Summary
This study evaluated the outcome of endovascular and conservative treatment for giant cavernous carotid artery aneurysms (CCAAs). We retrospectively reviewed a series of 35 consecutive giant CCAAs treated with endovascular and conservative treatment. All patients were evaluated by balloon occlusion test (BOT) before treatment. Patients who could tolerate BOT were treated by parent artery occlusion (PAO), those who could not tolerate BOT were treated by stent/coil or conservative methods.
Eight patients were treated conservatively, symptoms were worsened in four patients (50%), unchanged in three, and improved in one at 33.6±19.9 months (6~65 months) follow-up. In 27 aneurysms treated with endovascular methods, 17 aneurysms were treated by PAO, eight aneurysms were treated with stent-assisted coil embolization, and two aneurysms were embolized with coils. The initial post-procedure angiogram revealed complete occlusion, neck remnant, and incomplete occlusion in 81.5 %, 11.1 %, and 7.4 %, respectively. Procedure-related mortality and morbidity were 0 and 7.4 %, respectively. At 33.1±17.4 months (4~71 months) follow-up, a good clinical outcome (mRS 0-1) was observed in 25 (92.6%) patients, symptoms were resolved or improved in 20 (74.1%). Statistical analysis showed that risk factors for poor clinical outcome included age of 60 years and older (P=0.006), and conservative treatments (P=0.038).
Risk factors for poor clinical outcome of giant CCAAs included conservative treatment and age older than 60 years. A symptomatic giant cavernous carotid aneurysm should be treated. The outcome of endovascular treatment of giant CCAAs is promising.
Key words: endovascular treatment, conservative treatment, giant, cavernous carotid artery aneurysms
Introduction
Giant CCAAs are most often located outside the subarachnoid space. The mass effect attendant on CCAAs may produce compression of the adjacent third to sixth cranial nerves and result in symptoms such as headache, facial pain or ophthalmoplegia/paresis. Rupture may lead to symptomatic or asymptomatic direct cavernous-carotid fistula or severe intractable epistaxis. Subarachnoid hemorrhage can occur when the lesion erodes through the dura or dural rings of the carotid artery 1,2. Direct surgical obliteration of giant CCAAs has been possible but remains a formidable challenge. The intimate relationship between the intracavernous carotid artery and venous structures and the cranial nerves make surgical access difficult. Persistent morbidity with surgical therapy and steady advances in endovascular therapy have encouraged attempts at endovascular repair of giant CCAAs. Treatment strategy includes parent artery occlusion (PAO) and selective coiling with or without stent assistance. However, few large series have examined endovascular or conservative treatment of giant CCAAs. The goal of the present study was to investigate the risk factors and outcome of giant CCAAs treated by endovascular and conservative management.
Materials and Methods
Thirty-five consecutive patients with 35 giant CCAAs were enrolled between January 2006 and September 2011 in our center. There were 29 women (82.9%) and six men (17.1%), with a mean age of 52.4 years (range, 18-78 years). Twenty-six patients presented with cranial neuropathies, eight patients presented with headache, and one patient was asymptomatic (detected incidentally during neuroradiological imaging because of an unrelated medical condition). The median aneurysm size was 29.6 mm, ranging from 25 to 45 mm. All patients were evaluated by balloon occlusion test (BOT) before treatment as reported by previous authors 1-15.
Tolerance to test occlusion was assessed by a detailed neurologic examination consisting of evaluation of cranial nerve function, muscle strength, and language ability every five minutes or when a deficit was perceived. The test occlusion was considered positive if any new neurologic deficit occurred, that is clinically intolerant patients. If the patient tolerated 20 minutes of normal tension, the balloon was deflated for ten minutes, and then the test was repeated under hypotension after another 20 minutes. Hypotension was induced by the infusion of sodium nitroprusside (2.5 to 7.5 mg/kg body weight/min). After the mean arterial pressure was reduced to two thirds of baseline, hypotension was maintained for 20 minutes provided that the mean arterial blood pressure not less than 55 mmHg. If the patient tolerated BOT under hypotension, he/she was considered clinically able to tolerate parent vessel occlusion. The test was terminated immediately if any neurologic deficit developed during test occlusion under normotensive or hypotensive conditions.
Patients who could tolerate BOT were treated by PAO, those who could not tolerate BOT were treated by stent/coil or conservative methods, and the asymptomatic patient was treated by conservative methods.
