Abstract
Hepatic steatosis or fatty liver disease occurs when lipids accumulate within the liver and can lead to steatohepatitis, cirrhosis, liver cancer, and eventual liver failure requiring liver transplant. Conventional brightness mode (B-mode) ultrasound (US) is the most common noninvasive diagnostic imaging modality used to diagnose hepatic steatosis in clinics. However, it is mostly subjective or requires a reference organ such as the kidney or spleen with which to compare. This comparison can be problematic when the reference organ is diseased or absent. The current work presents an alternative approach to noninvasively detecting liver fat content using ultrasound-induced thermal strain imaging (US-TSI). This technique is based on the difference in the change in the speed of sound as a function of temperature between water- and lipid-based tissues. US-TSI was conducted using two system configurations including a mid-frequency scanner with a single linear array transducer (5-14 MHz) for both imaging and heating and a high-frequency (13-24 MHz) small animal imaging system combined with a separate custom-designed US heating transducer array. Fatty livers (n=10) with high fat content (45.6 ± 11.7%) from an obese mouse model and control livers (n=10) with low fat content (4.8± 2.9%) from wild-type mice were embedded in gelatin. Then, US imaging was performed before and after US induced heating. Heating time periods of ~3 s and ~9.2 s were used for the mid-frequency imaging and high-frequency imaging systems, respectively to induce temperature changes of approximately 1.5 °C. The apparent echo shifts that were induced as a result of sound speed change were estimated using 2D phase-sensitive speckle tracking. Following US-TSI, histology was performed to stain lipids and measure percentage fat in the mouse livers. Thermal strain measurements in fatty livers (−0.065±0.079%) were significantly (p<0.05) higher than those measured in control livers (−0.124±0.037%). Using histology as a gold standard to classify mouse livers, US-TSI had a sensitivity and specificity of 70% and 90%, respectively. The area under the receiver operating characteristic (ROC) curve (AUC) was 0.775. This ex vivo study demonstrates the feasibility of using US-TSI to detect fatty livers and warrants further investigation of US-TSI as a diagnostic tool for hepatic steatosis.
Keywords: Fatty liver disease, Obese mouse, Ultrasound thermal strain
1. Introduction
Hepatic steatosis is marked by the accumulation of triglycerides within hepatocytes. Non-alcoholic fatty liver disease (NAFLD) refers to the presence of hepatic steatosis without any other known cause (Strauss et al., 2007) and is estimated to affect up to 24% of the general population and 74% of obese people around the world (Angulo and Lindor, 2002). Hepatic steatosis, if left undiagnosed, can progress into more advanced, irreversible forms of the disease like cirrhosis (Ratziu et al., 2000; Teli et al., 1995). However, proper diagnosis and medical treatment of hepatic steatosis can prevent and reverse disease progression (Ueno et al., 1997). This clinical need suggests that a technology that could accurately identify and monitor early steatosis would be invaluable.
Currently, a needle biopsy is the gold standard for diagnosing hepatic steatosis (Angulo and Lindor, 2002). However, biopsies are prone to sampling error because only 1/50,000 of the whole volume of the liver is sampled and biopsies are associated with complications such as bleeding and perforation which make longitudinal monitoring impractical. Noninvasive methods such as computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound (US) are used to diagnose and assess the status of suspected fatty livers. However, CT exhibits a wide range of sensitivity (54-93%) and high specificity (95-100%) (Torres and Harrison, 2008), and exposes patients to a significant dose of ionizing radiation. The mean sensitivity and specificity ranges of MRI in diagnosing fatty livers are 82.0-97.4% and 76.1-95.3%, respectively (Bohte et al., 2011). While MRI does not use ionizing radiation, it is a much more expensive imaging modality as compared to CT and US.
Ultrasonography is considered the first choice when diagnosing hepatic steatosis due to its safety and low-cost. Common features of fatty livers in US B-mode images include hyperechoic hepatic patterns relative to a normal renal cortex and spleen, limited visibility of the diaphragm due to increased US attenuation, and blurred portal vein architecture (Joy et al., 2003). Previous studies reported a wide range of sensitivities (60–94%) and specificities (66–95%) for B-mode imaging in detecting hepatic steatosis in different groups of patients with livers of variable fat content (Schwenzer et al., 2009). However, most of these studies using B-mode imaging adopted subjective assessments that are operator dependent and have significant inter- and intra-observer variability (Strauss et al., 2007). Although (Webb et al 2009) tried to assess hepatic steatosis using the hepatorenal index, they could not apply their technique to patients with renal disease (e.g. ectopic or absent right kidneys) or in patients with single or multiple focal abnormalities that distort liver architecture. In addition, US B-mode of fibrotic livers exhibits diffusely increased echogenicity similar to images of hepatic steatosis which may affect the accuracy of detecting hepatic steatosis (Siegelman and Rosen, 2001).
