Abstract
Context
Cancer dramatically impacts human life expectancy and quality of life. Natural substances from vegetables, herbs and spices could be beneficial in the prevention or treatment of a variety of cancers. Crocus sativus, which has been used as a folk medicine for treating diseases for ages, showed obvious cancer chemoprevention potential.
Objective
This article focuses on the effects of Crocus sativus and its main ingredients, such as crocin, on cancer therapeutics.
Methods
We reviewed research data from saffron, a spice derived from the flower of Crocus sativus, and its constituents using the major databases, viz., Web of Science, SciFinder, and PubMed.
Results and conclusion
Saffron possesses free radical-scavenging properties and antitumor activities. Significant cancer chemopreventive effects have been shown in both in vitro and in vivo models. Based on current data, saffron and its ingredients could be considered as a promising candidate for clinical anticancer trials.
Keywords: Crocus sativus, saffron, crocin, natural substances, dietary supplements, cytotoxicity, anticancer, chemoprevention
Introduction
Cancer is one of the largest health threats to humans, claiming millions of lives each year. If cancer can be detected in its early stage, surgical intervention may be applicable as an efficacious therapeutic measure. Nevertheless, for better outcomes, many patients still need additional treatments such as chemotherapy and radiotherapy. In most cases, regular chemotherapy is unable to achieve satisfactory effects because of its severe side effects and dose-limiting toxicity. Even newly designated drug therapies with specific tumor targets are reported with many undesirable adverse effects (Venook, 2005; Wang et al., 2012). To date, no ideal approach has been found to obtain satisfactory effects against cancer.
An appropriate strategy for cancer prevention or treatment could be a combined approach, including the application of synthetic or natural agents to inhibit cancer development (Yang et al., 2011; Wang et al., 2012). Growing evidence shows that plants, such as vegetables, spices and herbs, have evolved as a solution for cancer chemoprevention and new drug development (Abdullaev, 2001; Johnson et al., 2011; Wu et al., 2012). Compared to traditional cancer therapies, natural remedies have advantages, including little or no toxicity and low cost (Lee & Park, 2003; Garodia et al., 2007; Xu et al., 2011). Herbal medications have already been used as an alternative treatment in cancer patients (Randhawa & Alghamdi, 2011; Wang et al., 2012). On an epidemiological basis, long-term consumption of certain botanicals, such as Asian ginseng, has been associated with reduced cancer incidence (Yun et al., 2010). The anticancer effects of ginseng have also been shown in experimental studies (Attele et al., 1999; Hwang et al., 2012). Research that explores new botanical candidates with potential anticancer effects is imperative, and it supplies new data for developing safer and efficacious anticancer therapies (Hemaiswarya & Doble, 2006; Lin et al., 2012).
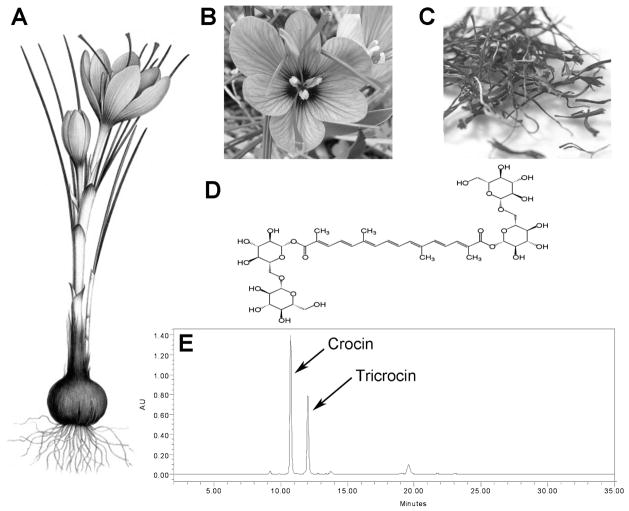
Crocus sativus, a plant in the iris family (Iridaceae), is mainly grown from the Mediterranean Sea through Persia to India, Tibet and other regions in China. Its flower contains various chemical constituents (Abdullaev & Espinosa-Aguirre, 2004), which have been used as a folk medicine for a long time (Figure 1). Saffron is a spice derived from the stigma of the flower of Crocus sativus, commonly known as the saffron crocus.
Figure 1.
