Anti-ADAMTS13 autoantibodies, predominantly of the IgG class, are found in plasma of patients with acquired thrombotic thrombocytopenic purpura (TTP)1 but also in approximately 5% of healthy individuals.1 Antibodies from TTP patients have previously been studied using various epitope mapping techniques.2–5 Applying patient-derived monoclonal antibodies and point mutated variants of ADAMTS13, these studies revealed that IgG autoantibodies target a major binding site on the surface of the spacer domain of ADAMTS13,6–8 and identified additional epitopes located in other domains of the protease.2,4,5,9 By contrast, ADAMTS13-specific autoantibodies from healthy individuals remain ill-defined and their binding specificities are unknown.
To determine which epitopes are recognized by anti-ADAMTS13 antibodies from healthy individuals, a plasma pool (approx. 1 L) of 45 randomly selected healthy donors (HD), donated by the Baxter plasma center in Vienna, Austria, was used as starting material. As a sufficient amount of plasma with a positive anti-ADAMTS13 IgG titer from one single healthy donor was not available, we assumed, based on the reported prevalence (5%)1 of elevated anti-ADAMTS13 IgG titers in healthy individuals, that a pool of 45 must contain at least 2–3 positive donors. IgG anti-ADAMTS13 autoantibodies were isolated in a 2-step chromatographic purification procedure using an ADAMTS13 affinity matrix and protein G. Plasma samples of 3 acquired TTP patients with positive IgG titers and known inhibitors served as a reference (Table 1). ADAMTS13-specific IgGs, recovered from the HD plasma pool and detected by ELISA, showed low affinity towards ADAMTS13 using Biacore, and were non-neutralizing in the FRETS-VWF73 activity assay. As expected, autoantibodies derived from the 3 TTP patients were of high affinity and inhibited ADAMTS13 activity (Table 1). A rough mapping of binding epitopes was achieved by testing the antibody preparations for interaction with full-length rADAMTS13 and a series of truncated variants thereof (Figure 1A and B). The antigen-antibody complexes that eventually formed were isolated by protein G, and co-immunoprecipitated ADAMTS13 fragments visualized by Western blot according to a published protocol.4,8 The purified IgGs from all 3 TTP patients clearly interacted with full-length ADAMTS13 and the N-terminal fragments up to the spacer domain (MDT and MDTCS) (Figure 1C–E). The stronger signal intensity for the MDTCS compared with the MDT fragment was in line with the presence of a major binding site within the spacer domain.6–8 The more distal C-terminal domains of ADAMTS13 (T2C and CUB) were only weakly recognized by the antibodies (Figure 1C–E). The HD antibody preparation gave rise to a similar profile, except that it bound stronger to the MDT than to the MDTCS fragment (Figure 1F). To narrow down the binding sites identified by immunoprecipitation, epitopes were additionally determined using peptide arrays (PEPperMAP®; PEPperPRINT GmbH, Heidelberg, Germany), a strategy previously used to identify antibodies’ linear binding epitopes.10 Incubation of these arrays, which had spotted the entire sequence of ADAMTS13 in the form of 13mer peptides with a 12 amino acid overlap, with a control monoclonal anti-ADAMTS13 antibody resulted in a single hit located in the metalloprotease domain of ADAMTS13 (data not shown). By contrast, all affinity-purified antibodies gave rise to multiple hits, indicative of their polyclonal nature (Table 2). The antigenic regions were clustered in the metalloprotease (M), disintegrin (D), spacer (S), thrombospondin 1-8 (T1-8) and CUB domains. No positive spots were detected with any antibody for the cysteine-rich or the T1-1, T1-3, T1-5 and T1-7 domains. Using the published crystal structure of an ADAMTS13 DTCS fragment and a modeled metalloprotease domain (based on the structures of human ADAMTS4/5), all but one (D173-Y177) of the epitopes were displayed on the surface of ADAMTS13 (Online Supplementary Figure S1), illustrating the accessibility of these binding sites to the isolated autoantibodies. Interestingly, the peptide recognition patterns of the antibody preparations from HD and the TTP patients were remarkably similar (Table 2). All 4 samples identified an epitope in the disintegrin domain (P296-P301) that also showed the strongest signals. Such an epitope has not yet been implicated in the literature as being immunogenic, but concurs with a reported antigenic site within the D/T1-1 region.9 A monoclonal antibody targeting an adjacent region (Y305-E327) strongly inhibited ADAMTS13 activity,11 which confirms the critical role of the D domain in binding to unfolded VWF.12 Thus, antibodies against the epitope identified here probably also interfere with VWF interaction.
