Summary
Agenesis of carotid or vertebrobasilar arteries with rete formation is rare. The anterior spinal artery or posterior spinal arteries supplying the posterior circulation with steno-occlusion or agenesis of bilateral vertebral arteries is also uncommon. Here, we describe a very rare case of concomitant segmental agenesis of bilateral carotid and vertebral arteries with collateral compensations from the prominent anterior spinal artery and posterior spinal arteries, as well as some transdural arterial networks which were considered a rete mirabile. We discuss its embryological and anatomic significance.
Key words: arterial agenesis, carotid, vertebrobasilar, rete mirabile, anterior spinal artery, posterior spinal artery
Introduction
A carotid rete mirabile (“wonderful net” in Latin) is rare in humans and is defined as a transdural arterial network occurring at the cavernous portion of the internal carotid artery (ICA) 1. Since it was not present during normal development of the cranial circulation in humans, the carotid rete mirabile was considered a collateral pathway developing as a consequence of segmental agenesis of the ICA 2,3. Agenesis of vertebrobasilar arteries with rete formation is even rarer than that of ICA. As far as we know, ten cases of concomitant segmental agenesis of bilateral carotid and vertebrobasilar arteries with rete formation have been reported in the literature to date.
Retrograde flow from the anterior spinal artery (ASA) or posterior spinal arteries (PSAs) reconstructing the intracranial vertebral artery (VA) and basilar artery (BA) is an uncommon collateral channel in case of bilateral VAs stenosis or occlusion. An enlarged ASA supplying the posterior circulation with segmental agenesis of bilateral VAs is exceptional.
Here, we describe a new case of concomitant segmental agenesis of bilateral carotid and vertebral arteries with collateral compensations from the prominent ASA, PSAs, and some transdural arterial networks which were considered a rete mirabile.
Case Report
An 18-year-old female presented with intermittent headache, the first period of which occurred when she was five years old. The patient was then asymptomatic until one year ago, when she suffered from recurrent head pain in the left temporal area. Her medical history was otherwise normal and neurologic examination was negative. Laboratory test results of complete blood count, C-reactive protein, erythrocyte sedimentation rate, anti-streptolysin O, antinuclear antibody and complements were also unremarkable. MRI scan of the brain demonstrated left frontal and periventricular lesions hyperintense on T2-weighted imaging which suggested previous ischemic infarction. TOF-MRA disclosed some anomalous flow voids in the region of the cavernous sinus and around the pons and medulla. Some prominent tortuous vessels were found in the craniocervical junction. Bilateral anterior cerebral arteries (ACAs) were hypoplastic, and bilateral middle cerebral arteries (MCAs) were opacified through a prominent BA and bilateral posterior communicating arteries (PComAs). Lack of flow in the bilateral carotid siphon was demonstrated. Cranial base CT revealed bilateral narrowed carotid canals (Figure 1).
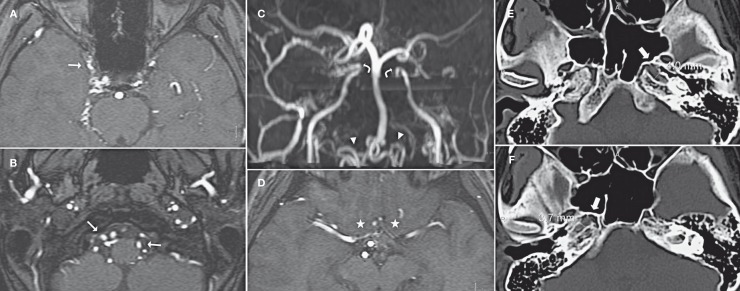
Figure 1.
TOF-MRA images (A-D) and Cranial base CT images (E.F). A network of thin vessels (thin arrow) was observed in the region of the cavernous sinus and around the pons and medulla. Some prominent tortuous vessels (arrowhead) were disclosed in the craniocervical junction. Both ICAs terminated at the level of cavernous sinus (curved arrow), and both MCAs and PCAs were supplied via a prominent BA and bilateral PComAs. Bilateral anterior cerebral arteries (ACAs) (asterisk) were hypoplastic. Bilateral narrowed carotid canals were also revealed (thick arrow).