Botanical and phytochemical profiles of Crocus sativus. Morphological characteristics of Crocus sativus plant (A), flower (B), and stigma (C). Chemical structure of a major compound, crocin (D), and chromatogram of saffron recorded at 442 nm (E) (Aung et al., 2007).
Saffron has served as antispasmodic, sedative, stomachic, stimulant and emmenagogue. Saffron contains crocin, crocetin, carotene and lycopene (Giaccio, 2004), and these compounds have a variety of pharmacological effects on different medical conditions, including antitumor effects via inhibition of cell growth (Abdullaev, 1994; Aung et al., 2007). Previous studies have shown that crocetin and crocetin di-glucose ester scavenged free radicals, especially superoxide anions, and thus protect cells from oxidative stress (Bors et al., 1982; Erben-Russ et al., 1987), responsible for many neurodegenerative disorders. Studies have also demonstrated that saffron extract and its major constituent, crocin, may have activities against various malignancies in addition to other pharmacological activities (Abdullaev, 2003; Aung et al., 2007).
Phytochemical composition of saffron
Saffron is characterized by its specific features including bitter taste, aromatic smell and intense red color. Its bitter taste originates from picrocrocin, a β-D-glucoside of hydroxysafranal. This bitter flavor substance can be crystallized and produces glucose and aldehyde safranal by hydrolysis (Wintherhalter & Straubinger, 2000). Two of the principal coloring pigments are crocin and tricrocin (Figure 1), which are easily soluble in water. In addition to the two compounds, saffron contains crocetin as a free agent and other small amounts of pigment, such as anthocianin, α-carotene, β-carotene, and zegxantin (Tarantilis & Polissiou, 1997; Abdulaev, 2003). The main aroma factor in saffron is safranal, which comprises about 60% of the volatile components of saffron. In fresh saffron, this substance exists as a stable picrocrocin. However, with heat and time it decomposes and releases the volatile aldehyde and safranal (Tarantilis & Polissiou, 1997).
Quality controlled saffron contains about 30% crocins, 5 to 15% picrocrocin and usually up to 2.5% of volatile compounds including safranal. A requirement for mature saffron is the analytical determination of picrocrocin, safranal and total crocin (Schmidt et al., 2007). The toxic effect of saffron has been found to be quite low. Animal studies indicated that the oral LD50 of saffron was approximately 20 g/kg (Abdulaev, 2003).
Cancer chemopreventive effects of saffron and its components
Studies of the effects of saffron on malignant cells have gathered attractive data. Increasing evidence indicates that saffron and its characteristic components possess antitumor activities using in vitro and in vivo models.
Cytotoxicity and cell inhibitory effects of saffron in vitro
Saffron had selective cytotoxic effects against tumor cells instead of healthy cells with a relatively low concentration range (Abdullaev, 2003; Schmidt et al., 2007). Incubation of HeLa cells with saffron extract resulted in obvious inhibition of colony formation and cellular DNA and RNA synthesis, with IC50 at 100–150 μg/ml (Abdullaev & Frenkel, 1992). A concentration-dependent inhibition of colony formation was observed in tumor cells, whereas proliferation of differentiation of normal cells remained unaffected. In other study using cancer cell lines A-549, WI-38 and VA-13 (SV-40 modified fetal lung fibroblasts), saffron extract showed much more sensitive effects on malignant cells than on normal cells. The crocetin, which was isolated from saffron, had an inhibitory effect on intracellular nucleic acid and protein synthesis in three malignant human cell lines, HeLa, A-549, and VA-13, but had no effect on colony formation (El-Daly, 1998).
Investigators also described the growth inhibition of human chronic myelogenous leukemia cells K562 and promyelocytic leukemia cells HL-60 by dimethyl-crocetin, crocetin, and crocin with ID50 at 0.8–2.0 μM (Morjani et al., 1990; Tarantilis et al., 1994). Cytotoxicity of dimethyl-crocetin and crocin on various cancer cell lines and human cancer cells obtained from surgical specimens (osteosarcoma, fibrosarcoma, and ovarian carcinoma) has also been reported (Nair et al., 1995).
