The childhood bone marrow failure disorders are a heterogeneous group of diseases of which the commonest ones are acquired severe aplastic anemia (SAA) and myelodysplastic syndromes. SAA is characterized by the presence of pancytopenia with a hypocellular marrow in the absence of malignant infiltration or fibrosis. Myelodysplastic syndromes are characterized by ineffective hematopoiesis, dyplasia and cytopenias. The commonest subset of childhood myelodysplastic syndrome is refractory cytopenia of childhood (RCC) accounting for half of all cases of childhood myelodysplastic syndrome. The majority of cases of RCC are hypocellular and distinguishing between SAA and hypocellular RCC can be challenging. Most cases of hypoplastic RCC lack a cytogenetic abnormality and conversely some patients with aplastic anemia have an abnormal cytogenetic clone. Thus careful morphological assessment is required to distinguish between SAA and RCC. Future use of next-generation sequencing to detect acquired somatic mutations typical of myeloid disorders may help to distinguish between the two conditions.1
It is postulated that, in both hypoplastic myelodysplastic syndrome and SAA, autoimmunity and T-cell-mediated suppression of hematopoiesis contribute to the pathogenesis of these disorders. In both sets of disorders, there is a plethora of data demonstrating oligoclonal T-cell expansion, leading to over-expression of cytokines, such as interferon-γ and tumor necrosis factor-α, which suppress hematopoiesis.2 In acquired SAA, there is absolute and functional deficiency of regulatory T cells.3,4 The best evidence for an immune pathogenesis is provided by the response to immune suppressive therapy (IST) with antithymocyte globulin (ATG) and cyclosporine. IST with ATG and cyclosporine is currently used as first-line treatment for children with SAA who do not have a matched sibling donor for bone-marrow transplantation. Historically, horse ATG was adopted as first line IST with rabbit ATG being used for relapse or refractory cases. Until 2007, there were two preparations of horse ATG; Lymphoglobulin (Genzyme) and ATGAM (Pfizer). Lymphoglobulin was withdrawn from the market in 2007 and because of the initial unavailability of ATGAM in most European countries, rabbit ATG (Thymoglobulin; Sanofi) was then adopted as first-line therapy. There have been numerous studies (Table 1) comparing the efficacy of rabbit ATG versus horse ATG. As described in this issue of the Journal, Jeong et al. performed a large retrospective comparison between Thymoglobulin and Lymphoglobulin in pediatric acquired SAA.5 While there was no difference in overall response rate at 6 months, patients in the horse ATG cohort had a superior overall survival and lower relapse rate. Thus so far, none of the seven published studies comparing horse ATG and rabbit ATG has demonstrated superiority of rabbit ATG.5–11 In five, rabbit ATG led to inferior overall survival or response rates5–7,9,10 and in the other two8,11 there was no significant difference between the two preparations.
Table 1.
Summary of studies of immunosuppression comparing horse ATG/cyclosporine and rabbit ATG/cyclosporine in acquired SAA. There were two horse ATG preparations (Lymphoglobulin or ATGAM). The rabbit ATG preparation was Thymoglobulin.