Thermal strain imaging (TSI) has been used in the field of medical imaging for applications relating to noninvasive tissue thermometry (Nasoni and Bowen 1989, Seip and Ebbini 1995, Varghese et al. 2002, Lai et al. 2010, Arthur et al. 2005, Souchon et al. 2005) and lipid detection in atherosclerotic plaques (Kim et al., 2008; Mahmoud et al., 2013; Seo et al., 2011; Shi et al., 2005). TSI relies on the observation that the speed of sound changes differently with respect to temperature for different tissue types (Bamber and Hill, 1979; Bamber, 2007). In particular, lipids exhibit a decreasing speed of sound with increasing temperature, whereas the opposite is true for water-based tissues (Bamber and Hill, 1979; Bamber, 2007). As the temperature increases, the US radiofrequency (RF) signal is received earlier or later due to an increase or a decrease in the sound speed, respectively. The derivative of these temporal shifts is proportional to the change in temperature and is referred to as thermal (or temporal) strain to reflect the fact that the source of the signal is from the change in temperature. Consequently, lipids and water-based tissues exhibit positive and negative thermal strains, respectively, upon temperature rise. However, it should be noted that the small temperature change (≤3°C) used in TSI relative to human body temperature (37°C) does not result in significant tissue thermal expansion and that the measured signal is mainly a result of changes in the speed of sound with temperature (Seo et al., 2011).
In this paper, we adopt a novel approach, as compared to current clinical practice, by applying US-TSI to differentiate between fatty and normal livers. US-TSI is a technique that exploits a unique and well-documented physical property of lipid-based tissues. US-TSI was implemented and used to image fatty and control mouse livers ex vivo on two US scanners. One scanner operates at medium frequencies (5-14 MHz) and uses a single linear array for both imaging and heating. The other scanner operates at high imaging frequencies (13-24 MHz) and uses a separate, custom-designed heating array that operates at 3.55 MHz. Two-dimensional phase-sensitive speckle tracking was used to estimate temporal shifts due to sound speed changes and then thermal strain maps were reconstructed and co-registered to B-scans. Oil red O histology was performed as a gold standard to verify and measure the lipid content in mouse livers. Then the sensitivity and specificity of US thermal strain measurements in identifying fatty livers were determined after comparison with histology.
2. Theory
This part describes the physical concept and definition of US thermal strain and explains its potential to detect fat in livers. The echo time from a tissue scatterer at depth z0 from the US transducer surface at the initial temperature T0 can be calculated as (MaassMoreno and Damianou, 1996):
| (1) |
where c(T0, z0) is the distribution of sound speed at T0. After inducing a slight temperature increase δT(z0) (≤3°C), the new sound speed distribution will be c(T(z0), z0) (Seo et al., 2011) resulting in a shift in echo time of
| (2) |
where β(z0) is the linear coefficient of thermal expansion. Within this range of temperature, a linear relationship can be assumed between sound speed and temperature change (Nasoni and Bowen, 1989) as
| (3) |
where α is the linear coefficient in (3) (Seo et al., 2011). Differentiating (2) with respect to z0, substituting (3), and assuming |α(z0) δT(z0)| << 1 leads to
| (4) |
Substituting , from (1), into (4) leads to the definition of thermal strain as
| (5) |
It was reported that β has a negligible effect compared to α for temperature range less than +50°C (MaassMoreno and Damianou, 1996; Seo et al., 2011). Thus, the thermal or temporal strain in (5) can be approximated as
| (6) |
The parameter α(°C−1) depends on the tissue composition and determines how the sound speed changes with temperature. It can be defined as and ranges from +0.7 × 10−3 to +1.3 × 10−3°C−1 in water-based tissue and −1.3 × 10−3 to −2.0 × 10−3°C−1 in lipids (Duck, 1990).
Livers consist of lipid-based tissue, water-based tissue, and residual components which include proteins, carbohydrates, minerals, and vitamins. According to the mixture law (Sehgal et al., 1986), the overall temperature-dependence of the speed of sound depends on the percentage of each composition. Based on this observation, (Miller et al., 2002) reported a noticeable difference in the temperature dependence of sound speed between normal and fatty human livers. However, the main objective of Miller et al. 2002 was to investigate the feasibility of detecting heating focal lesions during focused ultrasound surgeries (FUS) using computer simulation and they did not consider the effect of blood perfusion or physiological motion. Although Miller et al. 2004 extended their work to show the ex vivo feasibility of detecting hot spots during FUS using bovine livers, they did not include fatty livers. In this work, we hypothesize that fatty livers can be differentiated from normal livers using US-TSI.
3. Materials and methods
3.1 Animals
US-TSI was performed ex vivo to compare thermal strains measured in 10 fatty livers with those of 10 control livers. Fatty livers were excised from 10 obese (ob/ob) mice (7-13week-old). It was reported that the ob/ob mouse develops steatosis in 50% of its hepatocytes at 7 weeks and 85% of its hepatocytes at 13 weeks (Hines et al., 2010). Control livers were excised from 10 wild type (C57B6) mice (7-13 week-old) fed a normal diet.
Freshly harvested livers were embedded in 6% gelatin blocks which had dimensions approximately equal to 13.5 × 11.5 × 4.8 cm (G-2500, Sigma Aldrich Corp., St. Louis, MO). One percent cellulose (S3504, Sigma Aldrich Corp., St. Louis, MO) by weight was added to gelatin as US scatterers. Then, US-TSI was performed at room temperature as described below. The entire procedure including imaging lasted approximately 6 hours.