Saffron has been found to have non-mutagenic and non-antimutagenic activities against BP-induced mutagenicity. In the in vitro colony formation test system, saffron displayed a concentration-dependent inhibitory effect only against human malignant cells (Abdullaev et al., 2003). Aung et al. (2007) demonstrated that Crocus sativus and its major constituent, crocin, significantly inhibited the growth of colorectal cancer cell lines (HCT-116, HT-29, SW-480) and non-small cell lung cancer cell line (NSCLC). However, the extract did not affect non-cancer young adult mouse colon cells (YAMC) at concentrations used to inhibit malignant cells.
Extracts of different crocus species have been shown to inhibit cell proliferation in MDA-MB-231 and MCF-7 breast cancer cell lines, and this effect was independent of the status of the estrogen receptor (Chryssanthi et al., 2007). Another study showed that saffron extract and crocetin had a clear binding capacity at the PCP binding site of the NMDA receptor and at the sigma-1 receptor, while the crocins and picrocrocin had no effective binding effect (Lechtenberg et al., 2008). Moreover, saffron extract showed inhibitory effects on the human TCC 5637 cell line and mouse fibroblast cell line (L929) (Feizzadeh et al., 2008). Subsequently, another report demonstrated that saffron extract decreased cell viability after 48-h incubation in the MCF-7 breast cancer cell line. The saffron induced cell apoptosis was inhibited by pan-caspase inhibitor, which prompted its pro-apoptotic function (Mousavi et al., 2009).
Cancer chemopreventive effects of saffron in vivo
Saffron extract inhibited the initiation/promotion of 7,12-dimethylbenz [a] anthracene (DMBA)-induced skin tumors in mice, delaying the onset of papilloma formation and reducing the mean number of papillomas (Salomi et al., 1991). The oral administration of the same dose of saffron extract restricted the incidence of 20-methylcholanthrene (MCA)-induced soft tissue sarcomas in mice (Salomi et al., 1991).
Extract from saffron stigmas prolonged the life span of cisplatin-treated mice and partially regulated the decrease in body weight, leukocyte count and hemoglobin levels (Salomi et al., 1991; Nair et al., 1993, 1994). The protective effect of administration of a combined recipe with cysteine, vitamin E and saffron extract was shown against cisplatin-induced toxicity in rats (El-Daly, 1998). It was suggested that saffron rich in carotenoids might exert its chemopreventive effects by the modulation of lipid peroxidation, antioxidants, and a detoxification system. Crocetin from saffron also ameliorates bladder toxicity of the anticancer agent cyclophosphamide without altering its antitumor activity. The treatment with cysteine together with saffron extract in animals significantly reduced the toxic effects caused by cisplatin, such as nephrotoxicity and changes in enzyme activity (Nair et al., 1995; El-Daly, 1998).
Saffron treatment significantly reduced blood urea nitrogen, serum creatine level, blood glucose level and prevented many changes in serum enzyme activities. Pretreatment with the aqueous extract of saffron significantly inhibited the genotoxicity of cisplatin, cyclophosphamide, mitomycin, and urethane (Premkumar et al., 2001, 2003).
Regarding the tumoricidal effects, saffron was more active by oral administration. The effect might be improved by liposome encapsulation of the drug. Liposome encapsulation of saffron produced a significant inhibitory effect on the growth of transplanted tumor cells in mice (Nair et al., 1992). Another study showed that liposome encapsulation of saffron effectively enhanced its antitumor activity against S-180 and EAC solid tumors in mice (Nair et al., 1991). Oral administration of the saffron extract increased the life span of mice transplanted with tumors. Further study demonstrated that crocetin was effective in treating certain types of cancer treatable with all-trans retinoic acids (ATRA) in frog embryos. It was suggested that crocetin might be a safe alternative to treat ATRA-sensitive cancers in women of childbearing age (Martin et al., 2002).
The effects of long-term crocin treatment were evaluated in a rat model bearing colorectal tumors, induced by DHD/K12-PROb cells (rat adenocarcinoma cell line) injected subcutaneously. Crocin significantly increased survival time and decreased tumor growth, even more intensely in females (Garcia-Olmo et al., 1999). Saffron could play a protective role as an anti-genotoxic, antioxidant factor, which could be used as an adjuvant in chemotherapeutic medications (Prekumar et al., 2006). Evidence showed that another constituent of saffron, crotein, increased the serum level of lipid peroxidation and other marker enzymes, thus reversing the carcinogen induced lung cancer model to near normal conditions (Magesh et al., 2006). Subsequent research showed activities of saffron and crocin on pancreatic cancer (Dhar et al., 2009; Bakshi et al., 2010) and Dalton’s lymphoma (Bakshi et al., 2009) in animal models.