Cerebral angiogram demonstrated that both ICAs were hypoplastic beginning from the carotid bifurcation and terminating at the cavernous sinus without typical moyamoya-like vessel formation. Right internal maxillary artery (IMA) branches filled the right ophthalmic artery (OA) and distal segment of right ICA through a transdural arterial network, which was considered an atypical carotid rete mirabile. An anomalous occipital branch of the right external carotid artery (ECA) filled the BA via a collateral network at the level of C1 cervical vertebra and the prominent tortuous vessels at the craniocervical junction (Figure 2). The distal segment of the left ICA was mainly supplied by the reverse flow from the left PComA. A hypoglossal branch of the left ECA also supplied the BA with another intradural network formation (Figure 3). An autosynangiosis, recruiting a frontal branch of the right MMA, an occipital branch of the left MMA, as well as a right marginal tentorial artery, developed to supply the right posterior frontal lobe, left medial temporal lobe and left occipital lobe respectively.
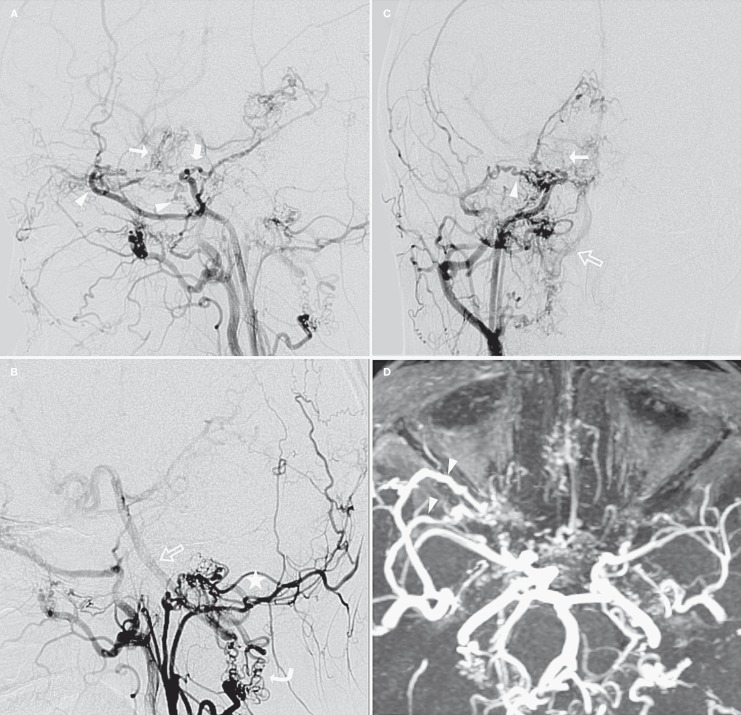
Figure 2.
Right carotid artery angiogram (A,C: RCCA in lateral and AP view; B: RECA in lateral view) and MIP images of TOF-MRA (D). The right ICA was hypoplastic beginning from the carotid bifurcation and terminating after sending off the right meningohypophyseal trunk (thick arrow). The 5th and 6th segments of the right ICA were not opacified. A transdural arterial network (atypical carotid rete) was demonstrated between the right OA and right MMA, deep temporal artery, foramen rotundum artery, and other distal branches of right IMA (arrowhead). The 7th segment of the right ICA (thin arrow), right MCA and ACA were faintly opacified by the arterial network and right OA. Note that an occipital branch of the right ECA supplied the BA (open arrow) via an anomalous collateral network at the level of the C1 cervical vertebra (curve arrow) and the prominent tortuous vessels at the craniocervical junction (asterisk).
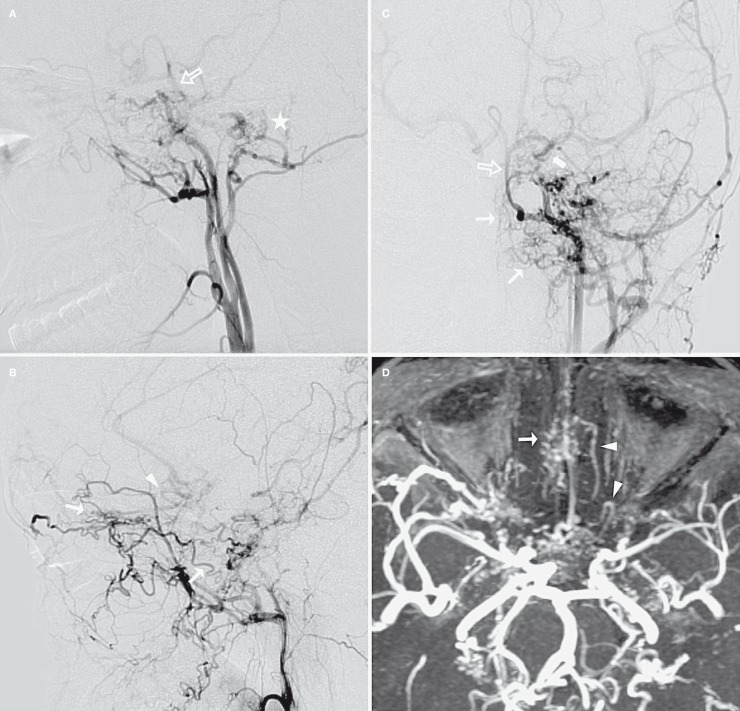
Figure 3.