Table 1.
ADAMTS13-related variables measured in plasma of 3 acquired TTP patients and an HD pool, and characteristics of the affinity-purified anti-ADAMTS13 IgGs derived thereof.
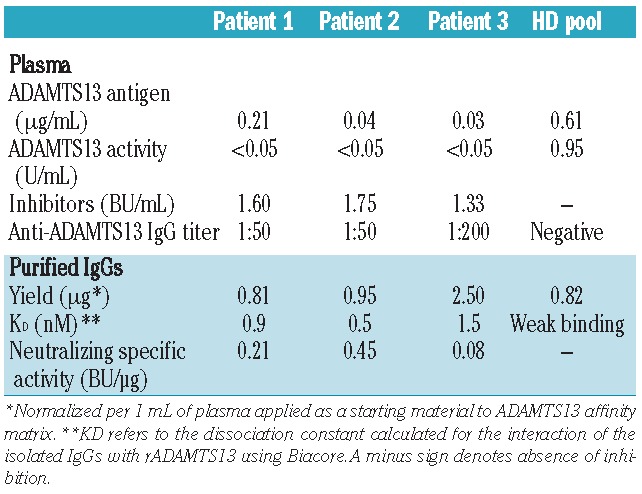
Figure 1.
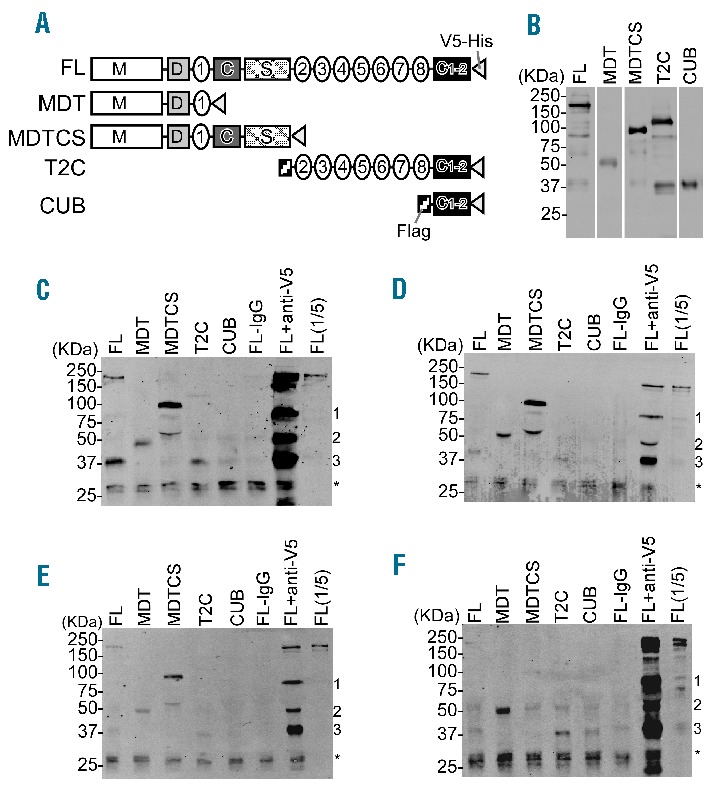
Epitope mapping of affinity-purified anti-ADAMTS13 IgG antibodies by immunoprecipitation and Western blotting. (A) Schematic domain representation of rADAMTS13 and variants used. FL: Full length; MDT: metalloprotease, disintegrin and 1st thrombospondin type 1 repeat domains; MDTCS: metalloprotease, disintegrin, 1st thrombospondin type 1 repeat, Cys-rich and spacer domains; T2C: 2nd – 8th thrombospondin type 1 repeat and two CUB domains; CUB: two CUB domains. (B) Detection of purified rADAMTS13 and variants (0.1 μg/lane) by SDS-PAGE and Western blotting with mouse anti-V5 IgG (1:5,000) (Invitrogen, Carlsbad, CA, USA) and IRDye 800-labeled goat anti-mouse IgG (1:10,000) (LICOR Bioscience, Lincoln, Nebraska, USA). (C–F) Binding pattern of the IgG preparations isolated from patient 1 (C), patient 2 (D), patient 3 (E) and an HD pool (F). 0.5 μg of the distinct IgG preparations and near equivalent amounts (0.5 μg) of either full length rADAMTS13 or variants were incubated with 30 μL protein G-conjugated Sepharose beads (GE Healthcare, Uppsala, Sweden) in 200 μL binding buffer (50 mM Tris-HCl, pH 7.6, 150 mM NaCl, 0.1% Triton X100 and 0.1% Tween20 containing 0.1% casein, 5 mM EDTA, and 0.2% protease inhibitor cocktail from Sigma) overnight at 4°C with rocking. After being washed, the antibody-antigen complex was eluted from the protein G beads with 30 μL 5X SDS buffer and heated for 10 min at 100°C. The bound ADAMTS13 and variants were determined by Western blotting as described in B. FL-IgG (incubation of FL ADAMTS13 in the absence of IgG) and FL+anti-V5 (incubation