Mechanisms of saffron for cancer chemoprevention
Several early hypotheses of antitumor activities of saffron and its components included the inhibitory effect of saffron on cellular DNA and RNA synthesis (Nair et al., 1995), the inhibitory effect on free radical chain reactions (Tseng et al., 1995), and that the saffron extract exerted the metabolic conversion of naturally occurring carotenoids into retinoids (Dufresne et al., 1997; Bors et al., 1982). However, another report indicated that the conversion of carotenoids to vitamin A was not a prerequisite procedure for the anticancer activity (Smith, 1998). Relatively, it was due to the interaction of carotenoids with topoisomerase II, an enzyme involved in multi cellular DNA-protein interactions. In addition, a glucoconjugate isolated from corm and callus of saffron could cause swelling and local plasma membrane evagination, which might address that cytotoxicity was mediated via extracellular fluid uptake (Escribano et al., 1999).
Another report showed that saffron contains lectins, which suggested that the antitumor activity of saffron is mediated by lectins (Escribano et al., 1999). Treatment of tumor cells with saffron resulted in an increase in the level of intracellular sulfhydryl compounds, and chemical investigation of γ-irradiated saffron was also conducted (Zareena et al., 2001). Recently, Amin et al. (2011) showed that saffron exerted a significant chemopreventive effect against liver cancer through inhibition of cell proliferation and induction of apoptosis. However, to date, the exact anticancer mechanisms of saffron and its main constituents are still largely unclear, and further studies are needed.
Summary and perspectives
Cancer chemoprevention, which can involve pharmacological intervention using synthetic and naturally originated agents alone or in combination, is a practical method for fighting against malignancies. Considerable evidence has suggested that plant-based dietary agents can inhibit the process of carcinogenesis effectively. Since ancient times, saffron was used as a folk medicine to treat different kinds of diseases including cancer (Li et al., 2004). A number of in vivo and in vitro experiments discussed above suggest that saffron and its main ingredients have great potential to reduce the risk of developing different types of cancer. Several monoterpenoids and a novel naturally occurring acid were isolated from the petals of saffron. Among them, three identified compounds, including crocusatin H, showed significant tyrosine inhibitory activity (Li & Wu, 2002), which could give us more opportunities to identify the effects of saffron.
Many herbal medicines with potential cancer chemopreventive effects have been reported recently (Zakaria et al., 2011; Lai et al., 2011; Kim et al., 2012; Shen et al., 2012). In addition to tumoricidal effects, anti-angiogenesis activity may also play a role in cancer chemoprevention (Lin et al. 2012). For those patients undergoing cancer chemotherapy, the efficacy and safety of botanicals has been evaluated (Yaal-Hahoshen et al., 2011; Yamaguchi et al., 2011). The effects of saffron have not yet been evaluated in human clinical trials. It would be interesting to compare the effects of saffron with other studied herbs in cancer patients. However, before saffron can be used in controlled clinical trials, more studies should be conducted, including the determination of active components in saffron and the mechanisms involved in cancer chemoprevention.
Acknowledgments
This work was supported in part by the NIH grants P01 AT004418 and K01 AT005362.
Footnotes
Declaration of interest
The authors declare no conflict of interest.