3.2 Experimental set-up and ultrasound systems
US-TSI requires US-RF imaging frame acquisition while gradually increasing tissue temperature. Two different US systems were used to perform US-TSI on mouse livers ex vivo. The first system was a mid-frequency US scanner (SonixTOUCH, Ultrasonix Medical Corporation, Richmond, BC, Canada) that allows customized pulse sequences and RF data access. Both US imaging and heating were performed using the same linear US transducer (L14-5/38, 5-14 MHz) (Huang et al., 2007). The interleaved imaging-heating pulse sequence described in (Huang et al., 2007) was adopted with an increased waiting period after each heating-imaging sequence to avoid tissue motion due to the acoustic radiation force. The US sequence begins with imaging for 5 ms, followed by heating for 192 ms, and a delay period up to 103 ms, and then repeats the entire sequence. This results in an imaging frame rate of at least 3.34 Hz. An US imaging frequency centered at 6 MHz was used resulting in a B-mode image resolution of approximately 190 μm (axial) × 300 μm (lateral) using 40 MHz sampling frequency. The mid-frequency system was used to image 4 fatty livers and 5 control livers.
The second system combined a high-frequency US imaging transducer (13-24 MHz) driven by a high-frequency small animal imaging system (Vevo2100, FUJIFILM VisualSonics Inc., Canada) with a custom-designed US heating transducer array that provides improved heating efficiency with a broad heating beam (Stephens et al., 2012). Figure 1(a) is a schematic of this experimental setup. A 3.55 MHz,1.414 Vp-p sine wave was generated using an arbitrary waveform generator (33250A, Agilent Technologies, CA) and was fed into an RF power amplifier (100A250A, Amplifier Research, PA) to provide a 282.8 Vp-p heating excitation signal (Gain=46 dB). Then, this was divided equally using a 1:8 power splitter to drive US heating elements. The heating array consists of a total of six elements arranged in sets of three elements on either side of the imaging array in a custom-designed manifold (lower panel, figure 1(a)) and were driven with about 50% average duty cycle every 300-500 ms (figure 1(b)). The imaging frame rate was 10 Hz and only frames obtained during no heating period were used for US-TSI. An US imaging frequency of 21 MHz was used resulting in an imaging resolution of approximately 70 μm (axial) × 180 μm (lateral) using 42 MHz sampling frequency. This system was used to image 6 fatty livers and 5 control livers.
Figure 1.
Experimental configuration for ultrasound thermal strain imaging (US-TSI) for the high-frequency small animal US system. (a) Schematic diagram of the experimental set-up for US-TSI. The image in the lower left corner shows the heating array manifold surrounding the high-frequency imaging array, and (b) timing diagram describing the imaging-heating sequence using the high-frequency configuration.
The pressure fields during US heating were measured in a water tank using a hydrophone (HNC, Onda Corp., Sunnyvale, CA), and were used to estimate the heating beam profiles for the mid-frequency (Dutta et al., 2013) and the custom-designed heating array systems (Stephens et al., 2012). The mid-frequency system produced a mean thermal strain of −0.15% within ~9.2 seconds which correspond to ~1.5 °C temperature rise in water-based tissues (Kim et al., 2008). The custom-designed heating transducer combined with the high-frequency imaging system achieved the same mean strain within ~3 seconds in water-based tissues. These heating periods were used for US-TSI liver experiments.
3.3 Signal Processing
A 2D phase-sensitive correlation-based speckle tracking algorithm (Lubinski et al., 1999) was applied to the US-RF data to estimate the echo shifts associated with changes in sound speed resulting from the change in temperature. These shifts can be seen in US frames as apparent displacements along the axis of US propagation. To measure these apparent displacements, a kernel that is approximately as large as the average speckles size, or full width at half maximum of the autocorrelation function (Lubinski et al., 1999), was used to estimate the complex cross-correlation coefficients between two frames. The kernel size used for mid-frequency system was approximately 212 μm (axial) × 593 μm (lateral) and the kernel size for the high-frequency small animal system was approximately 92 μm (axial) × 316 μm (lateral). A correlation filter about 1.5 times the kernel size was applied to reduce estimation variance and potential peak hopping errors for both systems (Lubinski et al., 1999). Axial displacements were initially estimated from the position corresponding to the maximum magnitude of the correlation coefficient. Then, the zero-crossing of the phase of the complex correlation function was used to refine the displacement estimate.
Thermal strains were computed as the spatial derivative of axial displacements along the axis of the US beam (Kim et al., 2008). For the mid-frequency system, median filters of 0.58 mm (axial) × 1.80 mm (lateral) and 0.12 mm (axial) × 1.80 mm (lateral) were applied to the displacements and strains, respectively. A 0.55 mm (axial) × 0.27 mm (lateral) and 0.11 mm (axial) × 0.27 mm (lateral) median filters were applied to the displacements and strains, respectively, for the high-frequency small animal imaging system. Thermal strain maps for heated regions within the liver were color coded such that red and blue indicated positive and negative strain, respectively. The color-coded thermal strain maps were then co-registered and superimposed on B-scan US images for visualization.