of FL ADAMTS13 with mouse anti-V5 IgG) refer to the negative and positive control, respectively. FL(1/5) refers to FL ADAMTS13 (0.1 μg) loaded directly on the gel. The loaded negative control “FL-IgG” in D and E refers to 1/5th of the amount loaded in panels C and F. The discernible 75 kDa (1), 50 kDa (2) and 37 kDa (3) bands refer to degradation products of ADAMTS13 constructs that still have the V5 at their C-terminus. A non-specific 25 kDa double band discernible in all lanes is indicated by an asterisk.
Table 2.
Linear ADAMTS13 peptide epitopes detected with isolated antibodies from acquired TTP patients and an HD pool.
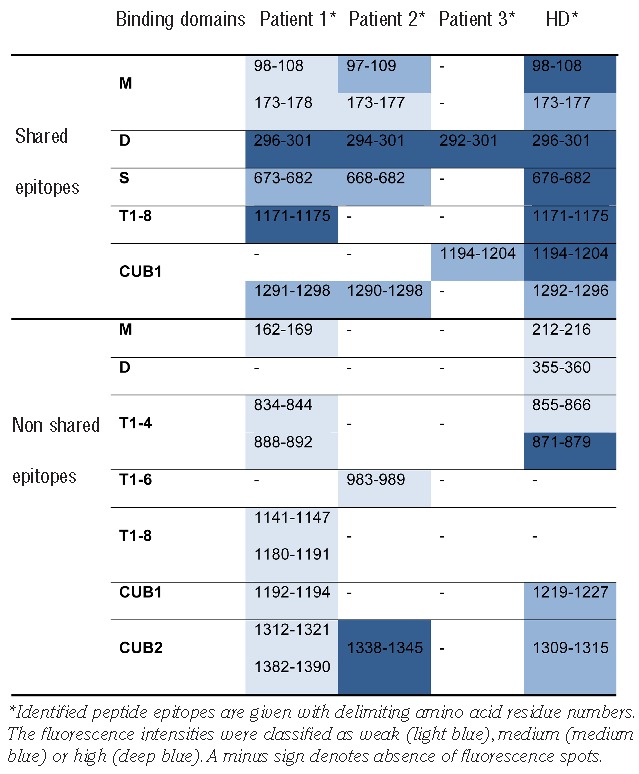
A sequence common to the HD pool and 2 patient samples was found in the spacer domain (T676-P682). This peptide is contained within the G662-V687 fragment that was isolated with autoantibodies from 5 of 13 TTP patients in a phage display screen where ADAMTS13 peptides with a length of 30–50 amino acids were expressed.5 The same ADAMTS13 fragment was also identified as the major VWF-binding peptide using a similar phage display-based approach.13 In the context of the larger MDTCS fragment, however, this epitope appears to be less important.3 Rather, it is the adjacent site comprising residues R660, Y661 and Y665 that is considered the main target for autoantibodies from many acquired TTP patients.6 No hit was observed for this peptide. Given that R568 and F592 contribute to the formation of this epitope’s antigenic surface,7,8 it is plausible that the peptide array, designed to identify linear antigenic regions, failed to detect this epitope. This concern notwithstanding, our data and those of Yamaguchi et al.5 suggest that the potential role of the adjacent region (T676 - P682) as an ancillary target site for ADAMTS13 autoantibodies deserves further attention.
