Abstract
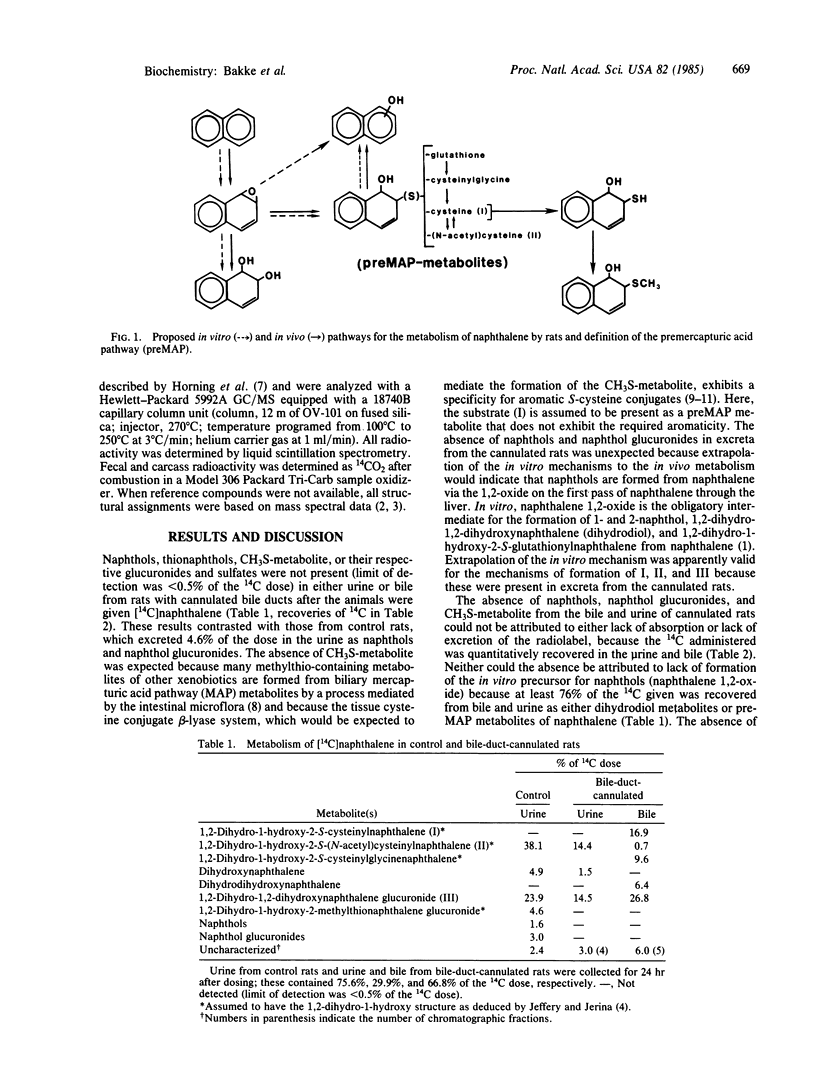
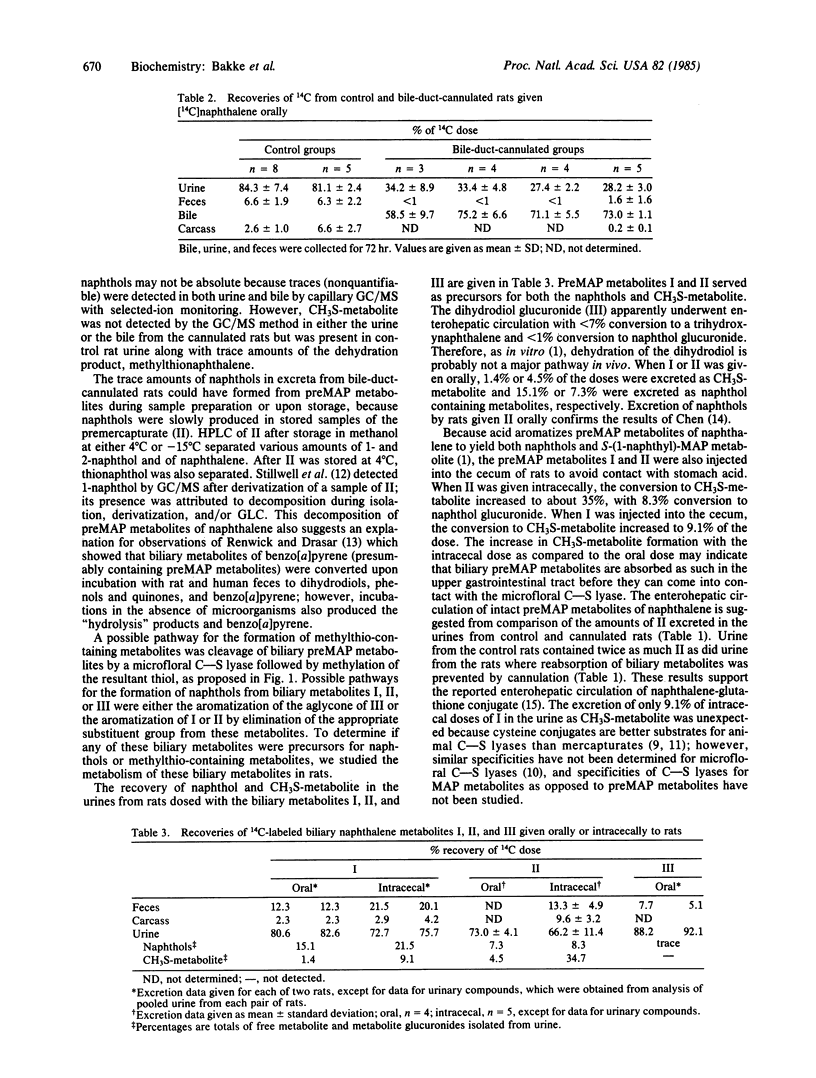
[14C]Naphthalene was given orally to rats with cannulated bile ducts and to germ-free rats. Bile and urine from the cannulated rats and urine from the germ-free rats contained no radioactive 1,2-dihydro-1-hydroxy-2-methylthionaphthalene and only trace amounts of radioactive naphthols or naphthol conjugates. Urine of control rats contained 4.6% of the 14C dose as naphthols and/or naphthol glucuronides. Appreciable quantities of 1- and 2-naphthol (7-20% of dose) and 1,2-dihydro-1-hydroxy-2-methylthionaphthalene (1-35% of dose) were in urine from rats dosed orally or intracecally with 1,2-dihydro-1-hydroxy-2-S-cysteinylnaphthalene and 1,2-dihydro-1-hydroxy-2-S-(N-acetyl)cysteinylnaphthalene. Apparently, in vivo, naphthols and methylthio-containing metabolites of naphthalene are formed during enterohepatic circulation of 1,2-dihydro-1-hydroxy-2-S-cysteinylnaphthalene and 1,2-dihydro-1-hydroxy-2-S-(N-acetyl)cysteinylnaphthalene in a process dependent upon intestinal microflora. A possible pathway for the formation of naphthols is aromatization of the precursor compounds by elimination of the appropriate substituent group from these metabolites. This discovery of the essential role of the intestinal microflora in the formation of naphthols from naphthalene indicates the existence of a novel pathway for hydroxylation of aromatic systems and challenges the current concept of the in vivo relevance of the in vitro production of naphthols from naphthalene 1,2-oxide.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakke J. E., Feil V. J., Struble C. Fragmentation patterns of trimethylsilyl derivatives of dihydrodiol glucuronides produced by metabolism of naphthalene and 1-methyl-N-naphthylcarbamate. Biomed Mass Spectrom. 1982 Jun;9(6):246–251. doi: 10.1002/bms.1200090605. [DOI] [PubMed] [Google Scholar]