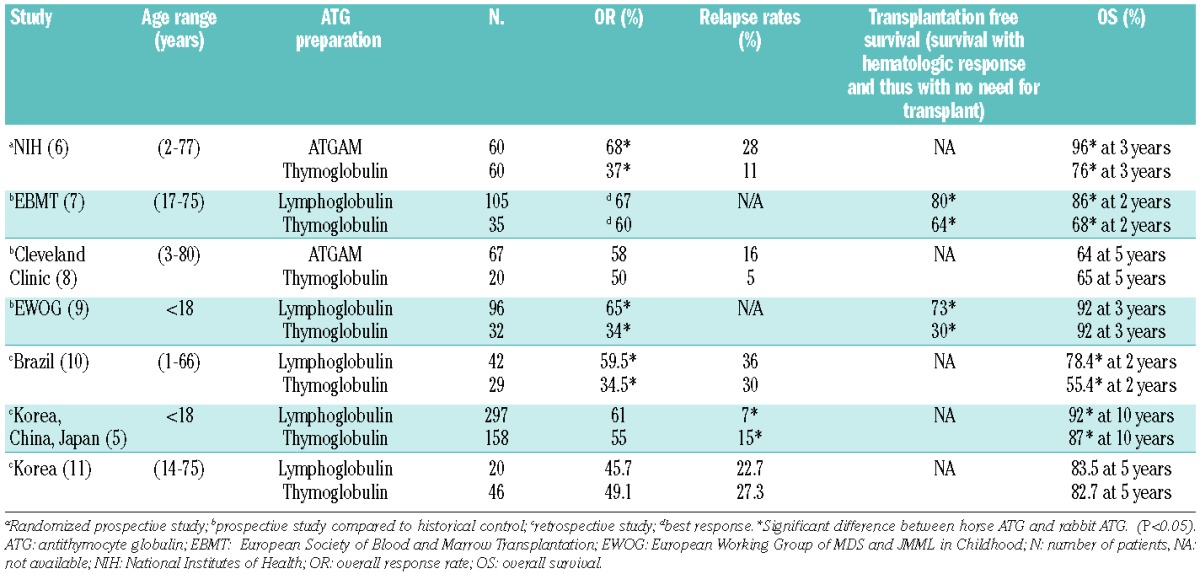
In a similar study published in this issue of the Journal, Yoshimi et al.12 prospectively evaluated responses following IST with horse ATG (Lymphoglobulin) until 2007 and rabbit ATG (Thymoglobulin) after 2007 in a selected cohort of children with RCC. All children enrolled in this study were carefully selected to maximize response to IST and minimize disease progression. Only children with a diagnosis of hypocellular RCC and karyotypes other than monosomy 7/7q- or three or more chromosomal abnormalities, were potentially eligible. As in the SAA studies, horse ATG led to a superior overall response at 6 months (74% for horse ATG versus 53% for rabbit ATG; P=0.04). This translated into superior transplantation-free and failure-free survival in the horse ATG group. The overall message from these comparison studies is clear: horse ATG with cyclosporine is the IST of choice in SAA and low-risk hypocellular RCC.
Rabbit ATG should only be used when horse ATG is not available. Further studies comparing the two preparations will be of limited value, whereas future efforts should concentrate on explaining the reasons for these differences, determining the optimal dose of the ATG preparations and enhancing existing response rates to IST. The reasons for the superiority of horse ATG is unclear. Rabbit ATG is more immunosuppressive and leads to lower absolute numbers of CD4+ regulatory T cells compared to horse ATG,6 but these findings may not necessarily explain the differences between the two preparations.
How can we improve the response to immune suppressive therapy?
The aforementioned studies demonstrate that IST with horse ATG and cyclosporine is still not fully satisfactory. The overall response rate is between 50–70%, relapse rates vary between 10–30% and there is a clonal evolution rate of 10–20%, with a significant risk of developing monosomy 7 aftter IST.1,13 Many of the responses are partial remissions and thus the ensuing subnormal blood counts may lead to restrictions to children’s quality of life. Long-term outcomes following IST with horse ATG and cyclosporine demonstrate that around 50% of children will require a second-line treatment (retreatment with IST or a transplant from an unrelated donor).9 Attempts to enhance remission rates seen with ATG and cyclosporine by adding sirolimus, mycophenolate mofetil or danazol have not improved responses rates.14 One potential agent that is currently being investigated which might improve IST responses is eltrombopag. Eltrombopag is an oral thrombopoietin receptor agonist and was originally developed to stimulate platelet production in immune thrombocytopenia. However, patients who lack the thrombopoietin receptor, c-mpl, develop a form of congenital bone marrow failure, called congenital amegakaryocytic thrombocytopenia. This suggests that thrombopoietin is critical to hematopoietic stem cell development and differentiation.
In a pilot dose escalation study in 25 adults with SAA who were refractory to IST, 44% of patients had hematologic responses to eltrombopag.15 In a subsequent update of this study, the National Institutes of Health group reported that in a total of 43 patients, 17 (40%) achieved responses at 3–4 months. Five patients were subsequently able to stop eltrombopag and have maintained stable blood counts despite stopping the drug at a median of 13 months off eltrombopag (range, 1–15 months).16 One concern about the use of the use of eltrombopag is the potential for clonal evolution. Eight of 43 patients (19%) developed new and early cytogenetic abnormalities, most by 3 months (range, 3–13 months) and most often monosomy 7. At present, it is too early to know whether eltrombopag increases the risk of clonal evolution. Randomized studies are underway or planned to determine the role of eltrombopag in combination with horse ATG/cyclosporine in acquired SAA. These studies will answer whether this agent can safely increase the response rates to IST beyond what is currently achievable.