Periprocedure Medications
When the use of a stent was planned, patients were premedicated with antiplatelet therapy consisting of aspirin 100 mg and clopidogrel 75 mg for three days before the procedure. After the procedure, clopidogrel (75 mg/day) was recommended for an additional 30 days, and aspirin (100 mg/day) was recommended for six months. After PAO, patients were treated with hypervolemia.
Results and Outcome Evaluation
The degree of the aneurysm occlusion was classified as: complete obliteration (dense coil packing with no contrast filling the aneurysm); neck remnant (contrast filling a very small “dog ear” portion at one side of the neck or within interstices between the coils at the level of the neck only); incomplete occlusion (coil packing is usually less dense). The length of the angiographic follow-up period was 11.7±15.2 months (range, 3-63 months). The clinical follow-up was classified by Modified Rankin Scale (mRS) scores at the last clinic visit or by telephone call.
Statistical Analysis
Logistic regression was performed to evaluate the association between sex, age (younger than 60 years vs 60 years and older), presentation, size (<35 mm vs ≥35 mm), treatment type (endovascular vs conservative treatment) and worsening outcome. The significance level was set at 0.05.
Results
Initial Results
Six patients failed BOT and refused endovascular or surgical treatment, one patient was asymptomatic, and one patient refused endovascular or surgical treatment because of old age (73 years old) (Table 1). Twenty-seven aneurysms were treated with endovascular methods. Seventeen aneurysms were treated by PAO (eight with coils, five with balloons, two with balloon and coils, two with coils and onyx), eight aneurysms were treated with stent-assisted coil embolization (one Neuroform, one Leo, six Enterprise), and two aneurysms were simply coiled (Table 2).
Table 1.
Conservatively treated patients demographics and outcomes
| Patient | Age | Sex |
Size (mm) |
Presentation |
Follow up (month) |
Status at last follow-up |
| 1 | 50 | F | 25×20 | Diplopia | 65 | Diplopia, ophthalmoparesis, Severe headache |
| 2 | 73 | F | 25×22 | Ophthalmoparesis, visual blurring |
55 | Ophthalmoparesis, ablepsia |
| 3 | 71 | F | 28×28 | Diplopia, ophthalmoparesis | 37 | Headache, Diplopia, Ophthalmoparesis |
| 4 | 69 | F | 36×28 | Visual blurring | 34 | Severe headache, ablepsia |
| 5 | 18 | M | 27×19 | Ophthalmoparesis, Diplopia | 31 | Asymptomatic |
| 6 | 56 | F | 25×20 | Asymptomatic | 31 | Asymptomatic |
| 7 | 64 | F | 30×26 | Headache, Diplopia | 10 | Headache, Diplopia |
| 8 | 53 | F | 35×35 | Diplopia, visual blurring | 6 | Diplopia, visual blurring |
Table 2.
Endovascularly treated patients' demographics, treatment and outcomes
| Patient | Age | Sex |
Size (mm) |
Presentation | BOT |
Treatment summary |
Treatment session |
Parent vessel preserved |
Occlusion | mRS | Outcome |
Follow-up (month) |
| 1 | 55 | F | 25×20 | Headache, ophthalmoparesis, diplopia | Fail | Stent/coil,coil | 2 | Yes | complete | 0 | Cured | 71 |