Other shared linear epitopes between the HD pool and at least 1 TTP patient sample were found in the M domain, the T1-8 domain and the CUB1 domain (Table 2). The identification of several common sites supports the idea that the antibodies isolated from an HD pool are indeed specific for ADAMTS13. Similar findings were reported for other autoimmune diseases such as anti-glomerular basement membrane (anti-GBM) disease, where anti-GBM autoantibodies recognize the same major epitopes in patients and healthy individuals,14 and systemic lupus erythematosus, where some common epitopes were likewise found for anti-caspase 8 antibodies.15
That the purified antibody preparations of the 3 TTP patients were inhibitory whereas those of the HD pool were not (despite recognizing common epitopes) is likely due to the vastly different affinities of the preparations towards ADAMTS13. The emergence of pathogenic autoantibodies may be caused by an enhanced frequency of somatic hypermutation of IgG memory B cells.16 Another mechanism that likely contributes to the development of pathogenic antibodies is epitope spreading where additional epitopes are acquired as the disease progresses.17 It will be interesting to see if healthy individuals with elevated anti-ADAMTS13 IgG plasma levels also test positive for the class 2 human leukocyte antigen haplotype HLA-DRB1*11, which was identified as risk factor for acquired TTP.18
Based on our combined data, it is tempting to suggest that anti-ADAMTS13 IgG autoantibodies occurring in healthy individuals provide the template for emergence of high affinity and pathogenic IgG autoantibodies in acquired TTP. Future studies should address whether the presence of anti-ADAMTS13 antibodies precedes clinical disease onset in TTP, as shown, for instance, in anti-GBM disease.19 This may not only improve our understanding of the pathophysiology of acquired TTP, but also help to identify biomarkers for early diagnosis and possible therapeutic intervention.
Footnotes
The online version of this article has a Supplementary Appendix.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Rieger M, Mannucci PM, Kremer Hovinga JA, Herzog A, Gerstenbauer G, Konetschny C, et al. ADAMTS13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated diseases. Blood. 2005;106(4):1262–7 [DOI] [PubMed] [Google Scholar]
- 2.Klaus C, Plaimauer B, Studt JD, Dorner F, Lammle B, Mannucci PM, et al. Epitope mapping of ADAMTS13 autoantibodies in acquired thrombotic thrombocytopenic purpura. Blood. 2004;103(12):4514–9 [DOI] [PubMed] [Google Scholar]
- 3.Luken BM, Turenhout EA, Kaijen PH, Greuter MJ, Pos W, Van Mourik JA, et al. Amino acid regions 572–579 and 657–666 of the spacer domain of ADAMTS13 provide a common antigenic core required for binding of antibodies in patients with acquired TTP. Thromb Haemost. 2006;96(3):295–301 [DOI] [PubMed] [Google Scholar]
- 4.Zheng XL, Wu HM, Shang D, Falls E, Skipwith CG, Cataland SR, et al. Multiple domains of ADAMTS13 are targeted by autoantibodies against ADAMTS13 in patients with acquired idiopathic thrombotic thrombocytopenic purpura. Haematologica. 2010;95(9):1555–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaguchi Y, Moriki T, Igari A, Nakagawa T, Wada H, Matsumoto M, et al. Epitope analysis of autoantibodies to ADAMTS13 in patients with acquired thrombotic thrombocytopenic purpura. Thromb Res. 2011;128(2):169–73 [DOI] [PubMed] [Google Scholar]