References
- Abdullaev FI, Frenkel GD. Effect of saffron on cell colony formation and cellular nucleic acid and protein synthesis. Biofactors. 1992;3:201–204. [PubMed] [Google Scholar]
- Abdullaev FI. Inhibitory effect of crocetin on intracellular nucleic acid and protein synthesis in malignant cells. Toxicol Lett. 1994;70:243–251. doi: 10.1016/0378-4274(94)90168-6. [DOI] [PubMed] [Google Scholar]
- Abdullaev FI. Plant-derived agents against cancer. In: Gupta SK, editor. Pharmacology and Therapeutics in the New Millennium. Narosa Publishing House; New Delhi: 2001. pp. 345–354. [Google Scholar]
- Abdullaev FI. Crocus sativus against cancer. Arch Med Res. 2003;34:354–363. doi: 10.1016/S0188-4409(03)00048-1. [DOI] [PubMed] [Google Scholar]
- Abdullaev FI, Espinosa-Aguirre JJ. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect Prev. 2004;28:426–432. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Amin A, Hamza AA, Bajbouj K, Ashraf SS, Daoud S. Saffron: A potential candidate for a novel anticancer drug against hepatocellular carcinoma. Hepatology. 2011;54:857–867. doi: 10.1002/hep.24433. [DOI] [PubMed] [Google Scholar]
- Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–93. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- Aung HH, Wang CZ, Ni M, Fishbein A, Mehendale SR, Xie JT, Shoyama CY, Yuan CS. Crocin from Crocus sativus possesses significant anti-proliferation effects on human colorectal cancer cells. Ex Oncol. 2007;29:175–180. [PMC free article] [PubMed] [Google Scholar]
- Bakshi HA, Sam S, Feroz A, Ravesh Z, Shah GA, Sharma M. Crocin from Kashmiri saffron (Crocus sativus) induces in vitro and in vivo xenograft growth inhibition of Dalton’s lymphoma (DLA) in mice. Asian Pac J Cancer Prev. 2009;10:887–890. [PubMed] [Google Scholar]
- Bakshi H, Sam S, Rozati R, Sultan P, Islam T, Rathore B, Lone Z, Sharma M, Triphati J, Saxena RC. DNA fragmentation and cell cycle arrest: A hallmark of apoptosis induced by crocin from kashmiri saffron in a human pancreatic cancer cell line. Asian Pac J Cancer Prev. 2010;11:675–9. [PubMed] [Google Scholar]
- Bors W, Saran M, Michel C. Radical intermediates involved in the bleaching of the carotenoid crocin. Hydroxyl radicals, superoxide anions and hydrated electrons. Int J Radiat Biol Relat Stud Phys Chem Med. 1982;41:493–501. doi: 10.1080/09553008214550571. [DOI] [PubMed] [Google Scholar]
- Chryssanthi DG, Lamari FN, Iatrou G, Pylara A, Karamanos NK, Cordopatis P. Inhibition of breast cancer cell proliferation by style constituents of different Crocus species. Anticancer Res. 2007;27:357–362. [PubMed] [Google Scholar]
- Dhar A, Mehta S, Dhar G, Dhar K, Banerjee S, Van Veldhuizen P, Campbell DR, Banerjee SK. Crocetin inhibits pancreatic cancer cell proliferation and tumor progression in a xenograft mouse model. Mol Cancer Ther. 2009;8:315–323. doi: 10.1158/1535-7163.MCT-08-0762. [DOI] [PubMed] [Google Scholar]
- Dufresne C, Cormier F, Dorion S. In vitro formation of crocetin glucosyl esters by Crocus sativus callus extract. Planta Med. 1997;63:150–153. doi: 10.1055/s-2006-957633. [DOI] [PubMed] [Google Scholar]
- El-Daly ES. Protective effect of cysteine and vitamin E, Crocus sativus and Nigella sativa extracts on cisplatin-induced toxicity in rats. J Pharm Belg. 1998;53:93–95. [PubMed] [Google Scholar]
- Erben-Russ M, Michel C, Bors W, Saran M. The reaction of sulfite radical anion with nucleic acid components. Free Radic Res Commun. 1987;2:289–294. doi: 10.3109/10715768709065293. [DOI] [PubMed] [Google Scholar]