| 2 | 55 | F | 25×22 | Headache, ophthalmoparesis, diplopia | Pass | Coil | 1 | No | complete | 1 | Improved | 57 |
| 3 | 64 | F | 28×28 | Headache, trigeminal sensory neuropathy | Fail | Coil | 1 | Yes | complete | 0 | Cured | 57 |
| 4 | 52 | F | 36×28 | Diplopia, headache, ophthalmoparesis | Pass | Coil | 1 | No | complete | 1 | Improved | 57 |
| 5 | 54 | F | 27×19 | Headache, diplopia, ophthalmoparesis | Pass | Coil | 1 | No | complete | 0 | Cured | 53 |
| 6 | 78 | M | 25×20 | Headache, diplopia, ophthalmoparesis | Fail | Coil | 1 | Yes | neck remnant | 4 | Worsed | 52 |
| 7 | 51 | F | 30×26 | Diplopia | Pass | Balloon | 1 | No | complete | 1 | Unchanged | 47 |
| 8 | 20 | F | 35×35 | Diplopia, headache, visual blurring | Pass | Balloon | 1 | No | complete | 0 | Cured | 45 |
| 9 | 53 | F | 30×20 | Diplopia | Pass | Balloon | 1 | No | complete | 1 | Improved | 35 |
| 10 | 57 | F | 25×20 | Diplopia | Fail | Stent/coil | 2 | Yes | complete | 1 | Improved | 37 |
| 11 | 56 | F | 25×16 | Headache, visual blurring | Fail | Stent/coil | 2 | Yes | incomplete | 1 | Improved | 35 |
| 12 | 25 | M | 28×28 | Headache | Pass | Coil | 1 | No | complete | 1 | Unchanged | 35 |
| 13 | 68 | F | 26×19 | Headache | Fail | Stent/coil | 2 | Yes | complete | 0 | Cured | 29 |
| 14 | 61 | F | 34×24 | Headache, diplopia | Pass | Coil /balloon | 1 | No | complete | 2 | Worsed | 33 |
| 15 | 38 | F | 38×31 | Headache | Pass | Coil/Onyx | 1 | No | complete | 1 | Cured | 33 |
| 16 | 36 | F | 29×23 | Headache | Pass | Coil/Onyx | 2 | No | complete | 0 | Cured | 28 |
| 17 | 38 | F | 45×45 | Headache | Pass | Coil | 1 | No | complete | 0 | Unchanged | 27 |
| 18 | 36 | F | 34×28 | Headache | Pass | Coil | 1 | No | complete | 0 | Cured | 24 |
| 19 | 78 | F | 28×26 | Headache, diplopia, ophthalmoparesis | Fail | Stent/coil | 1 | Yes | neck remnant | 1 | Cured | 23 |
| 20 | 61 | F | 25×22 | Headache, diplopia | Pass | Stent/coil | 1 | Yes | complete | 0 | Cured | 21 |
| 21 | 24 | M | 27×22 | Visual blurring | Pass | Coil | 1 | No | complete | 1 | Unchanged | 21 |
| 22 | 55 | F | 25×22 | headache | Fail | Stent/coil | 1 | Yes | incomplete | 1 | Unchanged | 19 |
| 23 | 58 | F | 32×29 | Diplopia, headache | Pass | Balloon/coil | 2 | No | complete | 1 | Improved | 17 |
| 24 | 58 | F | 30×20 | Diplopia | Pass | Coil | 1 | No | complete | 0 | Cured | 13 |
| 25 | 61 | F | 25×18 | Diplopia | Fail | Stent/coil | 1 | Yes | neck remnant | 0 | Cured | 4 |
| 26 | 46 | M | 25×13 | Diplopia | Pass | Balloon | 1 | No | complete | 1 | Improved | 8 |
| 27 | 43 | M | 44×25 | Headache, diplopia | Pass | Balloon | 1 | No | complete | 0 | Cured | 8 |
Of the 27 endovascularly treated giant CCAAs, the initial post-procedure angiograms revealed complete occlusion, neck remnant, and incomplete occlusion in 22 (81.5%), three (11.1%), and two (7.4%), respectively (Figures 1 and 2). Periprocedural infarcts occurred in two patients (7.4%), one caused by thromboembolic strokes after stent/coil and one as a result of hemodynamic insufficiency after PAO.
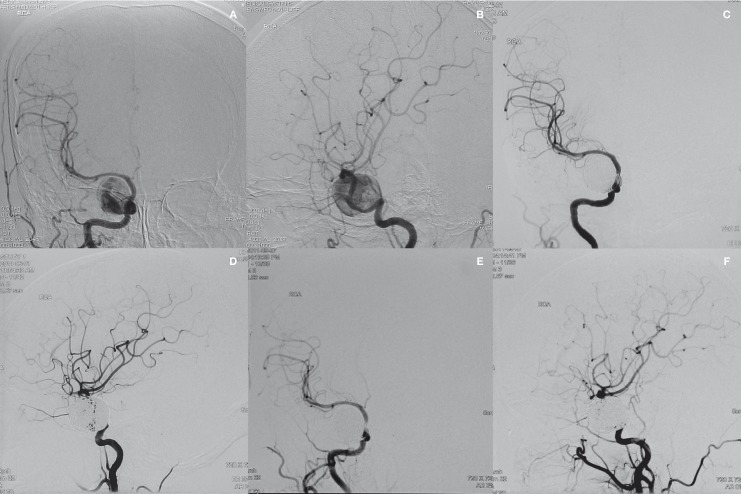
Figure 1.