3.4 Histology
After completing US-TSI, livers were cleaned and immediately fixed in formalin, embedded in molds of optimal cutting temperature (OCT) compound, and frozen at −80 C. Sections (8-10 μm thick) were stained using oil red O and hematoxylin counterstaining to identify steatosis morphologically. Using these stains, lipids appear red and water-based tissues appear blue. To quantify the percentage steatosis in each liver, a representative section was manually segmented and the area of red staining was computed and divided by the total area of the section. This analysis was conducted using Image J software (National Institutes of Health, Bethesda, MD). Livers with percentage steasosis ≥20 % were considered to be fatty.
3.5 Statistical analysis
All thermal strain values are expressed as the mean ± SD. For each liver, the thermal strain was measured as the mean strain in 3 mm (axial) × 3 mm (lateral) regions across 4 different elevational slices spaced 1-3 mm apart. A 3 mm × 3 mm region was chosen because it corresponds approximately to the smallest possible size for the heated region. Thermal strain measurements in control and fatty livers were compared using the non-parametric Wilcoxon Rank-Sum test. A p-value <0.05 was considered significant. Statistical analyses were performed using the Statistics Toolbox of Matlab 7.12.0. Receiver operating characteristic (ROC) curves were calculated using MedCalc for Windows, version 12.5 (MedCalc Software, Ostend, Belgium) to test the sensitivity and specificity of US-TSI in identifying fatty livers.
4. Results
Using representative oil red O slides, percentage fat was estimated in each liver. Fatty livers exhibited a high percentage fat (45.6± 11.7%) and a wide range of fat accumulation (range: 29.4-62.8%), whereas in control livers this percentage was 4.8 ± 2.9% (range: 2.0-9.5%). US-TSI measurements in control and fatty livers were significantly different (p<0.05). Thermal strains in fatty and control livers were −0.065±0.079% and −0.124±0.037%, respectively, for the aforementioned heating periods.
Figure 2(a) shows an US B-mode image for a typical control liver embedded in gelatin. US-TSI was performed using the mid-frequency system to generate this image. The approximate region heated with US is indicated with the dotted line in figure 2(a) and all subsequent figures. The surface boundary of the liver is indicated by the dashed outline and the strain map is superimposed on the B-mode image in figure 2(b) and all subsequent figures. The strain for this control liver was −0.158±0.037%. The percentage fat by oil red O staining (figure 2(c)) was 8.7%.
Figure 2.
Ultrasound images acquired using a mid-frequency US scanner for a control liver. (a) B-mode image of a typical cross-section in the liver. The dotted line shows the approximate size of the heating beam. (b) US-TSI for the liver section in (a) (dashed contour), and (c) oil red O histology for a cross-section within the liver.
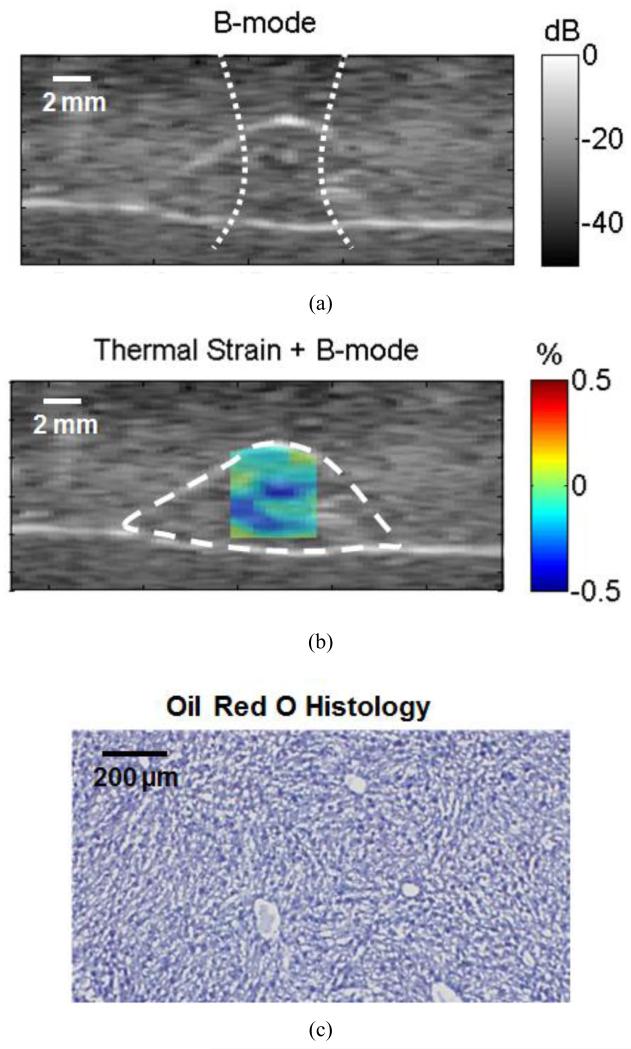
A representative US B-mode image of a fatty liver acquired using the same system is shown in figure 3(a).The corresponding US-TSI exhibited areas of slight positive and negative thermal strains as in figure 3(b). Higher strain of −0.070±0.007% was measured in this fatty liver. Quantification of oil red O on histology of this liver (figure 3(c)) showed a percentage fat of 29.4%.
Figure 3.
Ultrasound images acquired using a mid-frequency US scanner for a fatty liver. (a) B-mode image of a typical cross-section in the liver. The dotted line shows the approximate size of the heating beam. (b) US-TSI for the liver section in (a) (dashed contour), and (c) oil red O histology for a cross-section within the liver.
