Summary
Contrast staining of brain parenchyma identified on non-contrast CT performed after DSA in patients with acute ischemic stroke (AIS) is an incompletely understood imaging finding. We hypothesize contrast staining to be an indicator of brain injury and suspect the fate of involved parenchyma to be cerebral infarction.
Seventeen years of AIS data were retrospectively analyzed for contrast staining. Charts were reviewed and outcomes of the stained parenchyma were identified on subsequent CT and MRI.
Thirty-six of 67 patients meeting inclusion criteria (53.7%) had contrast staining on CT obtained within 72 hours after DSA. Brain parenchyma with contrast staining in patients with AIS most often evolved into cerebral infarction (81%). Hemorrhagic transformation was less likely in cases with staining compared with hemorrhagic transformation in the cohort that did not have contrast staining of the parenchyma on post DSA CT (6% versus 25%, respectively, OR 0.17, 95% CI 0.017 – 0.98, p = 0.02).
Brain parenchyma with contrast staining on CT after DSA in AIS patients was likely to infarct and unlikely to hemorrhage.
Key words: stroke recovery, radiology, angiography, contrast media, interventional neuroradiology, contrast stain
Introduction
In acute ischemic stroke (AIS) patients, brain parenchyma contrast staining on CT after recanalization therapy and DSA has been attributed to transient loss of BBB integrity1-4.
Several groups have linked a loss of BBB to increased risk of hemorrhagic transformation after recanalization therapy5,6, and particularly have linked brain parenchyma contrast staining on CT after intra-arterial (IA) contrast injection with hemorrhagic transformation2,7,9. One group found contrast staining to be an independent predictor of death10.
However, contrast staining does not always predict hemorrhagic transformation or clinical deterioration; in fact, resolution of the contrast staining may be a good prognostic indicator11,12.
Increasing frequency of intravenous (IV) and IA reperfusion therapy for AIS has led to an increased recognition of brain parenchyma contrast staining after stroke intervention. A better understanding of the clinical significance of this imaging sign may lead to improved prognostic information for patients following AIS interventions.
The aim of this study was to assess contrast staining on post-DSA CT to determine the fate of contrast-stained brain.
We also evaluated parameters of stroke patient management that may influence the likelihood of contrast staining on post-DSA CT. Our hypothesis was that contrast staining is an indicator of parenchyma damage, and ultimately will progress to infarction.
Methods
Our institution's Committee on Human Research approved this retrospective analysis of patient charts and imaging data.
Patient selection
Patients who received AIS interventions and DSA were retrospectively analyzed. Inclusion criteria for the study were: AIS patients with a head CT examination within 72 hours following DSA and a second, more delayed follow-up non-contrast head CT or brain MRI. Patients were excluded from the study if their imaging studies were incomplete or non-diagnostic due to motion or other artifacts. Patients with hemorrhage on CT prior to intervention were excluded from intervention.
Imaging analysis
Two independent American Board of Radiology certified, neuroradiology fellowship trained radiologists (MA and SH) independently reviewed all imaging studies blinded to each other's interpretations, details of the intervention performed, as well as patient outcomes. The initial CT after DSA was viewed to identify if there was parenchyma contrast staining. Parenchyma contrast staining was defined in Hounsfield units (HU) as high density (HU > 40) conforming to the boundaries of a normal anatomic structure, without mass effect or surrounding edema (Figure 1). Each reader recorded the average density of the contrast-stained brain parenchyma.