Right carotid angiogram on anteroposterior (A) and lateral (B) views from a 57-year-old woman with diplopia demonstrating a giant intracavernous carotid artery aneurysm. After 2 sessions of stent/coil embolization, right carotid angiogram on anteroposterior (C) and lateral (D) views demonstrating complete obliteration of the aneurysm. Right carotid angiogram on anteroposterior (E) and lateral (F) views at 3 months postembolization showing complete obliteration of the aneurysm.
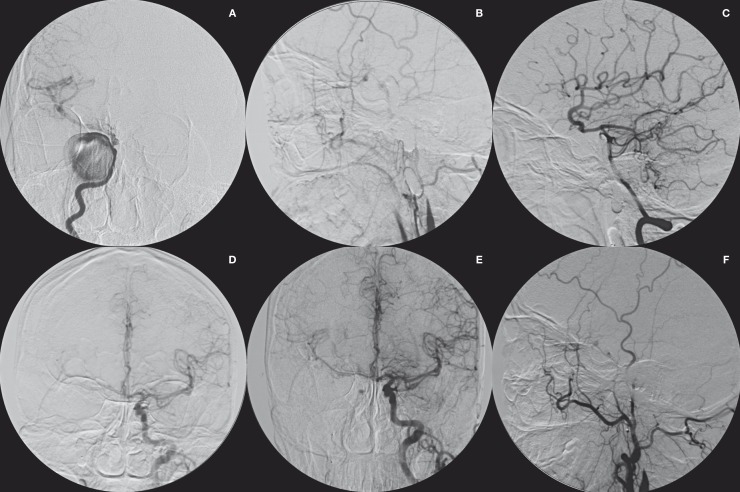
Figure 2.
Right carotid angiogram (A) from a 51-year-old woman with diplopia demonstrating a giant cavernous carotid artery aneurysm. After parent artery balloon occlusion, right carotid (B), vertebral (C) and left carotid (D) angiograms showing complete obliteration of the aneurysm. Left (E) and right (F) carotid angiograms at 6 months postembolization showing complete obliteration of the aneurysm.
Angiographic Follow-up
Twelve patients refused angiographic follow-up while angiographic follow-up was available in 15 (55.6%) patients treated endovascularly. Follow-up angiogram revealed complete occlusion, neck remnant, and incomplete occlusion in 13 (86.7 %), one (6.7 %), and one (6.7 %), respectively. All eight conservatively treated patients refused angiographic follow-up.
Clinical Follow-up
Of the eight conservatively treated giant CCAAs, symptoms were worsened in four patients (50%) (more than six years after symptom onset), three remained unchanged (less than three years after symptom onset), improved in one at 33.6±19.9 months (6~65 months) follow-up. Of the 27 endovascularly treated giant CCAAs, at 33.1±17.4 months (4~71months) follow-up examination symptoms were resolved in 13 (48.1%), improved in nine (33.3%), worsened in two (7.4%), and unchanged in three (11.1%), a good clinical outcome (mRS 0-1) was observed in 25 (92.6%) patients. Procedure-related morbidity and mortality was 7.4 % and 0, respectively.
Statistical Analysis
Patient sex, presentation, and size (<35 mm vs ≥35 mm) did not significantly correlate with worsening symptoms, risk factors for poor clinical outcome included age of 60 years and older (P=0.006), and conservative treatments (P= 0.038). There was no significant difference in the final outcome, whether the parent vessels were occluded or preserved (P=0.698).
Discussion
Most CCAAs are considered benign lesions, and have a natural history with a low risk of life-threatening complications. The management of CCAAs has been controversial 3-7. Diaz et al. 8 treated 32 symptomatic CCAAs. Nine received progressive ligation of the internal carotid artery in the neck with a Selverstone clamp and a surface superficial temporal artery-middle cerebral artery (STA-MCA) anastomosis, two (22.2%) developed transient neurological deficits. Seven underwent trapping of the internal carotid artery and a deep STA-MCA anastomosis. Two patients (28.6%) developed a cerebral infarction, one of whom died; 15 had direct clipping of the aneurysm. Two patients (13.3%) progressed from marked visual loss to blindness of the same side, and one (6.7%) developed an intraventricular hemorrhage during induction of anesthesia and died without surgery.
