereb. Blood Flow Metab. 2012;32:1188–1206. doi: 10.1038/jcbfm.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong LM, Zheng WB, Lian GP, Zhang HD. Acute effects of alcohol on the human brain: diffusion tensor imaging study. AJNR Am. J. Neuroradiol. 2012;33:928–934. doi: 10.3174/ajnr.A2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu. Rev. Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kubota M, Nakazaki S, Hirai S, Saeki N, Yamaura A, Kusaka T. Alcohol consumption and frontal lobe shrinkage: study of 1432 non-alcoholic subjects. J. Neurol. Neurosurg. Psychiatry. 2001;71:104–106. doi: 10.1136/jnnp.71.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, Fox PT. Behavioral interpretations of intrinsic connectivity networks. J. Cogn. Neurosci. 2011;23:4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Pietras CJ, Tcheremissine OV. Alcohol effects on human risk taking. Psychopharmacology (Berl) 2004;172:68–77. doi: 10.1007/s00213-003-1628-2. [DOI] [PubMed] [Google Scholar]
- Lau MA, Pihl RO, Peterson JB. Provocation, acute alcohol intoxication, cognitive performance, and aggression. J. Abnorm. Psychol. 1995;104:150–155. doi: 10.1037//0021-843x.104.1.150. [DOI] [PubMed] [Google Scholar]
- Leonard KE, Blane HT. Alcohol expectancies and personality characteristics in young men. Addict. Behav. 1988;13:353–357. doi: 10.1016/0306-4603(88)90041-x. [DOI] [PubMed] [Google Scholar]
- Levin JM, Ross MH, Mendelson JH, Kaufman MJ, Lange N, Maas LC, Mello NK, Cohen BM, Renshaw PF. Reduction in BOLD fMRI response to primary visual stimulation following alcohol ingestion. Psychiatry Res. 1998;82:135–146. doi: 10.1016/s0925-4927(98)00022-5. [DOI] [PubMed] [Google Scholar]
- Lindman RE, Lang AR. The alcohol-aggression stereotype: a cross-cultural comparison of beliefs. Int. J. Addict. 1994;29:1–13. doi: 10.3109/10826089409047365. [DOI] [PubMed] [Google Scholar]
- Lindman RE, Sjoholm BA, Lang AR. Expectations of alcohol-induced positive affect: a cross-cultural comparison. J. Stud. Alcohol. 2000;61:681–687. doi: 10.15288/jsa.2000.61.681. [DOI] [PubMed] [Google Scholar]
- Liu TT, Brown GG. Measurement of cerebral perfusion with arterial spin labeling: part 1. Methods. J. Int. Neuropsychol. Soc. 2007;13:517–525. doi: 10.1017/S1355617707070646. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J. Neurosci. 2003;23:3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchtmann M, Jachau K, Tempelmann C, Bernarding J. Alcohol induced region-dependent alterations of hemodynamic response: implications for the statistical interpretation of pharmacological fMRI studies. Exp. Brain Res. 2010;204:1–10. doi: 10.1007/s00221-010-2277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, Abi-Dargham A. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol. Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH. Regional cerebral blood flow changes associated with ethanol intoxication. Stroke. 1986;17:1156–1159. doi: 10.1161/01.str.17.6.1156. [DOI] [PubMed] [Google Scholar]
- Meda SA, Calhoun VD, Astur RS, Turner BM, Ruopp K, Pearlson GD. Alcohol dose effects on brain circuits during simulated driving: an fMRI study. Hum. Brain Mapp. 2009;30:1257–1270. doi: 10.1002/hbm.20591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M, Kelly C, Zuo XN, Di Martino A, Biswal BB, Castellanos FX, Milham MP. Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. Neuroimage. 2010;50:1690–1701. doi: 10.1016/j.neuroimage.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CM, Frederick BB, Renshaw PF. Brain biochemistry using magnetic resonance spectroscopy: relevance to psychiatric illness in the elderly. J. Geriatr. Psychiatry Neurol. 1999;12:107–117. doi: 10.1177/089198879901200304. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ, Collins MA. Moderate alcohol consumption and cognitive risk. Neuropsychiatr Dis. Treat. 2011;7:465–484. doi: 10.2147/NDT.S23159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlin DB, Golden CJ, Quaife M, Graber B. Effect of alcohol ingestion on regional cerebral blood flow. Int. J. Neurosci. 1982;17:145–150. doi: 10.3109/00207458208985916. [DOI] [PubMed] [Google Scholar]
- Newman JP, Wallace JF. Diverse pathways to deficient self-regulation: implications for disinhibitory psychopathology in children. Clin. Psychol. Rev. 1993;13:699–720. [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol. Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula CB, Simmons AN, Matthews SC, Robinson SK, Tapert SF, Schuckit MA, Paulus MP. Alcohol attenuates activation in the bilateral anterior insula during an emotional processing task: a pilot study. Alcohol Alcohol. 2011;46:547–552. doi: 10.1093/alcalc/agr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini D, White HR, Stouthamer-Loeber M. Early adolescent psychopathology as a predictor of alcohol use disorders by young adulthood. Drug Alcohol Depend. 2007;88(Suppl. 