- 6.Pos W, Crawley JT, Fijnheer R, Voorberg J, Lane DA, Luken BM. An autoantibody epitope comprising residues R660, Y661, and Y665 in the ADAMTS13 spacer domain identifies a binding site for the A2 domain of VWF. Blood. 2010;115(8):1640–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pos W, Sorvillo N, Fijnheer R, Feys HB, Kaijen PH, Vidarsson G, et al. Residues Arg568 and Phe592 contribute to an antigenic surface for anti-ADAMTS13 antibodies in the spacer domain. Haematologica. 2011;96(11):1670–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jian C, Xiao J, Gong L, Skipwith CG, Jin SY, Kwaan HC, et al. Gain-of-function ADAMTS13 variants that are resistant to autoantibodies against ADAMTS13 in patients with acquired thrombotic thrombocytopenic purpura. Blood. 2012;119(16):3836–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luken BM, Kaijen PH, Turenhout EA, Kremer Hovinga JA, Van Mourik JA, Fijnheer R, et al. Multiple B-cell clones producing antibodies directed to the spacer and disintegrin/thrombospondin type-1 repeat 1 (TSP1) of ADAMTS13 in a patient with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2006;4(11):2355–64 [DOI] [PubMed] [Google Scholar]
- 10.Shukla NM, Salunke DB, Balakrishna R, Mutz CA, Malladi SS, David SA. Potent adjuvanticity of a pure TLR7-agonistic imidazoquinoline dendrimer. PLoS One. 2012;7(8):e43612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igari A, Nakagawa T, Moriki T, Yamaguchi Y, Matsumoto M, Fujimura Y, et al. Identification of epitopes on ADAMTS13 recognized by a panel of monoclonal antibodies with functional or nonfunctional effects on catalytic activity. Thromb Res. 2012;130(3):e79–e83 [DOI] [PubMed] [Google Scholar]
- 12.Crawley JT, de Groot R, Xiang Y, Luken BM, Lane DA. Unraveling the scissile bond: how ADAMTS13 recognizes and cleaves von Willebrand factor. Blood. 2011;118(12):3212–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moriki T, Maruyama IN, Igari A, Ikeda Y, Murata M. Identification of ADAMTS13 peptide sequences binding to von Willebrand factor. Biochem Biophys Res Commun. 2010;391(1):783–8 [DOI] [PubMed] [Google Scholar]
- 14.Yang R, Cui Z, Hellmark T, Segelmark M, Zhao MH, Wang HY. Natural anti-GBM antibodies from normal human sera recognize alpha3(IV)NC1 restrictively and recognize the same epitopes as anti-GBM antibodies from patients with anti-GBM disease. Clin Immunol. 2007;124(2):207–12 [DOI] [PubMed] [Google Scholar]
- 15.Ueki A, Isozaki Y, Tomokuni A, Hatayama T, Ueki H, Kusaka M, et al. Intramolecular epitope spreading among anti-caspase-8 autoantibodies in patients with silicosis, systemic sclerosis and systemic lupus erythematosus, as well as in healthy individuals. Clin Exp Immunol. 2002;129(3):556–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mietzner B, Tsuiji M, Scheid J, Velinzon K, Tiller T, Abraham K, et al. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc Natl Acad Sci USA. 2008;105(28):9727–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roth AJ, Ooi JD, Hess JJ, van Timmeren MM, Berg EA, Poulton CE, et al. Epitope specificity determines pathogenicity and detectability in ANCA-associated vasculitis. J Clin Invest. 2013;123(4):1773–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coppo P, Busson M, Veyradier A, Wynckel A, Poullin P, Azoulay E, et al. HLA-DRB1*11: a strong risk factor for acquired severe ADAMTS13 deficiency-related idiopathic thrombotic thrombocytopenic purpura in Caucasians. J Thromb Haemost. 2010;8(4):856–9 [DOI] [PubMed] [Google Scholar]
- 19.Olson SW, Arbogast CB, Baker TP, Owshalimpur D, Oliver DK, Abbott KC, et al. Asymptomatic autoantibodies associate with future anti-glomerular basement membrane disease. J Am Soc Nephrol. 2011;22(10):1946–52 [DOI] [PMC free article] [PubMed] [Google Scholar]