Parent Artery Occlusion
Parent artery occlusion (PAO) is a therapeutic modality for patients who can tolerate BOT 9-12. Complications of PAO include early or late stroke and ‘de novo' aneurysm formation at a distant site because of hemodynamic changes in the circle of Willis 13-16. There remains a 5% to 10% risk of serious stroke with associated morbidity/mortality after PAO despite tolerated BOT 17. In our patient group, of the 17 aneurysms treated by PAO, one (5.9%) developed postocclusion ischemic infarction. No new aneurysms were found in our patients. However, longer term follow-up data will be needed to draw definitive conclusions regarding new aneurysm formation.
Stent-Assisted Coiling
The disadvantage of stent/coil therapy is the frequently incomplete occlusion of the aneurysm with the need for multiple treatments and follow-up angiography. Hauck et al. 18 reported 15 very large and giant unruptured ophthalmic and cavernous aneurysms treated with stenting and/or coiling, seven patients (47.7%) were completely or nearly completely occluded (90%-100%), including one patient with parent vessel sacrifice after stent complication. Eight patients (53.3%) had a significant residual aneurysm. Twelve patients required retreatment.
Heran et al. 19 had residual aneurysm in 50% of endovascularly treated aneurysms >1 cm. Malisch et al. 20 found a 33% recanalization rate in giant aneurysms that were part of their study in 1997. The overall rate of aneurysm recanalization after coil embolization may be significantly higher in giant and very large aneurysms compared with smaller lesions 21-23. Regrowth of the aneurysm, coil compaction, and coil migration into soft intra-aneurysmal thrombus are possible explanations for the faster recanalization 20.
Conservative Treatment
Lye and Jha 24 reported ten CCAAs managed conservatively (mean 6.9 years). Three (30%) improved, six (60%) were unchanged and one (10%) died following intracranial hemorrhage. Linskey et al. 3 observed 20 CCAAs without treatment (5 months ~13 years, median 2.4 years): symptoms were worsened in seven (35%), unchanged in nine (45%), and improved in four (20%). Goldenberg et al. 5 reported ten CCAAs without intervention, three (30%) remained stable, and seven (70%) worsened. In our study, the outcome of conservatively treated giant CCAAs was negative: four (50%) worsened, three (37.5%) were unchanged and one (12.5%) improved. Choulakian et al. 25 concluded that consideration should be given to the treatment of asymptomatic CCAAs 15 mm or larger due to the potential risks of cranial neuropathy and SAH. In our series, an asymptomatic giant aneurysm (the largest dimension 25 mm) was still unchanged after 31 months follow-up. Patients with asymptomatic giant CCAAs who cannot tolerate carotid artery occlusion should be treated with caution.
Vasconcellos et al. 26 reported five cases of giant CCAAs which evolved with spontaneous thrombosis of the internal carotid artery, and four patients had regression of deficit. They believe that spontaneous thrombosis of the internal carotid artery is a common outcome in giant CCAAs, and is related to a significant improvement of symptoms. In our series, spontaneous thrombosis of the internal carotid artery occurred in one patient, and symptoms were cured. We think thrombosis of the internal carotid artery evolved from a dissecting cavernous carotid artery aneurysm. This may be catastrophic for those patients without efficient collateral circulation.
Effect of Endovascular Treatment
Mass effect symptoms will probably improve with the shrinkage of aneurysms after embolization. Shrinkage of approximately 57% of initial volume after 18 months of endosaccular coiling has been reported 27. Gruber et al. 28 reported that 45.5% of patients with symptoms of neural compression improved after endosaccular embolization of giant and very large aneurysms. Niiro et al. 13 analyzed the results of the long-term follow-up of 11 patients with a giant or large cavernous sinus aneurysm treated by only proximal occlusion between 1975 and 1989. Eight of the 11 patients (72.7%) showed improvement of cranial nerves paresis or headache. Hassan et al. 29 observed 28 giant aneurysms treated by PAO with or without intra-aneurysmal occlusion: symptoms were resolved in 19 (68%), improved in four (14%), and unchanged in five (18%). In our series, symptoms were resolved or improved in 81.4%. Steibel-Kalish et al. 7 retrospectively reviewed 185 patients with 206 CCAAs. Seventy-four CCAAs underwent treatment, and 115 patients were followed for four years. They revealed that the treated group had a higher proportion of neurological and visual complications than those who were not treated. This result is different from ours. The reason probably was that 67 cases in the treated group were treated by PAO. Most of them were treated with balloons, and only five were treated with coils. The incidence of complications caused by coils in aneurysm treatment is lower than that caused by balloons 30. Their two groups of treated and untreated patients are not comparable because their selection was biased according to severity of symptoms. The sizes of their cavernous carotid artery aneurysms were not mentioned. Smaller CCAAs may be followed conservatively, and our study shows that giant symptomatic CCAAs should be treated.