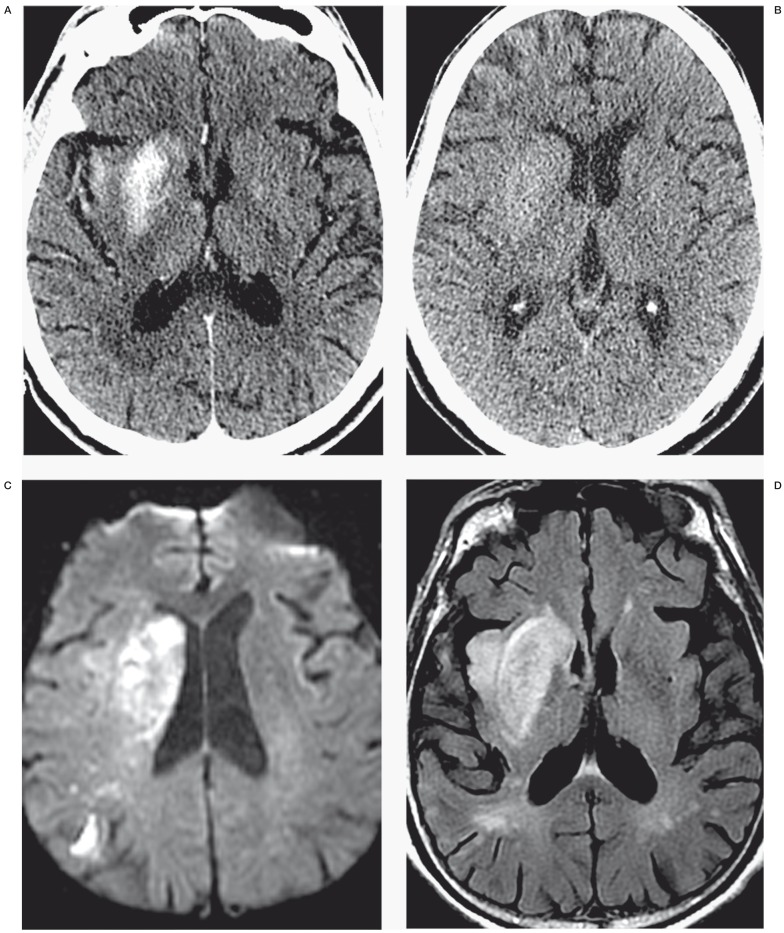
Figure 1.
Identification of contrast staining and differentiating contrast staining from hemorrhage. The first post-angiogram CT of four different AIS patients (panels A through D) demonstrates the difference between contrast staining (A,B) and parenchymal hemorrhage (C,D). Note high-density material corresponds to the right putamen and insula cortex in panel A and left lateral putamen and cortex of the left frontal and temporal lobes in panel B, without significant mass effect or surrounding edema. High density from parenchymal hemorrhage (C,D) is differentiated from contrast staining (A,B) by lack of conformation to anatomic boundaries, significant mass effect and surrounding edema. Cases presented in panels C and D also have subarachnoid hemorrhage including intraventricular hemorrhage (C), and subdural hemorrhage (D).
Once contrast staining was identified, its location was then classified. Staining was first classified as to the vascular territories involved: anterior circulation, posterior circulation, or both. The readers next determined if gray matter, white matter, or both appeared to be stained. If gray matter was identified as being involved, it was further classified as cortex, deep gray nuclei, or both.
Non-contrast CTs obtained prior to intervention were evaluated for CT evidence of ischemic stroke as per PROACT II trial definition of early CT signs of stroke in the areas of contrast staining. To prove that the high density (contrast staining) was not present prior to intervention, CTA and post contrast CT performed prior to angiography were evaluated for high density in the areas of contrast staining.
The second delayed follow-up CT, or MRI when available, was analyzed to determine the imaging fate of stained brain. Each of the classified areas of staining was independently graded as to its outcomes. For example, if the anterior circulation gray matter cortex and deep gray were stained, the cortex and deep gray structures were independently followed for outcome.
The potential outcomes were: persistent staining, hemorrhagic transformation, infarction, or return to normal structure. Disagreements on image interpretation between the two primary readers were settled by a third independent ABR certified, neuroradiology fellowship trained radiologist (DC).
Clinical data
To further elucidate if treatment methods predict post-angiographic contrast staining, the method of stroke intervention was recorded for each patient. These methods included: IV rtPA, IA rtPA, mechanical clot disruption with wire maceration, balloon angioplasty, and mechanical thrombectomy with Merci (Stryker Neurovascular, Fremont, CA, USA), Penumbra (Penumbra Inc., Alameda, CA, USA), or Solitaire FR (ev3, Irvine, CA, USA) devices.
The time period between stroke onset and DSA, as well as from DSA to first follow-up CT and second follow-up CT (or MRI) were recorded, as was the length of the DSA procedure.
Medical records were analyzed for modified Rankin Scores (mRS) at initial evaluation and at discharge.
Statistical analysis
Statistical analysis was performed using STATA SE version 12.1 (College Station, TX, USA). Binomial logistic regression was employed to analyze if the patients' mRS at presentation, timing from stroke onset to intervention, duration of DSA procedure, or timing from end of procedure to CT differed between patients with presence or absence of contrast staining.