- Escribano J, Piqueras A, Medina J, Rubio A, Alvarez-Orti M, Fernandez JA. Production of a cytotoxic proteoglycan using callus culture of saffron corms (Crocus sativus L.) J Biotechnol. 1999;73:53–59. doi: 10.1016/s0168-1656(99)00125-x. [DOI] [PubMed] [Google Scholar]
- Feizzadeh B, Afshari JT, Rakhshandeh H, Rahimi A, Brook A, Doosti H. Cytotoxic effect of saffron stigma aqueous extract on human transitional cell carcinoma and mouse fibroblast. Urol J. 2008;5:161–167. [PubMed] [Google Scholar]
- García-Olmo DC, Riese HH, Escribano J, Ontañón J, Fernández JA, Atiénzar M, García-Olmo D. Effects of long-term treatment of colon adenocarcinoma with crocin, a carotenoid from saffron (Crocus sativus L.): An experimental study in the rat. Nutr Cancer. 1999;35:120–126. doi: 10.1207/S15327914NC352_4. [DOI] [PubMed] [Google Scholar]
- Garodia P, Ichikawa H, Malani N, Sethi G, Aggarwal BB. From ancient medicine to modern medicine: Ayurvedic concepts of health and their role in inflammation and cancer. J Soc Integr Oncol. 2007;5:25–37. doi: 10.2310/7200.2006.029. [DOI] [PubMed] [Google Scholar]
- Giaccio M. Crocetin from saffron: An active component of an ancient spice. Crit Rev Food Sci Nutr. 2004;44:155–172. doi: 10.1080/10408690490441433. [DOI] [PubMed] [Google Scholar]
- Hemaiswarya S, Doble M. Potential synergism of natural products in the treatment of cancer. Phytother Res. 2006;20:239–249. doi: 10.1002/ptr.1841. [DOI] [PubMed] [Google Scholar]
- Hwang JW, Oh JH, Yoo HS, Lee YW, Cho CK, Kwon KR, Yoon JH, Park J, Her S, Lee ZW, Jang IS, Choi JS. Mountain ginseng extract exhibits anti-lung cancer activity by inhibiting the nuclear translocation of NF-κB. Am J Chin Med. 2012;40:187–202. doi: 10.1142/S0192415X12500152. [DOI] [PubMed] [Google Scholar]
- Johnson TE, Hermanson D, Wang L, Kassie F, Upadhyaya P, O’Sullivan MG, Hecht SS, Lu J, Xing C. Lung tumorigenesis suppressing effects of a commercial kava extract and its selected compounds in A/J mice. Am J Chin Med. 2011;39:727–42. doi: 10.1142/S0192415X11009202. [DOI] [PubMed] [Google Scholar]
- Kim SH, Huang CY, Tsai CY, Lu SY, Chiu CC, Fang K. The aqueous extract of Prunella vulgaris suppresses cell invasion and migration in human liver cancer cells by attenuating matrix metalloproteinases. Am J Chin Med. 2012;40:643–656. doi: 10.1142/S0192415X12500486. [DOI] [PubMed] [Google Scholar]
- Lai ZR, Ho YL, Huang SC, Huang TH, Lai SC, Tsai JC, Wang CY, Huang GJ, Chang YS. Antioxidant, anti-inflammatory and antiproliferative activities of Kalanchoe gracilis (L.) DC stem. Am J Chin Med. 2011;39:1275–90. doi: 10.1142/S0192415X1100955X. [DOI] [PubMed] [Google Scholar]
- Lechtenberg M, Schepmann D, Niehues M, Hellenbrand N, Wünsch B, Hensel A. Quality and functionality of saffron: Quality control, species assortment and affinity of extract and isolated saffron compounds to NMDA and sigma1 (sigma-1) receptors. Planta Med. 2008;74:764–772. doi: 10.1055/s-2008-1074535. [DOI] [PubMed] [Google Scholar]
- Lee BM, Park KK. Beneficial and adverse effects of chemopreventive agents. Mutat Res. 2003:523–524. 265–278. doi: 10.1016/s0027-5107(02)00342-1. [DOI] [PubMed] [Google Scholar]
- Li CY, Wu TS. Constituents of the stigmas of Crocus sativus and their tyrosine inhibitory activity. J Nat Prod. 2002;65:1452–1456. doi: 10.1021/np020188v. [DOI] [PubMed] [Google Scholar]
- Li CY, Lee EJ, Wu TS. Antitirosine principles and constituents of the petals of Crocus sativus. J Nat Prod. 2004;67:437–440. doi: 10.1021/np0302854. [DOI] [PubMed] [Google Scholar]