- COLUCCI D. F., BUYSKE D. A. THE BIOTRANSFORMATION OF A SULFONAMIDE TO A MERCAPTAN AND TO MERCAPTURIC ACID AND GLUCURONIDE CONJUGATES. Biochem Pharmacol. 1965 Apr;14:457–466. doi: 10.1016/0006-2952(65)90218-2. [DOI] [PubMed] [Google Scholar]
- GUSTAFSSON B. E. Lightweight stainless steel systems for rearing germfree animals. Ann N Y Acad Sci. 1959 May 8;78:17–28. doi: 10.1111/j.1749-6632.1959.tb53092.x. [DOI] [PubMed] [Google Scholar]
- Horning M. G., Stillwell W. G., Griffin G. W., Tsang W. S. Epoxide intermediates in the metabolism of naphthalene by the rat. Drug Metab Dispos. 1980 Nov-Dec;8(6):404–414. [PubMed] [Google Scholar]
- Jeffery A. M., Jerina D. M. Letter: Novel rearraNgements during dehydration of nucleophile adducts of arene oxides. A reappraisal of premercapturic acid structures. J Am Chem Soc. 1975 Jul 23;97(15):4427–4428. doi: 10.1021/ja00848a065. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Witkop B., Zaltzman-Nirenberg P., Udenfriend S. 1,2-naphthalene oxide as an intermediate in the microsomal hydroxylation of naphthalene. Biochemistry. 1970 Jan 6;9(1):147–156. doi: 10.1021/bi00803a019. [DOI] [PubMed] [Google Scholar]
- Renwick A. G., Drasar B. S. Environmental carcinogens and large bowel cancer. Nature. 1976 Sep 16;263(5574):234–235. doi: 10.1038/263234a0. [DOI] [PubMed] [Google Scholar]
- Stillwell W. G., Bouwsma O. J., Horning M. G. Formation in vivo of deuterated methylthio metabolites of naphthalene from L-methionine (methyl-d--3). Res Commun Chem Pathol Pharmacol. 1978 Nov;22(2):329–343. [PubMed] [Google Scholar]
- Stillwell W. G., Horning M. G., Griffin G. W., Tsang W. S. Identification of new sulfur-containing metabolites of naphthalene in mouse urine. Drug Metab Dispos. 1982 Nov-Dec;10(6):624–631. [PubMed] [Google Scholar]
- Tateishi M., Suzuki S., Shimizu H. Cysteine conjugate beta-lyase in rat liver. A novel enzyme catalyzing formation of thiol-containing metabolites of drugs. J Biol Chem. 1978 Dec 25;253(24):8854–8859. [PubMed] [Google Scholar]