The Wilcoxon rank-sum test was used to evaluate any differences in discharge mRS between patients with contrast staining and those without.
Intervention methods were evaluated with Fisher's exact test, case control odds ratios, and Pearson's test to determine significance. Fisher's exact test and case control odds ratios were also used to evaluate if patients with contrast staining of their parenchyma were more or less likely to have hemorrhagic transformation of their stroke.
Results
Patient population
Seventeen years of data from stroke interventions at our institution were retrospectively analyzed (April 1995 to June 2012). During that time period, 156 patients underwent recanalization therapy and DSA. Of those 156, 100 had a follow-up CT within 72 hours after their angiogram. Of these 100, 71 had a subsequent delayed follow-up CT or MRI. Four patients had incomplete follow-up CT scans and were excluded, yielding a cohort of 67 patients (Table 1).
Table 1.
Comparative demographics of acute ischemic stroke (AIS) patients without contrast staining on first CT after DSA and those with staining.
|
AIS patients without contrast stain |
AIS patients with contrast stain |
||
| N | 31 | 36 | |
| Female subjects | 20 | 22 | |
| Age in years (mean ± SD) | 62 ± 16.4 | 67 ± 14.6 | p = 0.39 |
|
Median initial mRS (25 %-ile, 75 %-ile) |
1 (0, 3) |
3 (0, 4) |
OR 1.32, 95% CI 0.99 − 1.75, p = 0.06 |
|
Initial mRS≤2
Initial mRS≥3 |
22 8 |
16 19 |
OR 0.31, 95% CI 0.093 − 0.98, p = 0.043 |
|
Median discharge mRS (25 %-ile, 75 %-ile) |
4 (3, 5.25) |
4 (3, 5) |
p=0.45 |
Patients with higher presentation mRS were marginally more likely to have contrast staining after recanalization therapy and DSA (OR 1.32, 95% CI 0.99 – 1.75, p = 0.06). Grouping the presentation mRS into two groups, mRS≤2 versus mRS>2, confirmed patients with higher presentation mRS were more likely to have contrast staining (OR 3.23, 95% CI 1.02 – 10.75, p=0.043). Conversely, contrast staining on post-DSA CT did not correlate with higher or lower mRS at discharge (p=0.45).
Identification of staining
None of the CTAs or post-contrast CTs performed prior to intervention demonstrated enhancement prior to intervention. Of the 67 patients who met the inclusion criteria, 36 (53.7%) had contrast staining of parenchyma on first follow-up non-contrast head CT. The readers independently identified 35 studies as demonstrating contrast staining with discordance on one (97% concordance). The average density of stained brain measured by the readers was 77.2 HU and 66.3 HU, respectively, with a range from 41 to 184 HU. Of the 36 patients with contrast staining, four cases (11.1%) of contrast staining had an average density measurement of greater than 90 HU. None of the patients with contrast staining measuring greater than 90 HU had hemorrhagic transformation. Whereas 84% of patients had no CT signs of stroke on pre-intervention non-contrast CT in the area of subsequent contrast staining, 16% of patients had loss of gray white differentiation in part of the territory ultimately affected by contrast staining.
Anatomic location of staining
Anatomic locations of contrast staining are summarized in Table 2. All cases of contrast staining demonstrated stain in the gray matter. Only 19% of cases also had white matter stain. No cases demonstrated stain in the white matter without involvement of gray matter. Cortex was more likely to demonstrate staining compared to the deep gray structures (81% and 48%, respectively). The majority of cases with deep gray nuclei staining also had cortex staining (71%).
Table 2 .
Location of contrast staining.
| Staining locations | |||||
| Anterior circulation | Posterior circulation | ||||
| Gray matter | White matter | Gray matter | White matter | ||
| 31& | 7* | 6# | 0 | ||
| Deep gray | Cortex | Deep gray | Cortex | ||
| 15 | 25 | 3 | 6 | ||
|
&: Six patients had deep gray staining without cortical staining in the anterior circulation, 0 had staining of deep gray without cortical stain in the posterior circulation; *: All patients with white matter staining also had gray matter staining; #: One patient with posterior cortex stain also had anterior gray stain. Review of the imaging demonstrates the posterior cortex stain to be in the MCA-PCA watershed zone. | |||||
Patients with anterior circulation AIS were more likely to have parenchyma contrast staining on follow-up CT than patients with a posterior circulation infarction (OR 3.41, 95% CI 0.91 – 14.23, p=0.048) (Table 3).