US-TSI was performed using a custom heating array to provide an efficient heating over a larger area, integrated with a high-frequency small animal imaging system for imaging with high spatial resolution. Anatomical details can be observed in the high-resolution B-mode image of the control liver in figure 4(a). The heating beam width using the custom-designed heating array (figure 4(a)) was at least 3 mm wider than the heating beam width of the mid-frequency system. The corresponding US-TSI in figure 4(b) shows mostly negative strains and the thermal strain was found to be −0.118±0.023% in this liver. Oil red O staining showed that the liver was predominantly water-based with only 2.9% fat (figure 4(c)). Figures 5(a) and 5(b) show high-resolution B-mode image and the corresponding US-TSI for a fatty liver cross-section, respectively. The thermal strain was +0.082±0.006% in this liver, which reflects the dominant areas of positive strain observed in the US-TSI (figure 5(b)). Oil red O histology of this liver showed strong red staining with 45.6% fat accumulation.
Figure 4.
Ultrasound images acquired using the high-frequency US configuration for a control liver. (a) B-mode image of a typical cross-section in the liver. The dotted line shows approximately the heated region. (b) US-TSI for the liver section in (a) (dashed contour), and (c) oil red O histology for a cross-section within the liver.
Figure 5.
Ultrasound images acquired using the high-frequency US configuration for a fatty liver. (a) B-mode image of a typical cross-section in the liver. The dotted line shows approximately the heated region. (b) US-TSI for the liver section in (a) (dashed contour), and (c) oil red O histology for a cross-section within the liver.
The ROC curve in figure 6 was reconstructed to evaluate the capability of US-TSI measurements to distinguish between 10 control and 10 fatty mouse livers. The area under the ROC curve (AUC) was 0.775 with a standard error of 0.116 (p<0.05). Using an optimal thermal strain cut-off level of −0.097%, the sensitivity and specificity were 70% and 90%, respectively.
Figure 6.
Receiver operating characteristics (ROC) curve for ultrasound thermal strain measurements in 10 fatty and 10 control mouse livers.
5. Discussion
This work demonstrated the feasibility of identifying hepatic steatosis in livers ex vivo using US-TSI. The change in speed of sound with respect to temperature depends heavily on the percentage fat in the liver (Bamber and Hill, 1979; Bamber, 2007). The greater the percentage fat, the more negative the value of α that governs the value of thermal strain (equation (6) in Theory). In this study, US-TSI was implemented and tested successfully on two different ultrasound scanners including a mid-frequency system and a high-frequency small animal imaging system. No significant differences in thermal strain measurements were observed in data acquired using the mid-frequency and high-frequency systems in fatty (p=0.637) and control (p=0.484) livers. Most of fatty livers showed slightly negative mean strains as exemplified by the liver in figure 3 instead of positive strains expected in fatty tissue. This is consistent with previous studies because even extremely “fatty” livers are not 100% fat. Using the mixture law and ignoring the residual component (Miller et al., 2002), we expect that only livers with fat concentration above ~39% to exhibit a positive thermal strain given a 1.5°C temperature rise and a starting temperature of 23 °C. Using the same theoretical calculations, a maximum thermal strain of only +0.079% is expected in a liver with 60% fat. Although our experimental findings did not show a perfect match with theoretical expectations due to several reasons discussed later, we observed a significant difference (p<0.05) in the measured thermal strain between fatty and control livers.
US-TSI identified livers designated as fatty (>20% fat) with 70% sensitivity, 90% specificity, and an AUC of 0.775. In order to compare the performance of US-TSI with B-mode techniques, we applied the B-mode technique described in (Webb et al., 2009) on the same livers using the gelatin background as a reference. The B-mode technique exhibited a higher sensitivity (90%), a lower specificity (80%), and larger AUC (0.900) compared to those of US-TSI. However, it is interesting that two livers that were misclassified using the B-mode technique, were correctly classified using US-TSI. This suggests that US-TSI may have the potential to be a complementary tool to conventional B-mode techniques.
Although attempts have been made to modify the beamforming of the mid-frequency transducer to perform both imaging and heating, there are still inherent limitations, such as limited heating efficiency and a small beam width since the array was originally designed for conventional US “imaging”. Further work is still required to design a heating transducer that is suitable for clinical use. In this effort, a previously developed prototype heating transducer was utilized with the small animal imaging system (Stephens et al., 2012). Our data showed more efficient heating (~3 times as fast) for US-TSI (Figure 4(b) and 5(b)). Currently, this high power heating array can deliver total acoustic power of 30 W to produce intensities greater than 15 W/cm2 in a tissue target depth from 20-30 mm (Stephens et al., 2012). For future clinical translation of this technology, the flexible design of this high power heating array can be adapted to increase the heating depth up to 60 mm.