Recently, flow diverters, such as the Pipeline embolization device (ev3, Irvine, CA, USA) and the Silk stent (Balt Extrusion, Montmorency, France), offer a novel therapeutic alternative for many of these same lesions 31-34. Although initial published results indicate a generally favorable risk-benefit profile for flow diverters, early and delayed complications, such as ipsilateral intraparenchymal hemorrhage and in-stent thrombosis, are increasingly reported 35-38. While these results provide a short-term benchmark versus flow diverters, the long-term comparison remains unstudied and these data do little to address the debate.
Conclusions
Risk factors for poor clinical outcome of giant CCAAs included conservative treatment and age older than 60 years. A symptomatic giant cavernous carotid aneurysm should be treated. The outcome of endovascular treatment of giant CCAAs is promising.
References
- 1.al-Rodhan NR, Piepgras DG, Sundt TM., Jr Transitional cavernous aneurysms of the internal carotid artery. Neurosurgery. 1993;33:997–998. doi: 10.1227/00006123-199312000-00006. doi: 10.1227/00006123-199312000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Date I, Ohmoto T. Long-term outcome of surgical treatment of intracavernous giant aneurysms. Neurol Med Chir (Tokyo) 1998;38(Suppl):62–69. doi: 10.2176/nmc.38.suppl_62. doi: 10.2176/nmc.38.suppl_62. [DOI] [PubMed] [Google Scholar]
- 3.Linskey ME, Sekhar LN, Hirsch WL, Jr, et al. Aneurysms of the intracavernous carotid artery: Natural history and indications for treatment. Neurosurgery. 1990;26:933–938. doi: 10.1227/00006123-199006000-00002. [PubMed] [Google Scholar]
- 4.Linskey ME, Sekhar LN, Horton JA, et al. Aneurysms of the intracavernous carotid artery: A multidisciplinary approach to treatment. J Neurosurg. 1991;75:525–534. doi: 10.3171/jns.1991.75.4.0525. doi: 10.3171/jns.1991.75.4.0525. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg-Cohen N, Curry C, Miller NR, et al. Long term visual and neurological prognosis in patients with treated and untreated cavernous sinus aneurysms. J Neurol Neurosurg Psychiatry. 2004;75:863–867. doi: 10.1136/jnnp.2003.020917. doi: 10.1136/jnnp.2003.020917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kupersmith MJ, Hurst R, Berenstein A, et al. The benign course of cavernous carotid artery aneurysms. J Neurosurg. 1992;77:690–693. doi: 10.3171/jns.1992.77.5.0690. doi: 10.3171/jns.1992.77.5.0690. [DOI] [PubMed] [Google Scholar]
- 7.Stiebel-Kalish H, Kalish Y, Bar-On RH, et al. Presentation, natural history, and management of carotid cavernous aneurysms. Neurosurgery. 2005;57:850–857. doi: 10.1227/01.neu.0000179922.48165.42. doi: 10.1227/01.NEU.0000179922.48165.42. [DOI] [PubMed] [Google Scholar]
- 8.Diaz FG, Ohaegbulam S, Dujovny M, et al. Surgical alternatives in the treatment of cavernous sinus aneurysms. J Neurosurg. 1989;71:846–853. doi: 10.3171/jns.1989.71.6.0846. doi: 10.3171/jns.1989.71.6.0846. [DOI] [PubMed] [Google Scholar]
- 9.Field M, Jungreis CA, Chengelis N, et al. Symptomatic cavernous sinus aneurysms: management and outcome after carotid occlusion and selective cerebral revascularization. Am J Neuroradiol. 2003;24:1200–1207. [PMC free article] [PubMed] [Google Scholar]
- 10.Barnett DW, Barrow DL, Joseph GJ. Combined extracranial-intracranial bypass and intraoperative balloon occlusion for the treatment of intracavernous and proximal carotid artery aneurysms. Neurosurgery. 1994;35:92–98. doi: 10.1227/00006123-199407000-00014. doi: 10.1227/00006123-199407000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Bavinzski G, Killer M, Ferraz-Leite H, et al. Endovascular therapy of idiopathic cavernous aneurysms over 11 years. Am J Neuroradiol. 1998;19:559–565. [PMC free article] [PubMed] [Google Scholar]