1):S38–S49. doi: 10.1016/j.drugalcdep.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul CA, Au R, Fredman L, Massaro JM, Seshadri S, Decarli C, Wolf PA. Association of alcohol consumption with brain volume in the Framingham study. Arch. Neurol. 2008;65:1363–1367. doi: 10.1001/archneur.65.10.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Pulido C, Schuckit MA. Alcohol attenuates load-related activation during a working memory task: relation to level of response to alcohol. Alcohol. Clin. Exp. Res. 2006;30:1363–1371. doi: 10.1111/j.1530-0277.2006.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina M, Azhar H, Love TM, Lu T, Fredrickson BL, Stohler CS, Zubieta JK. Personality trait predictors of placebo analgesia and neurobiological correlates. Neuropsychopharmacology. 2013;38:639–646. doi: 10.1038/npp.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin. Exp. Res. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Crusan K, Jernigan TL. Brain CT changes in alcoholics: effects of age and alcohol consumption. Alcohol. Clin. Exp. Res. 1988;12:81–87. doi: 10.1111/j.1530-0277.1988.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Deshmukh A, Sullivan E. Sex differences in the effects of alcohol on brain structure. Am. J. Psychiatry. 2001;158:188–197. doi: 10.1176/appi.ajp.158.2.188. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Fama R, Sassoon SA, Sullivan EV. Transcallosal white matter degradation detected with quantitative fiber tracking in alcoholic men and women: selective relations to dissociable functions. Alcohol. Clin. Exp. Res. 2010;34:1201–1211. doi: 10.1111/j.1530-0277.2010.01197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol. Clin. Exp. Res. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch. Gen. Psychiatry. 1998;55:905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK, O’Connor S. A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol. Clin. Exp. Res. 1999;23:617–623. [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, Damadzic R, Eskay R, Schoor M, Thorsell A, Schwandt ML, Sommer WH, George DT, Parsons LH, Herscovitch P, Hommer D, Heilig M. A genetic determinant of the striatal dopamine response to alcohol in men. Mol. Psychiatry. 2011;16:809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, de Wit H. Acute-alcohol effects on the Experiential Discounting Task (EDT) and a question-based measure of delay discounting. Pharmacol. Biochem. Behav. 2006;83:194–202. doi: 10.1016/j.pbb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Rickenbacher E, Greve DN, Azma S, Pfeuffer J, Marinkovic K. Effects of alcohol intoxication and gender on cerebral perfusion: an arterial spin labeling study. Alcohol. 2011;45:725–737. doi: 10.1016/j.alcohol.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, Snel J, Band GP. Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science. 2002;298:2209–2211. doi: 10.1126/science.1076929. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J. Pharmacol. Exp. Ther. 2003;305:1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- Sano M, Wendt PE, Wirsen A, Stenberg G, Risberg J, Ingvar DH. Acute effects of alcohol on regional cerebral blood flow in man. J. Stud. Alcohol. 1993;54:369–376. doi: 10.15288/jsa.1993.54.369. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Pierson J, Hesselbrock V, Bucholz KK, Kramer J, Kuperman S, Dietiker C, Brandon R, Chan G. The ability of the Self-Rating of the Effects of Alcohol (SRE) scale to predict alcohol-related outcomes five years later. J. Stud. Alcohol Drugs. 2007;68:371–378. doi: 10.15288/jsad.2007.68.371. [DOI] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Pfefferbaum A, Sullivan EV. Neurocircuitry of emotion and cognition in alcoholism: contributions from white matter fiber tractography. Dialogues Clin. Neurosci. 2010;12:554–560. doi: 10.31887/DCNS.2010.12.4/tschulte. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifritz E, Bilecen D, Hanggi D, Haselhorst R, Radu EW, Wetzel S, Seelig J, Scheffler K. Effect of ethanol on BOLD response to acoustic stimulation: implications for neuropharmacological fMRI. Psychiatry Res. 2000;99:1–13. doi: 10.1016/s0925-4927(00)00054-8. [DOI] [PubMed] [Google Scholar]
- Smith SM, Miller KL, Moeller S, Xu J, Auerbach EJ, Woolrich MW, Beckmann CF, Jenkinson M, Andersson J, Glasser MF, Van Essen DC, Feinberg DA, Yacoub ES, Ugurbil K. Temporally-independent functional modes of spontaneous brain activity. Proc. Natl. Acad. Sci. U. S. A. 2012;109:3131–3136. doi: 10.1073/pnas.1121329109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada CS, Angstadt M, McNamara P, King AC, Phan KL. Effects of alcohol on brain responses to social signals of threat in humans. Neuroimage. 2011;55:371–380. doi: 10.1016/j.neuroimage.2010.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava V, Buzas B, Momenan R, Oroszi G, Pulay AJ, Enoch MA, Hommer DW, Goldman D. Association of SOD2, a mitochondrial antioxidant enzyme, with gray matter volume shrinkage in alcoholics. Neuropsychopharmacology. 2010;35:1120–1128. doi: 10.1038/npp.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, Hawkins M, Rao SM, Bandettini PA, Bloom AS. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am. J. Psychiatry. 1998;155:1009–1015. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neuroimaging of the Wernicke-Korsakoff syndrome. Alcohol Alcohol. 2009;44:155–165. doi: 10.1093/alcalc/agn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol. Clin. Exp. Res. 2000;24:611–621. [PubMed] [Google Scholar]