Left carotid artery angiogram (A,C: LCCA in lateral and AP view; B: LECA in lateral view) and MIP images of TOF-MRA (D). The left ICA was also hypoplastic beginning from the carotid bifurcation, the 5th and 6th segments were tapered, and the 7th segment was mainly supplied by the reverse flow from left PComA (thick arrow). Abundant thin arterial collaterals from sphenopalatine, inferior orbital and angular branches of the left IMA were observed in the nasal cavity, ethmoidal sinus and ethmoidal planum (thin arrow). The A2 segment of the left ACA was also opacified by those collaterals through their anastomoses with a fronto-orbital branch of the left ACA. Note that the fronto-orbital artery made a peculiar “hair-pin” turn (arrowhead) in its proximal segment. A hypoglossal branch of the left ECA also supplied the BA (open arrow) with another intradural network formation (asterisk).
Bilateral VAs terminated before entering the cranium. The ASA and right posterior spinal artery (PSA) were both prominent and meandering, and they were supplied by several radiculomedullary arteries which set off from the right cervical VA at the level of the seventh, second (ASA) and fifth (PSA) cervical vertebra. The left PSA were supplied by several other radiculomedullary arteries which set off from the left VA at the level of the fourth and second cervical vertebra. The BA was supplied by the ASA and bilateral PSAs, instead of bilateral VAs, via some collateral vessels at the level of the C1 cervical vertebra and the prominent tortuous vessels at the craniocervical junction (vertebral rete mirabile). The prominent BA then constituted the major route of blood supply to bilateral MCAs via bilateral PComAs (Figure 4). Bilateral PCAs and the right anterior inferior cerebellar artery (AICA) were opacified through the BA flow, while bilateral superior cerebellar arteries, posterior inferior cerebellar arteries and left AICA were not observed. The cerebellum was mostly supplied by the bilateral posterior meningeal arteries, which set off from both ascending pharyngeal arteries.
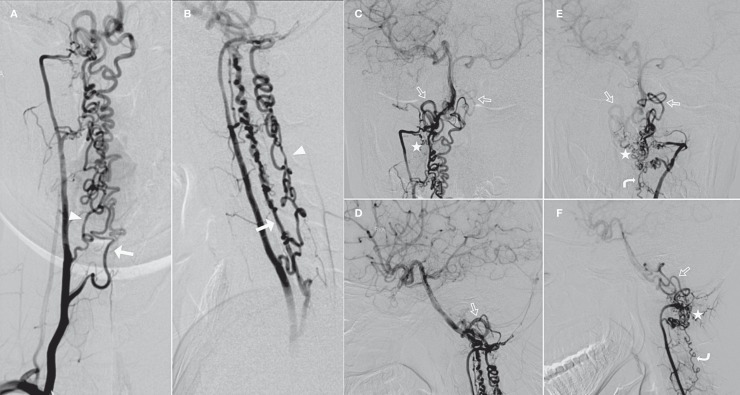
Figure 4.
Bilateral VAs and BA angiogram. (A,C: Right VA in AP view; B,D: Right VA in lateral view; E: Left VA in AP view; F: Left VA in lateral view). Bilateral VAs occluded before entering the cranium. The ASA (arrow) and the right PSA (arrowhead) were both prominent and meandering and were fed by several radiculomedullary arteries which set off from the right cervical VA at the level of the 7th, 2nd (ASA) and 5th (PSA) cervical vertebra. The enlarged left PSAs (curved arrow) were supplied by several other radiculomedullary arteries which set off from left VA at the level of the 4th and 2nd cervical vertebra. The BA was reconstituted by the prominent ASA and PSAs via some collateral networks (asterisk) at the level of C1 cervical vertebra and the prominent tortuous vessels at the craniocervical junction (open arrow) (vertebral rete). Bilateral MCAs and PCAs were opacified through the prominent BA.