- Lin CM, Chen YH, Ong JR, Ma HP, Shyu KG, Bai KJ. Functional role of wogonin in anti-angiogenesis. Am J Chin Med. 2012;40:415–27. doi: 10.1142/S0192415X12500322. [DOI] [PubMed] [Google Scholar]
- Magesh V, Singh JP, Selvendiran K, Ekambaram G, Sakthisekaran D. Antitumour activity of crocetin in accordance to tumor incidence, antioxidant status, drug metabolizing enzymes and histopathological studies. Mol Cell Biochem. 2006;287:127–135. doi: 10.1007/s11010-005-9088-0. [DOI] [PubMed] [Google Scholar]
- Martin G, Goh E, Neff AW. Evaluation of the developmental toxicity of crocetin on Xenopus. Food Chem Toxicol. 2002;40:959–964. doi: 10.1016/s0278-6915(02)00040-6. [DOI] [PubMed] [Google Scholar]
- Morjani H, Tarantilis P, Polissiou M, Manfait M. Growth inhibition and induction of crythroid differentiation activity by crocin, dimethylcrocetine and β-carotene on K562 tumor cells. Anticancer Res. 1990;10:1398–1406. [Google Scholar]
- Mousavi SH, Tavakkol-Afshari J, Brook A, Jafari-Anarkooli I. Role of caspases and Bax protein in saffron-induced apoptosis in MCF-7 cells. Food Chem Toxicol. 2009;47:1909–1913. doi: 10.1016/j.fct.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Nair SC, Panikkar B, Panikkar KR. Antitumor activity of saffron (Crocus sativus) Cancer Lett. 1991;57:109–114. doi: 10.1016/0304-3835(91)90203-t. [DOI] [PubMed] [Google Scholar]
- Nair SC, Salomi MJ, Varghese CD, Panikkar B, Panikkar KR. Effect of saffron on thymocute proliferation, intracellular gluthation levels and its antitumor activity. BioFactors. 1992;4:51–54. [PubMed] [Google Scholar]
- Nair SC, Panikkar KR, Parathod RK. Protective effects of crocetin on bladder cytotoxicity induced by cyclophosphamide. Cancer Biother. 1993;8:339–343. doi: 10.1089/cbr.1993.8.339. [DOI] [PubMed] [Google Scholar]
- Nair SC, Varghese CD, Pannikar KR, Kurumboor SK, Parathod RK. Effects of saffron in vitamin A levels and its antitumor activity on the growth of solid tumors in mice. Int J Pharmacog. 1994;32:105–114. [Google Scholar]
- Nair SC, Kurumboor SK, Hasegawa JH. Saffron chemoprevention in biology and medicine: A review. Cancer Biother. 1995;10:257–264. doi: 10.1089/cbr.1995.10.257. [DOI] [PubMed] [Google Scholar]
- Premkumar K, Abraham SK, Santhiya ST, Gopinath PM, Ramesh A. Inhibition of genotoxicity by saffron (Crocus sativus L.) in mice. Drug Chem Toxicol. 2001;24:421–428. doi: 10.1081/dct-100106266. [DOI] [PubMed] [Google Scholar]
- Premkumar K, Abraham SK, Santhiya ST, Ramesh A. Protective effects of saffron (Crocus sativus L.) on genotoxin-induced oxidative stress in Swiss mice. Phytother Res. 2003;17:614–617. doi: 10.1002/ptr.1209. [DOI] [PubMed] [Google Scholar]
- Premkumar K, Thirunavukkarasu C, Abraham SK, Santhiya ST, Ramesh A. Protective effect of saffron (Crocus sativus L.) aqueous extract against genetic damage induced by anti-tumor agents in mice. Hum Exp Toxicol. 2006;25:79–84. doi: 10.1191/0960327106ht589oa. [DOI] [PubMed] [Google Scholar]
- Randhawa MA, Alghamdi MS. Anticancer activity of Nigella sativa (black seed) - a review. Am J Chin Med. 2011;39:1075–91. doi: 10.1142/S0192415X1100941X. [DOI] [PubMed] [Google Scholar]
- Salomi MJ, Nair SC, Panikkar KR. Inhibitory effects of Nigella sativa and saffron (Crocus sativus) on chemical carcinogenesis in mice. Nutr Cancer. 1991;16:67–72. doi: 10.1080/01635589109514142. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Betti G, Hensel A. Saffron in phytotherapy: Pharmacology and clinical uses. Wien Med Wochenschr. 2007;157:315–319. doi: 10.1007/s10354-007-0428-4. [DOI] [PubMed] [Google Scholar]