Time to consider upfront transplantation from a matched unrelated donor?
While there has been no improvement in overall or failure-free survival rates in the last two decades following IST,17 results of matched unrelated donor hematopoietic stem cell transplantation have improved dramatically over this period. This has been due to improvements in supportive care, the development of high resolution HLA typing and less toxic conditioning regimens. Results of matched unrelated donor hematopoietic stem cell transplantation in childhood acquired SAA and RCC in children now approach those of matched sibling donor hematopoietic stem cell transplantation.18
With the excellent outcomes currently seen in unrelated donor hematopoietic stem cell transplantation in pediatric idiopathic SAA/RCC, it is unclear whether newly diagnosed children lacking a matched sibling donor should receive IST with horse ATG or proceed directly to transplantation from an unrelated donor. With the disappointing results seen following rabbit ATG, there has been an increasing move towards upfront matched unrelated donor hematopoietic stem cell transplantation in children. Compared to IST, transplantation offers a more complete restoration of hematopoiesis, lower relapse rates and better protection against secondary cancers.19 The major potential drawbacks of upfront matched unrelated donor transplantation are: (i) difficulties in finding donors; (ii) the time from diagnosis to stem cell donation; (iii) graft-versus-host disease/graft rejection; (iv) donors opting to donate peripheral blood stem cells rather than bone marrow; and (v) treatment-related mortality.
In the study by Yoshimi et al., the median time from diagnosis to administration of IST was 60 days while it was 134 days in those who underwent upfront matched unrelated donor hematopoietic stem cell transplantation.12 While IST can, undoubtedly, always be started earlier than an upfront matched unrelated donor transplant, the time to cellular recovery is slow following IST (typically 3–6 months). In contrast, cellular recovery following matched unrelated donor transplantation is much quicker (normally a month). Thus, time to cellular recovery following IST or upfront matched unrelated donor hematopoietic stem cell transplantation is often similar if a matched unrelated donor can be found quickly. With the advent of FCC (fludarabine, cyclophosphamide and alemtuzumab) conditioning regimens for acquired SAA, the rates of extensive chronic graft-versus-host disease and graft rejection are now less than 5%.18,20 Thus, the current EBMT SAA recommendations are that if a matched unrelated donor can be found quickly, then transplantation could be considered a potential first-line option in those children who lack a matched sibling donor. The decision to proceed with horse ATG-based IST or an upfront transplant from a matched unrelated donor will, therefore, depend on the preferences of patients and physicians and donor availability until further data become available.
Conclusion
Survival outcomes for low-risk RCC and acquired SAA in children following IST with horse ATG/cyclosporine are excellent. Future strategies will now need to focus more on quality of life and failure-free survival so that further improvements can be made. This can only be achieved with well-designed prospective clinical studies.