Table 3.
Outcome of contrast staining on last imaging study.
| Outcome | N | % |
| Infarction | 31 | 86% |
| Hemorrhage | 2 | 6% |
| Normal | 7 | 19% |
| Still Stained | 7 | 19% |
Outcome of staining
Contrast-stained brain parenchyma evolved into cerebral infarction in 31 of 36 cases (86%). Continued staining was demonstrated in seven cases (19%). Parenchyma with staining had a normal imaging appearance in follow-up in seven cases (19%) (Table 3, Figure 2). Hemorrhagic transformation of infarction was less likely in patients with contrast staining than in those without contrast staining (6% versus 26%, respectively, OR 0.17, 95% CI 0.017 – 0.98, p = 0.02).
Figure 2.
Example of contrast staining and imaging follow-up with MRI. A 73-year-old man presenting with left hemiparesis and left gaze preference underwent mechanical embolectomy for a right carotid terminus clot four hours after he was last seen normal. CT performed two hours after DSA (A) demonstrates high-density material conforming to the right anterior insular cortex, right putamen and to a lesser extent the head of the right caudate. Follow-up CT eight hours later demonstrates decreased conspicuity of the density and loss of gray-white differentiation in these regions. MRI performed 24 hours after DSA demonstrates the high density in the insula, putamen and caudate have reduced diffusion (C) and hyperintensity on the T2 FLAIR (D) consistent with infarction.
Timing of intervention/duration of DSA/DSA to follow-up imaging
Patients with staining had shorter time intervals between stroke symptom onset and DSA (p=0.03), as well as shorter time intervals between DSA and follow-up CT (p = 0.03). Duration of the DSA procedure in patients with staining was similar to those without staining (p = 0.38) (Table 4).
Table 4.
Comparison of treatment and imaging timing variables in patients with and without contrast staining.
| Timing Variable (hours) | No Contrast Stain | Contrast Stain | T-test |
| Time to DSA, median (± SD) |
5.3 ± 18.0 | 3.7 ± 4.0 | p = 0.03 |
| Duration of DSA, mean (± SD) |
3.7 ± 1.1 | 4.0 ± 1.2 | p = 0.38 |
| Time from DSA to first follow-up CT, mean (± SD) |
14.5 ± 9.3 | 9.4 ± 5.9 | p = 0.03 |
Method of intervention
IV rtPA and IA rtPA use were not correlated with contrast staining on CT after DSA (p=0.33 and 0.14 IV and IA, respectively). Mechanical disruption of clot (via clot retrieval device, wire maceration, angioplasty, or stent) was similarly unrelated to the presence of contrast staining on follow-up CT (p = 0.10).
Discussion
Contrast-stained brain parenchyma on CT after DSA in acute ischemic stroke (AIS) patients most often progressed to infarction in our study. Hemorrhagic transformation was less likely in patients with contrast staining on CT after DSA than in those patients who did not have any contrast staining. We found an incidence of contrast staining of 53.7%, which falls within the previously published range of 31.2 to 60%7,11,13-16.
Six per cent of our patients had hemorrhagic transformation of their region of high density. Previously published reports of hemorrhagic transformation of contrast-stained brain range from 0% to 60%7,11. This wide disparity may be related to non-standardized imaging criteria for identifying contrast staining, difficulty in differentiating staining from hemorrhage, and differences in the definitions used for hemorrhagic transformation.
Previous authors have described high density in the brain after reperfusion therapy, which may be either hemorrhage or contrast7,11,13-16. Our study is different in that we attempt to differentiate hemorrhage from contrast. We defined contrast staining as high density (>40 HU) on post-DSA CT, which conformed to the anatomic boundaries of normal structures, lacked surrounding edema, and had no mass effect. Conformation to normal anatomic boundaries and lack of surrounding edema or mass effect allowed for consistent and reproducible differentiation of contrast staining from parenchymal hemorrhage and the radiologic spot sign of active contrast extravasation 17. The outcome of contrast staining, as distinguished from parenchymal hemorrhage, identified on the first CT examination after DSA, has not been previously evaluated.