Discussion
Carotid agenesis is a rare developmental anomaly. According to Lasjaunias et al. 4, when segmental agenesis of the ICA occurs between the primitive maxillary artery and the dorsal ophthalmic artery of the dorsal aorta in the human embryo, the “carotid rete mirabile” can be observed to reconstruct the distal flow of the ICA. The carotid rete mirabile has several unique angiographic features: 1) a rich arterial network supplied mostly by the branches of the ECA, especially those of the IMA; 2) hypoplasia of the ICA beginning from the carotid bifurcation; 3) the supraclinoid ICA is not hypoplastic and is fed by the arterial network and dilated OA; 4) no other abnormal vessels such as moyamoya are found in the intradural circulation; 5) bilateral lesions 5. According to the aforementioned criteria, the transdural collateral in the right cavernous portion in our case can be considered a carotid rete, although it was unilateral and did not seem as remarkable as those in other reports. We postulated that this might be because the distal right ICA and MCA were mainly supplied by the prominent BA and right PComA, instead of that transdural collateral.
Agenesis of vertebrobasilar arteries with rete compensation is even rarer than that of the ICA. Hyogo et al. 3 first reported a case of bilateral VAs agenesis with an abnormal arterial network of intracranial VAs, which they called “vertebral rete mirabile”, supplying the distal VAs and the BA. To the best of our knowledge, only ten cases of vertebrobasilar rete have been reported in the literature, and all of them were also associated with carotid rete (Table 1) 3,6,7-14. Among those cases, bilateral segmental agenesis of ICAs was observed in all; six cases had bilateral transdural VAs agenesis with transcranial rete formation, one had rete formation at the proximal segment of the right intracranial VA and duplication of the left VA, and four had segmental agenesis of the BA with various types of intradural rete. Here, a new case of concomitant segmental agenesis of bilateral transdural ICAs and VAs with rete formation in the skull base is reported.
Table 1.
Literature reports of concomitant agenesis of carotid and vertebrobasilar arteries with rete formation.
| Author | Age | Gender | ICA agenesis |
Vertebrobasilar arteries agenesis |
Blood supply of vertebrobasilar rete |
| Itoyama et al. | 40 | M | Bilateral ICAs | Transdural segment (V3/V4) of bilateral VAs |
V3 and V4 muscular branches of bilateral VAs |
| Hyogo et al. | 37 | F | Bilateral ICAs | Proximal V4 segment of right VA |
V4 segment of right VA |
| Mahadevan et al. | 39 | F | Bilateral ICAs | Transdural segment (V3/V4) of bilateral VAs |
ASA, lateral spinal artery, PICA |
| Weon et al. | 14 | F | Bilateral ICAs | V4 segment of bilateral VAs | Proximal BA |
| Kim et al. | 38 | M | Bilateral ICAs | BA | V3 and V4 meningeal branches of bilateral VAs |
| Li et al. | 57 | F | Bilateral ICAs | Transdural segment (V3/V4) of bilateral VAs |
ASA |
| Sahin et al. | 32 | M | Bilateral ICAs | Proximal BA | V4 segment of bilateral VAs |
| Castro et al. | 34 | F | Bilateral ICAs | Distal BA | Collaterals between PICAs and SCAs |
| Hong et al. | 45 | F | Bilateral ICAs | Transdural segment (V3/V4) of bilateral VAs |
ASA, V3 muscular branches of bilateral VAs |
| Lee and Cha | 28 | M | Bilateral ICAs | Transdural segment (V3/V4) of bilateral VAs |
ASA, V4 segment of bilateral VAs |
| Present case | 18 | F | Bilateral ICAs | Transdural segment (V3/V4) of bilateral VAs |
ASA, PSAs, an occipital branch of right ECA |
According to Padget 2, the carotid rete is not present in normal human development. Mahadevan et al. 8 considered the absence of the ICA occurred by regression after its development, and the carotid rete formed in early development as a secondary collateral pathway per se, when the embryonic carotid-vertebrobasilar anastomoses such as primary trigeminal artery and hypoglossal artery had already regressed. They believed the cause of agenesis and rete formation was similar for the ICA and VA, since they shared the same common venous environment for the transdural portions. For the present case, we also suppose the vertebral rete might develop as a result of the regression of the transdural segments of both VAs in the fetal or perinatal period. These can be compared with Burger's cases of bilateral vertebrobasilar junction agenesis, in which the collateral compensation was provided by a prominent persistent (not regressed) carotid-basilar anastomosis 15. Burger et al. 15 believed that the cause of vertebrobasilar junction agenesis for their two patients, might be the congenital absence of the anterior radicular artery of C1, and might be related to a common, as yet unknown, mechanism interfering with the development of such vascular segments at a specific moment of fetal life. Woodcock et al. 16 also reported a case of persistent bilateral primitive proatlantal arteries with absence of both vertebral arteries at their origin.