- Tolentino NJ, Wierenga CE, Hall S, Tapert SF, Paulus MP, Liu TT, Smith TL, Schuckit MA. Alcohol effects on cerebral blood flow in subjects with low and high responses to alcohol. Alcohol. Clin. Exp. Res. 2011;35:1034–1040. doi: 10.1111/j.1530-0277.2011.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TE, Bucholz R, Chang A, Chen L, Corbetta M, Curtiss SW, Della Penna S, Feinberg D, Glasser MF, Harel N, Heath AC, Larson-Prior L, Marcus D, Michalareas G, Moeller S, Oostenveld R, Petersen SE, Prior F, Schlaggar BL, Smith SM, Snyder AZ, Xu J, Yacoub E. The Human Connectome Project: a data acquisition perspective. Neuroimage. 2012;62:2222–2231. doi: 10.1016/j.neuroimage.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wolf AP, Logan J, Fowler JS, Christman D, Dewey SL, Schlyer D, Burr G, Vitkun S, et al. Acute effects of ethanol on regional brain glucose metabolism and transport. Psychiatry Res. 1990;35:39–48. doi: 10.1016/0925-4927(90)90007-s. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Kim SW, Wang GJ, Alexoff D, Logan J, Muench L, Shea C, Telang F, Fowler JS, Wong C, Benveniste H, Tomasi D. Acute alcohol intoxication decreases glucose metabolism but increases acetate uptake in the human brain. Neuroimage. 2013;64:277–283. doi: 10.1016/j.neuroimage.2012.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Ma Y, Zhu W, Fowler JS, Li J, Rao M, Mueller K, Pradhan K, Wong C, Wang GJ. Moderate doses of alcohol disrupt the functional organization of the human brain. Psychiatry Res. 2008;162:205–213. doi: 10.1016/j.pscychresns.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould L, Adler SS, Guynn RW, Overall JE, Dewey S. Effects of acute alcohol intoxication on cerebral blood flow measured with PET. Psychiatry Res. 1988;24:201–209. doi: 10.1016/0165-1781(88)90063-7. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Franceschi D, Fowler JS, Thanos PP, Maynard L, Gatley SJ, Wong C, Veech RL, Kunos G, Kai Li T. Low doses of alcohol substantially decrease glucose metabolism in the human brain. Neuroimage. 2006;29:295–301. doi: 10.1016/j.neuroimage.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J. Neurosci. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Franceschi D, Wong CT, Pappas NR, Netusil N, Zhu W, Felder C, Ma Y. Alcohol intoxication induces greater reductions in brain metabolism in male than in female subjects. Alcohol. Clin. Exp. Res. 2003;27:909–917. doi: 10.1097/01.ALC.0000071740.56375.BA. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Franceschi D, Fowler JS, Thanos PK, Scherbaum N, Pappas N, Wong CT, Hitzemann RJ, Felder CA. Regional brain metabolism during alcohol intoxication. Alcohol. Clin. Exp. Res. 2000;24:822–829. [PubMed] [Google Scholar]
- Wilkinson D, Halligan P. The relevance of behavioural measures for functional-imaging studies of cognition. Nat. Rev. Neurosci. 2004;5:67–73. doi: 10.1038/nrn1302. [DOI] [PubMed] [Google Scholar]
- Yeh PH, Simpson K, Durazzo TC, Gazdzinski S, Meyerhoff DJ. Tract-Based Spatial Statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: abnormalities of the motivational neurocircuitry. Psychiatry Res. 2009;173:22–30. doi: 10.1016/j.pscychresns.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Constantinescu CC, Kareken DA, Normandin MD, Cheng TE, O’Connor SJ, Morris ED. Heterogeneous effects of alcohol on dopamine release in the striatum: a PET study. Alcohol. Clin. Exp. Res. 2007;31:965–973. doi: 10.1111/j.1530-0277.2007.00390.x. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Kaufman KL, Harper CG. Clinical and pathological features of alcohol-related brain damage. Nat. Rev. Neurol. 2011;7:284–294. doi: 10.1038/nrneurol.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Orduna J, Luong R, Sullivan EV, Pfefferbaum A. A mechanism of rapidly reversible cerebral ventricular enlargement independent of tissue atrophy. Neuropsychopharmacology. 2013;38(6):1121–1129. doi: 10.1038/npp.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoethout RW, Delgado WL, Ippel AE, Dahan A, van Gerven JM. Functional biomarkers for the acute effects of alcohol on the central nervous system in healthy volunteers. Br. J. Clin. Pharmacol. 2011;71:331–350. doi: 10.1111/j.1365-2125.2010.03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]