Intracranial hemorrhage definitions have also varied among studies evaluating the likelihood of hemorrhagic transformation in cases of contrast staining. If we were to utilize the lexicon used by the European Cooperative Acute Stroke Study (ECASS) I and II14,18,19 that was later translated into clinically significant hemorrhage by Berger et al.20 we are evaluating the likelihood of patients developing hemorrhagic infarct (HI) specifically parenchymal hemorrhage, which is differentiated from hemorrhagic transformation by mass effect. Parenchymal hemorrhage (specifically type 2) was shown by Berger et al. to be the only hemorrhagic infarction that independently causes clinical deterioration and impairs prognosis. Our study follows this guideline using PH2 as the definition for the primary outcome of hemorrhage. Using this as our definition of significant hemorrhagic transformation, only 6% of patients with contrast staining subsequently developed hemorrhage.
A recent article on contrast staining by Parrilla et al. also found few cases of contrast staining to progress to symptomatic intracranial hemorrhage13. Patients were excluded from that 2012 study if they received rtPA. Our experience is more generalizable in that it demonstrated the method of intervention, both mechanical and pharmacological, did not have an impact on the incidence of contrast staining.
Nakano et al.'s is probably the benchmark article to note an increased incidence of hemorrhagic transformation in patients with contrast staining3. The disparity between our results and those of Nakano et al. can be partially explained by differences in definitions of hemorrhagic transformation. In their study, persistent contrast staining beyond 24 hours was defined as hemorrhagic transformation of infarction. Our experience demonstrated contrast staining may persist beyond 24 hours, and there may be little or no hemorrhagic transformation in these cases. It is possible that our findings of high density persisting beyond 24 hours could include cases of hemorrhagic infarction types 1 and 2. In cases when an MRI was available, it served as a benchmark evaluation to exclude subclinical hemorrhage. It is possible that our study overlooked some cases of subclinical hemorrhage (HI), but clinically significant hemorrhage is a much more meaningful outcome to patient care and only PH2 (associated with mass effect) has been found to independently predict negative clinical outcome20. Our results are a more clinically oriented outcome focused on evaluating for hemorrhagic events that are significant to patient prognosis.
Mericle et al. proposed contrast staining measuring less than 90 HU differed from contrast extravasation measuring greater than 90 HU based on their experience12. Yoon et al. applied the 90 HU cut-off criteria for distinguishing extravasation from contrast staining to their cohort of anterior circulation infarction cases, and found an incidence of contrast staining of 22.6% with a contrast extravasation incidence of 11.3% (seven patients out of 62)15. Our study found only four cases out of our 36 patients with contrast staining to have an average density measurement greater than 90 HU, none of whom went on to demonstrate hemorrhagic transformation on follow-up imaging. We propose a more robust method for differentiating contrast staining from early hemorrhagic transformation by emphasizing the importance of associated radiologic findings of conforming to normal anatomic structure boundaries and lack of mass effect.
Contrast staining was more likely in cases of anterior circulation ischemia. Vasoreactivity and autoregulation are well known to differ between the anterior and posterior circulations21,22. While there is still debate as to the exact pathophysiology of reversible posterior leukoencephalopathy syndrome (RPLS) or posterior reversible encephalopathy syndrome (PRES), the relative insensitivity of the posterior circulation to autoregulation has been established in transcranial Doppler studies23. Perhaps this insensitivity to autoregulation in cases of stroke helps limit the amount of contrast seepage into the parenchyma.
The longer the time period between stroke symptom onset and DSA, the more likely staining was to be found on follow-up CT. One could postulate staining occurs in severely ischemic parenchyma that may have already suffered from infarction at the time of the dense intra-arterial contrast injection. Staining thus might, in some cases, be an early CT imaging finding of acute infarction.
Contrast staining also appears to be a transient phenomenon. Contrast staining was more likely to be identified if the time interval between the DSA and the follow-up CT was shorter. Blood often reaches areas of parenchyma that have already been damaged by acute stroke. It is well known that areas of infarction may have hyperperfusion, which has been termed “luxury perfusion”. It is possible that this return of perfusion to these areas may help to account for the transient nature of contrast enhancement. Additionally, while contrast-stained parenchyma most often progresses to infarction, in some cases the area of staining returned to normal brain.