Footnotes
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.Jiang J, Smith AE, Mohamedali AM, Gandhi SA, Czepulkowski B, Marsh J, et al. Somatic mutations implicated in myeloid malignancies are frequent in idiopathic aplastic anaemia and its relevance to disease classification and treatment- a comprehensive analysis of 150 patients. Blood. 2013. 2013;122(21):803 [Google Scholar]
- 2.Dufour C, Ferretti E, Bagnasco F, Burlando O, Lanciotti M, Ramenghi U, et al. Changes in cytokine profile pre- and post-immunosuppression in acquired aplastic anemia. Haematologica. 2009;94(12):1743–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomou EE, Rezvani K, Mielke S, Malide D, Keyvanfar K, Visconte V, et al. Deficient CD4+ CD25+ FOXP3+ T regulatory cells in acquired aplastic anemia. Blood. 2007;110(5):1603–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kordasti S, Marsh J, Al-Khan S, Jiang J, Smith A, Mohamedali A, et al. Functional characterization of CD4+ T cells in aplastic anemia. Blood. 2012;119(9):2033–43 [DOI] [PubMed] [Google Scholar]
- 5.Jeong DC, Chung NG, Cho B, Zou Y, Ruan M, Takahashi Y, et al. Long-term outcome after immunosuppressive therapy with horse or rabbit antithymocyte globulin and cyclosporine for severe aplastic anemia in children. Haematologica. 2014;99(4):664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheinberg P, Nunez O, Weinstein B, Biancotto A, Wu CO, Young NS. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365(5):430–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marsh JC, Bacigalupo A, Schrezenmeier H, Tichelli A, Risitano AM, Passweg JR, et al. Prospective study of rabbit antithymocyte globulin and cyclosporine for aplastic anemia from the EBMT Severe Aplastic Anaemia Working Party. Blood. 2012;119(23):5391–6 [DOI] [PubMed] [Google Scholar]
- 8.Afable MG, 2nd, Shaik M, Sugimoto Y, Elson P, Clemente M, Makishima H, et al. Efficacy of rabbit anti-thymocyte globulin in severe aplastic anemia. Haematologica. 2011;96(9):1269–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshimi A, Niemeyer CM, Fuhrer MM, Strahm B. Comparison of the efficacy of rabbit and horse antithymocyte globulin for the treatment of severe aplastic anemia in children. Blood. 2013;121(5):860–1 [DOI] [PubMed] [Google Scholar]
- 10.Atta EH, Dias DS, Marra VL, de Azevedo AM. Comparison between horse and rabbit antithymocyte globulin as first-line treatment for patients with severe aplastic anemia: a single-center retrospective study. Ann Hematol. 2010;89(9):851–9 [DOI] [PubMed] [Google Scholar]
- 11.Shin SH, Yoon JH, Yahng SA, Lee SE, Cho BS, Eom KS, et al. The efficacy of rabbit antithymocyte globulin with cyclosporine in comparison to horse antithymocyte globulin as a first-line treatment in adult patients with severe aplastic anemia: a single-center retrospective study. Ann Hematol. 2013;92(6):817–24 [DOI] [PubMed] [Google Scholar]
- 12.Yoshimi A, van den Heuvel-Eibrink MM, Baumann I, Schwarz S, Simonitsch-Klupp I, de Paepe P, et al. Comparison of horse and rabbit antithymocyte globulin in immunosuppressive therapy for refractory cytopenia of childhood. Haematologica. 2014;99(4):656–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheinberg P, Wu CO, Nunez O, Young NS. Long-term outcome of pediatric patients with severe aplastic anemia treated with antithymocyte globulin and cyclosporine. J Pediatr. 2008;153(6):814–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheinberg P, Wu CO, Nunez O, Boss C, Sloand EM, Young NS. Treatment of severe aplastic anemia with a combination of horse antithymocyte globulin and cyclosporine, with or without sirolimus: a prospective randomized study. Haematologica. 2009;94(3):348–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olnes MJ, Scheinberg P, Calvo KR, Desmond R, Tang Y, Dumitriu B, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367(1):11–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desmond R, Townsley DM, Dumitriu B, Olnes MJ, Scheinberg P, Bevans M, et al. Eltrombopag restores tri-lineage hematopoiesis in refractory severe aplastic anemia which can be sustained on discontinuation of drug. Blood. 2013. December 17 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Passweg JR, Tichelli A. Immunosuppressive treatment for aplastic anemia: are we hitting the ceiling¿. Haematologica. 2009;94(3):310–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samarasinghe S, Steward C, Hiwarkar P, Saif MA, Hough R, Webb D, et al. Excellent outcome of matched unrelated donor transplantation in paediatric aplastic anaemia following failure with immunosuppressive therapy: a United Kingdom multicentre retrospective experience. Br J Haematol. 2012;157(3):339–46 [DOI] [PubMed] [Google Scholar]
- 19.Locasciulli A, Oneto R, Bacigalupo A, Socie G, Korthof E, Bekassy A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation (EBMT). Haematologica. 2007;92(1):11–8 [DOI] [PubMed] [Google Scholar]
- 20.Marsh JC, Gupta V, Lim Z, Ho AY, Ireland RM, Hayden J, et al. Alemtuzumab with fludarabine and cyclophosphamide reduces chronic graft-versus-host disease after allogeneic stem cell transplantation for acquired aplastic anemia. Blood. 2011;118(8):2351–7 [DOI] [PubMed] [Google Scholar]