Safety is an important consideration for the future in vivo application of US-TSI in preclinical or clinical studies. According to the American Institute of Ultrasound in Medicine (AIUM), no significant adverse biological effects were observed due to temperature increases of less than or equal to 2°C above normal for exposure durations up to 50 hours. Also, when the maximum heat exposure time is limited to 10 s, the maximum allowable safe temperature increase is 8.5 °C above normal (O’Brien et al., 2008). The temperature rise induced in this study is well within these limits. In addition, the TI was calculated (Abbott, 1999) for both systems using pressure data collected during previous water tank experiments (Dutta et al., 2013; Stephens et al., 2012). For the mid-frequency system, it was found to be 0.1 and for the custom-designed heating transducer, it was found to be 57.9. Although the TI for the custom-designed heating transducer is higher than typical clinical imaging systems, it should be noted that the TI only provides a measure of relative risk and does not take into account the time over which the heating occurs (Abbott, 1999; Bigelow et al., 2011). The temperature rise induced by US-TSI is transient and lasts for only a few seconds. For this reason, the aforementioned standards proposed by the AIUM and (O’Brien et al., 2008) might be more appropriate measures of the absolute thermal risk.
The peak rarefactional (negative) pressure of the US heating pulse was measured experimentally to estimate the mechanical index (MI), which was calculated as the derated peak rarefactional pressure in MPa divided by the square root of the transmitted center frequency in MHz (O’Brien, 2007). An attenuation coefficient of 0.3 dB/cm.MHz was used to calculate the derated pressure in soft tissue (O’Brien, 2007). The peak derated pressure amplitude was −1.82 MPa at 2.3 cm imaging depth and −1.86 MPa at 2.6 cm imaging depth for the mid-frequency system and the custom-designed heating transducer, respectively. Consequently, the MI was calculated to be 0.74 and 0.99 for the mid-frequency and custom-designed systems, respectively, which are below the FDA maximum allowance (MI < 1.9) to avoid adverse biological effects (O’Brien, 2007). These acoustic measurements in water tank suggest that US-TSI is safe to use; however, an important next step would be to conduct extensive monitoring for the acoustic parameters during in vivo animal studies.
One major challenge that must be overcome before this technology can be translated into clinics is to address the physiological motion artifacts arising from breathing and cardiac pulsations. These physiological motions may cause translational motion and/or mechanical strain components in the liver and need to be separated from thermal strain (Bell et al., 2012). They may also cause speckle decorrelation due to the out-of-imaging plane motion which would result in a poor displacement estimation using speckle tracking. Respiratory motion might be addressed by asking patients to initiate a breath hold for a few seconds (<5 s). The cardiac motion could be addressed by synchronizing US-TSI frame acquisition with an ECG trigger to compensate for cardiac pulsations. Alternatively, additional signal processing technique based on time series analyses could be used to separate the thermal strains which are monotonically increasing from cyclic mechanical strains (Dutta et al., 2013). Furthermore, 3D speckle tracking using a 2D US array could be applied to track the elevational motion and further overcome the limitation with speckle decorrelation (Bell et al., 2012). Reflections and shadowing artifacts from the ribs can also be challenging when the upper lobes of the liver are imaged. This problem can be avoided by carefully placing the US transducer below the ribs with a cephalad imaging angle. Blood perfusion in the liver can affect tissue temperature during the in vivo application of US-TSI. This effect was not included in this ex vivo feasibility study. Further simulation and in vivo studies are needed to determine and compensate this effect before translating this technology into the clinical setting.
In this study, US-TSI exhibited relatively low sensitivity as compared to B-mode imaging. This might have resulted from the experimental design, and/or the additional TSI processing steps. First, because of the small size of mouse livers, it was difficult to find homogeneous segments in fatty livers for strain calculations. Some segments may have included blood vessels and bile ductules that were difficult to exclude and might have resulted in underestimation of thermal strain. This issue can be tested by using a bigger animal model for fatty liver such as high-cholesterol fed rabbits or pigs. Second, fatty livers were imaged at room temperature (~21-25°C). In this temperature range, the speed of sound in livers may have only exhibited a small, positive or negative dependence on temperature which helps explain the absence of the distinctive positive strain typically associated with fatty tissue (Miller et al., 2002). The electronic noise of commercial ultrasound systems could be another factor affecting the estimation of nanosecond scale temporal shifts in fatty livers. This can be minimized by applying temporal averaging for subsequent frames acquired at a high frame rate before applying speckle tracking.
There are also two key steps in the TSI process that might affect the accuracy of the estimates. First, a homogenous beam must be applied to heat the region of interest because the spatial profile of the induced temperature change is directly proportional to the spatial profile of the heating beam. Since thermal strain is directly proportional to the change in temperature, a non-uniform heating beam will make a homogeneous medium appear inhomogeneous which might bias the final estimate of thermal strain. Second, displacement estimators must be robust over a wide range of displacements. Thermal strain is proportional to the derivative of displacement and generates a wide range of apparent displacements (−8μm to +20μm). Displacement estimators currently being used, including 2D speckle tracking, have variance properties that depend heavily on the displacement that is generated (Pinton et al., 2006). Ongoing studies are being conducted to address these issues.
6. Conclusion
This study demonstrates ex vivo the feasibility of identifying hepatic steatosis using US-TSI. Thermal strains measured in the fatty livers of obese mice were significantly different from those measured in control mouse livers. This warrants further in vivo preclinical investigation. This novel US-TSI approach may have potential to provide a complementary diagnostic tool of fatty liver disease to the conventional B-mode techniques. Ongoing investigation in our laboratory focuses on the in vivo testing of US-TSI along with further safety measurements.