We acknowledge several limitations of our study. Our study was designed as a retrospective analysis of an imaging finding. A more powerful study would be to prospectively identify contrast staining on CT after DSA and subsequently obtain an MRI to determine the fate of the high-density parenchyma. While several of our cases did have MRI follow-up with T2* imaging to definitively determine the presence of hemorrhage in the area of interest, the majority of AIS cases treated more than five years ago did not. CT is less sensitive than MRI with T2* imaging in the detection of small parenchymal brain hemorrhage. However, CT has been shown to be able to detect clinically significant hemorrhage in stroke patients after reperfusion therapy, and CT was more widely available in our patient cohort. Also, given that many patients with large vessel occlusion ischemic strokes have cardiac arrhythmias (and therefore pacemakers), MRI is not possible in all AIS patients. Therefore, CT-based methods for predicting ultimate infarct size remain useful in many clinical settings.
Additionally, the amount of contrast used in each procedure may correlate with the presence of contrast staining. When reviewing the records of procedures greater than 15 years, we found the volume of contrast estimated to be unreliable. However, the duration of the procedure was reliably recorded. Procedure length was calculated from the time of the patient entry to physically exiting the suite. Even though this includes patient intubation time, preparing the groin site for access, draping the patient, transferring the patient to and from the gurney, etc., we estimated these preparatory and finalizing procedures required a similar amount of time for each of the procedures and large fluctuations in the duration of the procedure could be attributed to a longer intervention. While procedure length is not a direct measurement of the amount of contrast used, it serves as an estimate. The amount of contrast may be a confounding factor in this study.
Subacute infarction is another possible cause for enhancement of brain parenchyma. Although in theory, subacute infarctions could enhance, we should not expect to see enhancement since the timing of these infarctions is acute. Additionally, no enhancement was demonstrated on pre-procedure contrast-enhanced CT (an additional delayed CT acquisition obtained in the venous phase after the CTA) that would suggest a delayed presentation of a patient in the subacute phase of infarction.
The physiology behind contrast staining of parenchyma in stroke is not clearly understood. Several authors have postulated brain tissue experiencing a high concentration of contrast, usually via direct intra-arterial injection past a thrombus, would be likely to stain secondary to a combination of breakdown of the BBB and the inherent toxic effects of contrast1,2,11. Transient loss of the BBB is strongly predictive of impending hemorrhagic transformation5,6. However, the paucity of hemorrhagic transformation in patients with parenchyma staining relative to the group without staining seems to argue against the theory of breakdown of the BBB. We hypothesize a no-reflow phenomenon at the capillary level may help account for high-density parenchyma on CT after DSA26-30. Lack of sufficient revascularization at the capillary level in cerebral infarction (even in cases with large vessel recanalization), and thus a lack of blood flowing through the territory at the capillary levels to “wash out” contrast, could be a contributing factor to contrast staining of brain parenchyma. The no-reflow phenomenon was initially described as a contributing factor, along with duration of ischemia, by Ames et al. in their evaluation of severity of stroke using a rabbit stroke model published in 196826. In these experiments, there was an impaired ability to return blood flow to the parenchyma level even when the largest vessels were completely recanalized, which was attributed to a combination of factors resulting in occlusion of blood flow in the capillaries even when the larger vessels are visibly patent. One could postulate a similar phenomenon is happening in cases of contrast staining. In the ischemic brain, cerebral vasculature autoregulation results in maximum vasodilation. When vessel recanalization is attempted in the angiography suite, the maximally dilated capillaries are filled with contrast. During angiography, the operator may see hyperemia in the region of ischemia. Perhaps a combination of factors including cellular swelling limits the ability of blood, as contrast is progressively cleared from the blood pool, to continue to perfuse these capillary beds leading to contrast that is not “washed out” from the affected tissue.
Conclusion
In summary, we defined criteria to differentiate brain parenchyma contrast staining from hemorrhagic transformation on CT after DSA in patients with AIS. Areas of contrast staining were likely to infarct and unlikely to hemorrhage.
Acknowledgments
We acknowledge the support of the UCSF Dean's Office Medical Student Research Program. We also thank Drs Alisa Gean and Wade Smith for helpful discussions.