- 12.Drake CG, Peerless SJ, Ferguson GG. Hunterian proximal arterial occlusion for giant aneurysms of the carotid circulation. J Neurosurg. 1994;81:656–665. doi: 10.3171/jns.1994.81.5.0656. doi: 10.3171/jns.1994.81.5.0656. [DOI] [PubMed] [Google Scholar]
- 13.Shimozuru T, Kadota K, et al. Long-term follow-up study of patients with cavernous sinus aneurysm treated by proximal occlusion. Neurol Med Chir (Tokyo) 2000;40:88–97. doi: 10.2176/nmc.40.88. doi: 10.2176/nmc.40.88. [DOI] [PubMed] [Google Scholar]
- 14.Wolf RL, Imbesi SG, Galetta SL, et al. Development of a posterior cerebral artery aneurysm subsequent to occlusion of the contralateral internal carotid artery for giant cavernous aneurysm. Neuroradiology. 2002;44:443–446. doi: 10.1007/s00234-001-0723-5. doi: 10.1007/s00234-001-0723-5. [DOI] [PubMed] [Google Scholar]
- 15.Vazquez Añon V, Aymard A, Gobin YP, et al. Balloon occlusion of the internal carotid artery in 40 cases of giant intracavernous aneurysm: technical aspects, cerebral monitoring, and results. Neuroradiology. 1992;34:245–251. doi: 10.1007/BF00596347. doi: 10.1007/BF00596347. [DOI] [PubMed] [Google Scholar]
- 16.Briganti F, Cirillo S, Caranci F, et al. Development of “de novo” aneurysms following endovascular procedures. Neuroradiology. 2002;44:604–609. doi: 10.1007/s00234-001-0732-4. doi: 10.1007/s00234-001-0732-4. [DOI] [PubMed] [Google Scholar]
- 17.Carter BS, Ogilvy CS, Putman C, et al. Selective use of extracranial-intracranial bypass as an adjunct to therapeutic internal carotid artery occlusion. Clin Neurosurg. 2000;46:351–362. [PubMed] [Google Scholar]
- 18.Hauck EF, Welch BG, White JA, et al. Stent/coil treatment of very large and giant unruptured ophthalmic and cavernous aneurysms. Surg Neurol. 2009;71:19–24. doi: 10.1016/j.surneu.2008.01.025. doi: 10.1016/j.surneu.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 19.Heran NS, Song JK, Kupersmith MJ, et al. Large ophthalmic segment aneurysms with anterior optic pathway compression: assessment of anatomical and visual outcomes after endosaccular coil therapy. J Neurosurg. 2007;106:968–975. doi: 10.3171/jns.2007.106.6.968. doi: 10.3171/jns.2007.106.6.968. [DOI] [PubMed] [Google Scholar]
- 20.Malisch TW, Guglielmi G, Viñuela F, et al. Intracranial aneurysms treated with the Guglielmi detachable coil: midterm clinical results in a consecutive series of 100 patients. J Neurosurg. 1997;87:176–183. doi: 10.3171/jns.1997.87.2.0176. doi: 10.3171/jns.1997.87.2.0176. [DOI] [PubMed] [Google Scholar]
- 21.Lv X, Jiang C, Li Y, et al. Treatment of giant intracranial aneurysms. Interv Neuroradiol. 2009;15(2):135–144. doi: 10.1177/159101990901500201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayakawa M, Murayama Y, Duckwiler GR, et al. Natural history of the neck remnant of a cerebral aneurysm treated with the Guglielmi detachable coil system. J Neurosurg. 2000;93:561–568. doi: 10.3171/jns.2000.93.4.0561. doi: 10.3171/jns.2000.93.4.0561. [DOI] [PubMed] [Google Scholar]
- 23.Rad J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398–1403. doi: 10.1161/01.STR.0000073841.88563.E9. doi: 10.1161/01.STR.0000073841.88563.E9. [DOI] [PubMed] [Google Scholar]
- 24.Lye RH, Jha AN. Unruptured aneurythe intracavernous internal carotid artery: outcome following carotid ligation or conservative treatment. Br J Neurosurg. 1989;3:181–188. doi: 10.3109/02688698909002793. doi: 10.3109/02688698909002793. [DOI] [PubMed] [Google Scholar]
- 25.Choulakian A, Drazin D, Alexander MJ. Endovascular treatment of 113 cavernous carotid artery aneurysms. J Neurointerv Surg. 2010;2:359–362. doi: 10.1136/jnis.2010.003137. doi: 10.1136/jnis.2010.003137. [DOI] [PubMed] [Google Scholar]