Acknowledgments
This work was supported by the National Institute of Health (NIH) grant R01 HL098230-01A1 and the small animal imaging system (Vevo2100) was supported by the NIH grant 1S10RR027383-01. Student training was supported by NIH grant T32 HL076124.
References
- Abbott JG. Rationale and derivation of MI and TI—a review. Ultrasound Med. Biol. 1999;25:431–441. doi: 10.1016/s0301-5629(98)00172-0. [DOI] [PubMed] [Google Scholar]
- Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2002;17:S186–S190. doi: 10.1046/j.1440-1746.17.s1.10.x. [DOI] [PubMed] [Google Scholar]
- Arthur RM, Straube WL, Trobaugh JW, Moros EG. Non-invasive estimation of hyperthermia temperatures with ultrasound. Int. J. Hyperthermia. 2005;21:589–600. doi: 10.1080/02656730500159103. [DOI] [PubMed] [Google Scholar]
- Bamber JC. Encyclopedia of Acoustics. John Wiley & Sons, Inc.; 2007. Acoustical Characteristics of Biological Media; pp. 1703–1726. [Google Scholar]
- Bamber JC, Hill CR. Ultrasonic attenuation and propagation speed in mammalian tissue as a function of Temperature. Ultrasound Med. Biol. 1979;5:149–158. doi: 10.1016/0301-5629(79)90083-8. [DOI] [PubMed] [Google Scholar]
- Bell MAL, Byram BC, Harris EJ, Evans PM, Bamber JC. In vivo liver tracking with a high volume rate 4D ultrasound scanner and a 2D matrix array probe. Phys. Med. Biol. 2012;57:1359–1374. doi: 10.1088/0031-9155/57/5/1359. [DOI] [PubMed] [Google Scholar]
- Bigelow TA, Church CC, Sandstrom K, Abbott JG, Ziskin MC, Edmonds PD, Herman B, Thomenius KE, Teo TJ. The Thermal Index Its Strengths, Weaknesses, and Proposed Improvements. J. Ultrasound Med. 2011;30:714–734. doi: 10.7863/jum.2011.30.5.714. [DOI] [PubMed] [Google Scholar]
- Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and H-1-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur. Radiol. 2011;21:87–97. doi: 10.1007/s00330-010-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duck FA. Physical Properties of Tissue: A Comprehensive Reference Book. Academic; 1990. [Google Scholar]
- Dutta D, Mahmoud AM, Leers SA, Kim K. Motion artifact reduction in ultrasound based thermal strain imaging of atherosclerotic plaques using time-series analysis. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2013;60:1660–1668. doi: 10.1109/TUFFc.2013.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines CDG, Yu H, Shimakawa A, McKenzie CA, Warner TF, Brittain JH, Reeder SB. Quantification of Hepatic Steatosis with 3-T MR Imaging: Validation in ob/ob Mice. Radiology. 2010;254:119–128. doi: 10.1148/radiol.09090131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S-W, Kim K, Witte RS, Olafsson R, O’Donnell M. Inducing and Imaging thermal strain using a single ultrasound linear array. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2007;54:1718–1720. doi: 10.1109/tuffc.2007.454. [DOI] [PubMed] [Google Scholar]
- Joy D, Thava VR, Scott BB. Diagnosis of fatty liver disease: is biopsy necessary.? Eur. J. Gastroenterol. Hepatol. 2003;15:539–543. doi: 10.1097/01.meg.0000059112.41030.2e. [DOI] [PubMed] [Google Scholar]
- Kim K, Huang S, Hall TL, Witte RS, Chenevert TL, O’Donnell M. Arterial vulnerable plaque characterization using ultrasound-induced thermal strain imaging (TSI) IEEE Trans. Biomed. Eng. 2008;55:171–180. doi: 10.1109/TBME.2007.900565. [DOI] [PubMed] [Google Scholar]
- Lai C-Y, Kruse DE, Caskey CF, Stephens DN, Sutcliffe PL, Ferrara KW. Noninvasive Thermometry Assisted by a Dual-Function Ultrasound Transducer for Mild Hyperthermia. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2010;57:2671–2684. doi: 10.1109/TUFFC.2010.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubinski MA, Emelianov SY, O’Donnell M. Speckle tracking methods for ultrasonic elasticity imaging using short-time correlation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1999;46:82–96. doi: 10.1109/58.741427. [DOI] [PubMed] [Google Scholar]
- MaassMoreno R, Damianou CA. Noninvasive temperature estimation in tissue via ultrasound echo-shifts .1. Analytical model. J. Acoust. Soc. Am. 1996;100:2514–2521. doi: 10.1121/1.417359. [DOI] [PubMed] [Google Scholar]
- Mahmoud AM, Dutta D, Lavery L, Stephens DN, Villanueva FS, Kim K. Noninvasive detection of lipids in atherosclerotic plaque using ultrasound thermal strain imaging: in vivo animal study. J. Am. Coll. Cardiol. 2013 doi: 10.1016/j.jacc.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NR, Bamber JC, Meaney PM. Fundamental limitations of noninvasive temperature imaging by means of ultrasound echo strain estimation. Ultrasound Med. Biol. 2002;28:1319–1333. doi: 10.1016/s0301-5629(02)00608-7. [DOI] [PubMed] [Google Scholar]