References
- 1.Ito U, Tomita H, Kito K, et al. CT enhancement after prolonged high-dose contrast infusion in the early stage of cerebral infarction. Stroke. 1986;17:424–430. doi: 10.1161/01.str.17.3.424. doi: 10.1161/01.STR.17.3.424. [DOI] [PubMed] [Google Scholar]
- 2.Numaguchi Y, Fleming MS, Hasuo K, et al. Blood-brain barrier disruption due to cerebral arteriography: CT findings. J Comput Assist Tomogr. 1984;8(5):936–939. doi: 10.1097/00004728-198410000-00024. [DOI] [PubMed] [Google Scholar]
- 3.Nakano S, Iseda T, Yoneyama T, et al. Early CT signs in patients with acute middle cerebral artery occlusion: incidence of contrast staining and haemorrhagic transformations after intra-arterial reperfusion therapy. Clin Radiol. 2006;61(2):156–162. doi: 10.1016/j.crad.2005.08.016. doi: 10.1016/j.crad.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Roberts HC, Dillon WP, Furlan AJ, et al. Computed tomographic findings in patients undergoing intra-arterial thrombolysis for acute ischemic stroke due to middle cerebral artery occlusion: results from the PROACT II trial. Stroke. 2002;33(6):1557–1565. doi: 10.1161/01.str.0000018011.66817.41. doi: 10.1161/01.STR.0000018011.66817.41. [DOI] [PubMed] [Google Scholar]
- 5.Kassner A, Mandell DM, Mikulis DJ. Measuring permeability in acute ischemic stroke. Neuroimaging Clin N Am. 2011;21(2):315–325. doi: 10.1016/j.nic.2011.01.004. doi: 10.1161/01.STR.0000018011.66817.41. [DOI] [PubMed] [Google Scholar]
- 6.Lee M, Saver JL, Alger JR, et al. Blood-brain barrier permeability derangements in posterior circulation ischemic stroke: frequency and relation to hemorrhagic transformation. J Neurol Sci. 2012;313(1-2):142–146. doi: 10.1016/j.jns.2011.08.048. doi: 10.1016/j.jns.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakano S, Iseda T, Kawano H, et al. Parenchymal hyperdensity on computed tomography after intra-arterial reperfusion therapy for acute middle cerebral artery occlusion: incidence and clinical significance. Stroke. 2001;32(9):2042–2048. doi: 10.1161/hs0901.095602. doi: 10.1161/hs0901.095602. [DOI] [PubMed] [Google Scholar]
- 8.Yokogami K, Nakano S, Ohta H, et al. Prediction of hemorrhagic complications after thrombolytic therapy for middle cerebral artery occlusion: value of pre- and post-therapeutic computed tomographic findings and angiographic occlusive site. Neurosurgery. 1996;39(6):1102–1107. doi: 10.1097/00006123-199612000-00006. doi: 10.1097/00006123-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Hornig CR, Dorndorf W, Agnoli AL. Hemorrhagic cerebral infarction--A prospective study. Stroke. 1986;17:179–185. doi: 10.1161/01.str.17.2.179. doi: 10.1161/01.STR.17.2.179. [DOI] [PubMed] [Google Scholar]
- 10.Becker KJ, Baxter AB, Bybee HM, et al. Extravasation of radiographic contrast is an independent predictor of death in primary intracerebral hemorrhage. Stroke. 1999;30:2025–2032. doi: 10.1161/01.str.30.10.2025. doi: 10.1161/01.STR.30.10.2025. [DOI] [PubMed] [Google Scholar]
- 11.Wildenhain SL, Jungreis CA, Barr J, et al. CT after intracranial intraarterial thrombolysis for acute stroke. Am J Neuroradiol. 1994;15(3):487–492. [PMC free article] [PubMed] [Google Scholar]
- 12.Mericle RA, Lopes DK, Fronckowiak MD, et al. A grading scale to predict outcomes after intra-arterial thrombolysis for stroke complicated by contrast extravasation. Neurosurgery. 2000;46(6):1307–1314. doi: 10.1097/00006123-200006000-00005. discussion 14-15. doi: 10.1097/00006123-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Parrilla G, Garcia-Villalba B, Espinosa de Rueda M, et al. Hemorrhage/contrast staining areas after mechanical intra-arterial thrombectomy in acute ischemic stroke: imaging findings and clinical significance. Am J Neuroradiol. 2012;33(9):1791–1796. doi: 10.3174/ajnr.A3044. doi: 10.3174/ajnr.A3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolpert SM, Bruckmann H, Greenlee R, et al. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator. The rt-PA Acute Stroke Study Group. Am J Neuroradiol. 1993;14(1):3–13. [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon W, Seo JJ, Kim JK, et al. Contrast enhancement and contrast extravasation on computed tomography after intra-arterial thrombolysis in patients with acute ischemic stroke. Stroke. 2004;35(4):876–881. doi: 10.1161/01.STR.0000120726.69501.74. doi: 10.1161/01.STR.0000120726.69501.74. [DOI] [PubMed] [Google Scholar]