Moreover, we assume the transdural portions of ICAs in our case regressed simultaneously with the VAs for some unclear reason. Due to the paucity of blood flow in the forward direction from bilateral extracranial VAs and that of the reverse direction from PComAs and ICAs, the ASA as well as PSAs reconstituted the BA via an alternative vertebral rete mirabile. That might explain why the ASA and PSAs became enlarged and meandering. Only five cases of bilateral carotid and vertebrobasilar agenesis with the ASA or PSAs supplying the BA by rete mirabile have been reported in the literature including the present one (Table 1).
Retrograde flow from the ASA and/or PSA reconstructing the intracranial VA and BA is an uncommon collateral channel in patients with steno-occlusion or aplasia of bilateral VAs. Acquired and congenital compensation should be differentiated. For the acquired condition, most reported cases were middle-aged or elderly patients with symptoms of posterior circulation ischemia. Cerebral angiogram showed atherosclerotic occlusion of bilateral VAs and the collateral supply from the PComAs was lacking or incomplete. Other intracranial vessels often demonstrated diffuse atherosclerotic disease as well. The ASA and PSAs were usually thin and ran straight 17-19. The patient in our case was young and had no risk factor of atherosclerosis. The level of segmental agenesis of VAs was bilaterally symmetrical. The enlarged ASA and PSAs were supplied by multiple cervical branches of both VAs, and provided the majority of the blood flow into the BA and even the anterior circulation. The prominence and meandering of the ASA, PSAs and the anomaly vessels at the craniocervical junction indicated that they might have been an important part of the cerebral circulation since the embryonic stage. In other words, they imply congenital aspects in the present case.
References
- 1.Karasawa J, Touho H, Ohnishi H, et al. Rete mirabile in humans - Case report. Neuro Med Chir (Tokyo) 1997;37(2):188–192. doi: 10.2176/nmc.37.188. doi: 10.2176/nmc.37.188. [DOI] [PubMed] [Google Scholar]
- 2.Padget DH. The development of the cranial arteries in the human embryo. Contrib Embryol. 1948;32:7–262. [Google Scholar]
- 3.Hyogo T, Nakagawara J, Nakamura J, et al. Multiple segmental agenesis of the cerebral arteries: case report. Neuroradiology. 1996;38(5):433–436. doi: 10.1007/BF00607267. doi: 10.1007/BF00607267. [DOI] [PubMed] [Google Scholar]
- 4.Lasjaunias P, Santoyo-Vazquez A. Segmental agenesis of the internal carotid artery: angiographic aspects with embryologic discussion. Anat Clin. 1984;6(2):133–141. doi: 10.1007/BF01773165. doi: 10.1007/BF01773165. [DOI] [PubMed] [Google Scholar]
- 5.Araki Y, Imai S, Saitoh A, et al. [A case of carotid rete mirabile associated with pseudoxanthoma elasticum: a case report] No To Shinkei. 1986;38(5):495–500. [Article in Japanese] [PubMed] [Google Scholar]
- 6.Castro S, Abreu P, Azevedo E, et al. A new pattern of arterial rete compensation of segmental basilar agenesis associated with carotid retia mirabilia: a case report (2010: 1b) Euro Radiol. 2010;20(4):1024–1028. doi: 10.1007/s00330-009-1491-6. doi: 10.1007/s00330-009-1491-6. [DOI] [PubMed] [Google Scholar]
- 7.Itoyama Y, Kitano I, Ushio Y. Carotid and vertebral rete mirabile in man - Case report. Neuro Med Chir (Tokyo) 1993;33:181–184. doi: 10.2176/nmc.33.181. doi: 10.2176/nmc.33.181. [DOI] [PubMed] [Google Scholar]