- 26.Vasconcellos LPP, Flores JA, Conti ML, et al. Spontaneous thrombosis of internal carotid artery: a natural history of giant carotid cavernous aneurysms. Arq Neuropsiquiatr. 2009;67:278–283. doi: 10.1590/s0004-282x2009000200020. doi: 10.1590/S0004-282X2009000200020. [DOI] [PubMed] [Google Scholar]
- 27.Tsuura M, Terada T, Nakamura Y, et al. Magnetic resonance signal intensity and volume changes after endovascular treatment of intracranial aneurysms causing mass effect. Neuroradiology. 1998;40:184–188. doi: 10.1007/s002340050565. doi: 10.1007/s002340050565. [DOI] [PubMed] [Google Scholar]
- 28.Gruber A, Killer M, Bavinzski G, et al. Clinical and angiographic results of endovascular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery. 1999;45:793–804. doi: 10.1097/00006123-199910000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Hassan T, Hamimi A. Successful endovascular management of brain aneurysms presenting with mass effect and cranial nerve palsy. Neurosurg Rev. 2012 doi: 10.1007/s10143-012-0404-3. [Epub ahead of print]. doi: 10.1007/s10143-012-0404-3. [DOI] [PubMed] [Google Scholar]
- 30.van der Schaaf EH, Buskens E, Rinkel G. Endovascular treatment of aneurysms in the cavernous sinus: A systematic review on balloon occlusion of the parent vessel and embolization with coils. Stroke. 2002;33:313–318. doi: 10.1161/hs0102.101479. doi: 10.1161/hs0102.101479. [DOI] [PubMed] [Google Scholar]
- 31.Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the Pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009;64:632–642. doi: 10.1227/01.NEU.0000339109.98070.65. doi: 10.1227/01.NEU.0000339109.98070.65. [DOI] [PubMed] [Google Scholar]
- 32.Byrne JV, Beltechi R, Yarnold JA, et al. Early experience in the treatment of intra-cranial aneurysms by endovascular flow diversion: a multicentre prospective study. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012492. doi: 10.1371/journal.pone.0012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szikora I, Berentei Z, Kulcsar Z, et al. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: the Budapest experience with the Pipeline embolization device. Am J Neuroradiol. 2010;31:1139–1147. doi: 10.3174/ajnr.A2023. doi: 10.3174/ajnr.A2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson PK, Lylyk P, Szikora I, et al. The Pipeline embolization device for the intracranial treatment of aneurysms trial. Am J Neuroradiol. 2011;32:34–40. doi: 10.3174/ajnr.A2421. doi: 10.3174/ajnr.A2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lubicz B, Collignon L, Raphaeli G, et al. Flow-diverter stent for the endovascular treatment of intracranial aneurysms: a prospective study in 29 patients with 34 aneurysms. Stroke. 2010;41:2247–2253. doi: 10.1161/STROKEAHA.110.589911. doi: 10.1161/STROKEAHA.110.589911. [DOI] [PubMed] [Google Scholar]
- 36.Chow M, McDougall C, O'Kelly C, et al. Delayed spontaneous rupture of a posterior inferior cerebellar artery aneurysm following treatment with flow diversion: a clinicopathologic study. Am J Neuroradiol. 2011 doi: 10.3174/ajnr.A2532. [Epub ahead of print]. doi: 10.3174/ajnr.A2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klisch J, Turk A, Turner R, et al. Very late thrombosis of flow-diverting constructs after the treatment of large fusiformposterior circulation aneurysms. Am J Neuroradiol. 2011;32:627–632. doi: 10.3174/ajnr.A2571. doi: 10.3174/ajnr.A2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiorella D. Pipeline in clinical practice in 2011. Neuroradiology. 2012;54:277–278. doi: 10.1007/s00234-011-0957-9. doi: 10.1007/s00234-011-0957-9. [DOI] [PubMed] [Google Scholar]