- 16.Jang YM, Lee DH, Kim HS, et al. The fate of high-density lesions on the non-contrast CT obtained immediately after intra-arterial thrombolysis in ischemic stroke patients. Korean J Radiol. 2006;7(4):221–228. doi: 10.3348/kjr.2006.7.4.221. doi: 10.3348/kjr.2006.7.4.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delgado Almandoz JE, Yoo AJ, Stone MJ, et al. The spot sign score in primary intracerebral hemorrhage identifies patients at highest risk of in-hospital mortality and poor outcome among survivors. Stroke. 2010;41:54–60. doi: 10.1161/STROKEAHA.109.565382. doi: 10.1161/STROKEAHA.109.565382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274(13):1017–1025. doi: 10.1001/jama.274.13.1017. [PubMed] [Google Scholar]
- 19.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352(9136):1245–1251. doi: 10.1016/s0140-6736(98)08020-9. doi: 10.1016/S0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 20.Berger C, Fiorelli M, Steiner T, et al. Hemorrhagic transformation of ischemic brain tissue: asymptomatic or symptomatic? Stroke. 2001;32(6):1330–1335. doi: 10.1161/01.str.32.6.1330. doi: 10.1161/01.STR.32.6.1330. [DOI] [PubMed] [Google Scholar]
- 21.Covarrubias DJ, Luetmer PH, Campeau NG. Posterior reversible encephalopathy syndrome: prognostic utility of quantitative diffusion-weighted MR images. Am J Neuroradiol. 2002;23:1038–1048. [PMC free article] [PubMed] [Google Scholar]
- 22.McKinney AM, Sarikaya B, Gustafson C, et al. Detection of microhemorrhage in posterior reversible encephalopathy syndrome using susceptibility-weighted imaging. Am J Neuroradiol. 2012;33(5):896–903. doi: 10.3174/ajnr.A2886. doi: 10.3174/ajnr.A2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haubrich C, Wendt A, Diehl RR, et al. Dynamic autoregulation testing in the posterior cerebral artery. Stroke. 2004;35:848–852. doi: 10.1161/01.STR.0000120729.99039.B6. doi: 10.1161/01.STR.0000120729.99039.B6. [DOI] [PubMed] [Google Scholar]
- 24.Kidwell CS, Chalela JA, Saver JL, et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA. 2004;292(15):1823–1830. doi: 10.1001/jama.292.15.1823. doi: 10.1001/jama.292.15.1823. [DOI] [PubMed] [Google Scholar]
- 25.The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. doi: 10.1161/01.STR.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 26.Ames A, 3rd, Wright RL, Kowada M, et al. Cerebral ischemia. II. The no-reflow phenomenon. Am J Pathol. 1968;52:437–453. [PMC free article] [PubMed] [Google Scholar]
- 27.del Zoppo GJ, Hallenbeck JM. Advances in the vascular pathophysiology of ischemic stroke. Thromb Res. 2000;98:73–81. doi: 10.1016/s0049-3848(00)00218-8. doi: 10.1016/S0049-3848(00)00218-8. [DOI] [PubMed] [Google Scholar]
- 28.Ito H. No-reflow phenomenon in patients with acute myocardial infarction: its pathophysiology and clinical implications. Acta Med Okayama. 2009;63:161–168. doi: 10.18926/AMO/31817. [DOI] [PubMed] [Google Scholar]
- 29.Kowada M, Ames A, 3rd, Majno G, et al. Cerebral ischemia. I. An improved experimental method for study; cardiovascular effects and demonstration of an early vascular lesion in the rabbit. J Neurosurg. 1968;28(2):150–157. doi: 10.3171/jns.1968.28.2.0150. [DOI] [PubMed] [Google Scholar]
- 30.Molina CA, Alvarez-Sabin J. Recanalization and reperfusion therapies for acute ischemic stroke. Cerebrovasc Dis. 2009;27(Suppl 1):162–167. doi: 10.1159/000200455. doi: 10.1159/000200455. [DOI] [PubMed] [Google Scholar]