- 8.Mahadevan J, Batista L, Alvarez H, et al. Bilateral segmental regression of the carotid and vertebral arteries with rete compensation in a Western patient. Neuroradiology. 2004;46(6):444–449. doi: 10.1007/s00234-003-1086-x. doi: 10.1007/s00234-003-1086-x. [DOI] [PubMed] [Google Scholar]
- 9.Weon YC, Chung JI, Kim HJ, et al. Agenesis of bilateral internal carotid arteries and posterior fossa abnormality in a patient with facial capillary hemangioma: presumed incomplete phenotypic expression of PHACE syndrome. Am J Neuroradiol. 2005;26(10):2635–2639. [PMC free article] [PubMed] [Google Scholar]
- 10.Kim MS, Lee SJ, Lee CH, et al. Bilateral segmental absence of the internal carotid artery with rete compensation associated with absence of basilar artery: case report. Surg Neurol. 2006;65(6):615–619. doi: 10.1016/j.surneu.2005.07.072. doi: 10.1016/j.surneu.2005.07.072. [DOI] [PubMed] [Google Scholar]
- 11.Li G, Jayaraman MV, Lad SP, et al. Carotid and vertebral rete mirabile in man presenting with intraparenchymal hemorrhage: a case report. J Stroke Cerebrovasc Dis. 2006;15(5):228–231. doi: 10.1016/j.jstrokecerebrovasdis.2006.05.005. doi: 10.1016/j.jstrokecerebrovasdis.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Sahin H, Cinar C, Oran I. Carotid and vertebrobasilar rete mirabile: a case report. Surg Radiol Anat. 2010;32(2):95–98. doi: 10.1007/s00276-009-0531-x. doi: 10.1007/s00276-009-0531-x. [DOI] [PubMed] [Google Scholar]
- 13.Hong JM, Lee JH, Yong SW. Neurological picture. Asymptomatic rete mirabile in carotid and vertebral circulation systems. J Neurol Neurosurg Psychiatry. 2010;81(8):1022–1023. doi: 10.1136/jnnp.2009.178368. doi: 10.1136/jnnp.2009.178368. [DOI] [PubMed] [Google Scholar]
- 14.Lee SY, Cha SH. Bilateral carotid and vertebral rete mirabile presenting with a prominent anterior spinal artery mimicking a spinal dural AV fistula at MRI. Korean J Radiol. 2011;12(6):740–744. doi: 10.3348/kjr.2011.12.6.740. doi: 10.3348/kjr.2011.12.6.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burger IM, Siclari F, Gregg L, et al. Bilateral segmental agenesis of the vertebrobasilar junction: developmental and angiographic anatomy. Am J Neuroradiol. 2007;28(10):2017–2022. doi: 10.3174/ajnr.A0719. Epub 2007 Sep 26. doi: 10.3174/ajnr.A0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodcock RJ, Cloft HJ, Dion JE. Bilateral type 1 proatlantal arteries with absence of vertebral arteries. Am J Neuroradiol. 2001;22(2):418–420. [PMC free article] [PubMed] [Google Scholar]
- 17.Kang HS, Han MH, Kim SH, et al. Anterior spinal artery as a collateral channel in cases of bilateral vertebral arterial steno-occlusive disease. Am J Neuroradiol. 2007;28(2):222–225. [PMC free article] [PubMed] [Google Scholar]
- 18.Yamakawa H, Yoshimura S, Iwama T. Anterior spinal artery as a collateral channel in patients with acute bilateral vertebral artery occlusions. Two case reports. Neurol Med Chir (Tokyo) 2009;49(8):354–358. doi: 10.2176/nmc.49.354. doi: 10.2176/nmc.49.354. [DOI] [PubMed] [Google Scholar]
- 19.Hott JS, Vishteh G, Wallace R, et al. Anterior spinal artery supplying posterior circulation. Neurology. 2004;62(3):468. doi: 10.1212/01.wnl.0000106825.66718.e4. doi: 10.1212/01.WNL.0000106825.66718.E4. [DOI] [PubMed] [Google Scholar]