- Miller NR, Bamber JC, ter Haar GR. Imaging of temperature-induced echo strain: preliminary in vitro study to assess feasibility for guiding focused ultrasound surgery. Ultrasound Med. Biol. 2004;30:345–356. doi: 10.1016/j.ultrasmedbio.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Nasoni R, Bowen T. Ultrasonic speed as a parameter for non-invasive thermometry. Non-Invasive Temp. Meas. 1989:95–107. [Google Scholar]
- O’Brien WD. Ultrasound–biophysics mechanisms. Prog. Biophys. Mol. Biol. 2007;93:212–255. doi: 10.1016/j.pbiomolbio.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien WD, Deng CX, Harris GR, Herman BA, Merritt CR, Sanghvi N, Zachary JF. The risk of exposure to diagnostic ultrasound in postnatal subjects - Thermal effects. J. Ultrasound Med. 2008;27:517–535. doi: 10.7863/jum.2008.27.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton GF, Dahl JJ, Trahey GE. Rapid tracking of small displacements with ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2006;53:1103–1117. doi: 10.1109/tuffc.2006.1642509. [DOI] [PubMed] [Google Scholar]
- Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, Khalil L, Turpin G, Opolon P, Poynard T. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–+. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J. Hepatol. 2009;51:433–445. doi: 10.1016/j.jhep.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Sehgal CM, Brown GM, Bahn RC, Greenleaf JF. Measurement and use of acoustic nonlinearity and sound speed to estimate composition of excised livers. Ultrasound Med. Biol. 1986;12:865–874. doi: 10.1016/0301-5629(86)90004-9. [DOI] [PubMed] [Google Scholar]
- Seip R, Ebbini E. Noninvasive estimation of tissue Temperature response to heating fields using diagnostic ultrasound. IEEE Trans. Biomed. Eng. 1995;42:828–839. doi: 10.1109/10.398644. [DOI] [PubMed] [Google Scholar]
- Seo CH, Shi Y, Huang S-W, Kim K, O’Donnell M. Thermal strain imaging: a review. Interface Focus. 2011a;1:649–664. doi: 10.1098/rsfs.2011.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo CH, Shi Y, Huang S-W, Kim K, O’Donnell M. Thermal strain imaging: a review. Interface Focus. 2011b;1:649–664. doi: 10.1098/rsfs.2011.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Witte RS, O’Donnell M. Identification of vulnerable atherosclerotic plaque using IVUS-based thermal strain imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2005;52:844–850. doi: 10.1109/tuffc.2005.1503971. [DOI] [PubMed] [Google Scholar]
- Siegelman ES, Rosen MA. Imaging of hepatic steatosis. Semin. Liver Dis. 2001;21:71–80. doi: 10.1055/s-2001-12930. [DOI] [PubMed] [Google Scholar]
- Souchon R, Bouchoux G, Maciejko E, Lafon C, Cathignol D, Bertrand M, Chapelon J-Y. Monitoring the formation of thermal lesions with heat-induced echo-strain imaging: A feasibility study. Ultrasound Med. Biol. 2005;31:251–259. doi: 10.1016/j.ultrasmedbio.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Mahmoud AM, Ding X, Lucero S, Debaditya D, Yu FTH, Chen X, Kim K. Flexible Integration of Both High Imaging Resolution and High Power Arrays for Ultrasound-Induced Thermal Strain Imaging (US-TSI) IEEE Trans. Ultrason. Ferroelectr. Freq. Control. doi: 10.1109/TUFFC.2013.2863. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, Mahmoud AM, Dutta D, Lucero S, Kim K. Flexible Integration of Both High Imaging Resolution and High Power Arrays for TSI; Presented at the the IEEE International Ultrasonics Symposium; Dresden, Germany. 2012. [Google Scholar]
- Strauss S, Gavish E, Gottlieb P, Katsnelson L. Interobserver and Intraobserver variability in the sonographic assessment of fatty liver. Am. J. Roentgenol. 2007;189:W320–W323. doi: 10.2214/AJR.07.2123. [DOI] [PubMed] [Google Scholar]
- Teli MR, James OFW, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: A follow-up study. Hepatology. 1995;22:1714–1719. [PubMed] [Google Scholar]
- Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–1698. doi: 10.1053/j.gastro.2008.02.077. [DOI] [PubMed] [Google Scholar]
- Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, Torimura T, Inuzuka S, Sata M, Tanikawa K. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J. Hepatol. 1997;27:103–107. doi: 10.1016/s0168-8278(97)80287-5. [DOI] [PubMed] [Google Scholar]
- Varghese T, Zagzebski JA, Chen Q, Techavipoo U, Frank G, Johnson C, Wright A, Lee FT. Ultrasound monitoring of temperature change during radiofrequency ablation: Preliminary in-vivo results. Ultrasound Med. Biol. 2002;28:321–329. doi: 10.1016/s0301-5629(01)00519-1. [DOI] [PubMed] [Google Scholar]
- Webb M, Yeshua H, Zelber-Sagi S, Santo E, Brazowski E, Halpern Z, Oren R. Diagnostic Value of a Computerized Hepatorenal Index for Sonographic Quantification of Liver Steatosis. Am. J. Roentgenol. 2009;192:909–914. doi: 10.2214/AJR.07.4016. [DOI] [PubMed] [Google Scholar]