- Shen KH, Chen ZT, Duh PD. Cytotoxic effect of Eucalyptus citriodora resin on human hepatoma HepG2 cells. Am J Chin Med. 2012;40:399–413. doi: 10.1142/S0192415X12500310. [DOI] [PubMed] [Google Scholar]
- Smith TA. Carotenoids and cancer: Prevention and potential therapy. Br J Biomed Sci. 1998;55:268–275. [PubMed] [Google Scholar]
- Tarantilis PA, Morjani HM, Polissiou M, Manfait M. Inhibition of growth and induction of differentiation of promyelocytic leukemia (HL-60) by carotenoids from Crocus sativus L. Anticancer Res. 1994;14:1913–1918. [PubMed] [Google Scholar]
- Tarantilis PA, Polissiou M. Isolation and identification of the aroma constituents of saffron (Crocus sativa) J Agri Food Chem. 1997;45:459–462. [Google Scholar]
- Tseng TH, Chu CY, Huang JM, Shiow SJ, Wang CJ. Crocetin protects against oxidative damage in rat primary hepatocytes. Cancer Lett. 1995;97:61–67. doi: 10.1016/0304-3835(95)03964-x. [DOI] [PubMed] [Google Scholar]
- Venook A. Critical evaluation of current treatments in metastatic colorectal cancer. Oncologist. 2005;10:250–261. doi: 10.1634/theoncologist.10-4-250. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Calway T, Yuan CS. Herbal medicines as adjuvants for cancer therapeutics. Am J Chin Med. 2012;40:657–69. doi: 10.1142/S0192415X12500498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintherhalter P, Straubinger M. Saffron – renewed interest in an ancient spice. Food Rev Int. 2000;16:39–59. [Google Scholar]
- Wu G, Qian Z, Guo J, Hu D, Bao J, Xie J, Xu W, Lu J, Chen X, Wang Y. Ganoderma lucidum extract induces g1 cell cycle arrest, and apoptosis in human breast cancer cells. Am J Chin Med. 2012;40:631–42. doi: 10.1142/S0192415X12500474. [DOI] [PubMed] [Google Scholar]
- Xu Z, Chen X, Zhong Z, Chen L, Wang Y. Ganoderma lucidum polysaccharides: Immunomodulation and potential anti-tumor activities. Am J Chin Med. 2011;39:15–27. doi: 10.1142/S0192415X11008610. [DOI] [PubMed] [Google Scholar]
- Yaal-Hahoshen N, Maimon Y, Siegelmann-Danieli N, Lev-Ari S, Ron IG, Sperber F, Samuels N, Shoham J, Merimsky O. A prospective, controlled study of the botanical compound mixture LCS101 for chemotherapy-induced hematological complications in breast cancer. Oncologist. 2011;16:1197–202. doi: 10.1634/theoncologist.2011-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Miyahara E, Hihara J. Efficacy and safety of orally administered Lentinula edodes mycelia extract for patients undergoing cancer chemotherapy: A pilot study. Am J Chin Med. 2011;39:451–9. doi: 10.1142/S0192415X11008956. [DOI] [PubMed] [Google Scholar]
- Yang SW, Wang W, Xie XY, Zhu WP, Li FQ. In vitro synergistic cytotoxic effect of triptolide combined with hydroxycamptothecin on pancreatic cancer cells. Am J Chin Med. 2011;39:121–34. doi: 10.1142/S0192415X11008695. [DOI] [PubMed] [Google Scholar]
- Yun TK, Zheng S, Choi SY, Cai SR, Lee YS, Liu XY, Cho KJ, Park KY. Non-organ-specific preventive effect of long-term administration of Korean red ginseng extract on incidence of human cancers. J Med Food. 2010;13:489–94. doi: 10.1089/jmf.2009.1275. [DOI] [PubMed] [Google Scholar]
- Zakaria ZA, Mohamed AM, Jamil NS, Rofiee MS, Hussain MK, Sulaiman MR, Teh LK, Salleh MZ. In vitro antiproliferative and antioxidant activities of the extracts of Muntingia calabura leaves. Am J Chin Med. 2011;39:183–200. doi: 10.1142/S0192415X11008749. [DOI] [PubMed] [Google Scholar]
- Zareena AV, Variar PS, Gholar AS, Bongirwar DF. Chemical investigation of gamma-irradiated saffron (Crocus sativus L.) J Agric Food Chem. 2001;49:687–691. doi: 10.1021/jf000922l. [DOI] [PubMed] [Google Scholar]