Summary
A flow-diverting stent such as the Pipeline embolization device (PED, ev3 Endovascular, Plymouth, MN, USA) and Silk flow-diverting stent (Balt Extrusion, Montmorency, France) offers an acceptable alternative for the treatment of difficult aneurysms according to their morphologies, including giant, wide-necked, fusiform, and blister types. However, complications arising from the use of these stents have frequently been reported including several cases of branch artery occlusion and delayed occlusion of the stented parent vessel shortly after antiplatelet medications were discontinued, highlighting the potential need for long-term antiplatelet therapy, and disastrous bleeding complications in unruptured aneurysm. In addition, these microcell stents are difficult to use in distal aneurysms located over the ICA bifurcation and basilar tip because of the stiffness of the device, and perforating vessel occlusion is more likely to occur due to the characteristics of the stent. Before the era of flow-diverting microcell stents, large cell intracranial stents like the Neuroform stent (Boston Scientific/Target Therapeutic, Fremont, CA, USA) and Enterprise stent (Cordis Neurovascular, Miami, FL, USA) without coiling were used to provide flow-diverting effects for complex intracranial aneurysms. Sole stenting has been used even in cases of ruptured aneurysm, with patients on different antiplatelet medications. However, no single endovascular institute has embraced sole stenting using large cell intracranial stents as a systemized treatment for ruptured intracranial aneurysms. Here we designed this study to evaluate the possibility of safely treating very small aneurysms using one or two stents without coiling during the period of subarachnoid hemorrhage (SAH). This retrospective study was conducted with eight patients who had rupture of very small intracranial aneurysms (less than 3 mm in size). All were treated using the Neuroform and the Enterprise stents; there was single stenting in five, in-stent telescopic stenting in two, and Y-configured stenting in one. The angiographic results with clinical outcomes were collected and analyzed. Complete aneurysm obliteration was observed in three cases, and size reduction or stable angiographic findings was found in five cases on the last follow-up angiography. No growing aneurysm or rebleeding was found on any follow-up angiography. Thromboembolic complications were found in one patient. It is difficult to make conclusions on the long-term efficacy of this technique with such a small number of cases, however sole stenting with a large cell intracranial stent for the treatment of very small aneurysms may be used safely as an alternative treatment even during an episode of SAH.
Key words: sole stenting, very small intracranial aneurysm, subarachnoid hemorrhage
Introduction
Very small ruptured intracranial aneurysms, including blood-blister like aneurysms, are undoubtedly one of the most challenging morphological and pathological entities to be treated. Although advances in endovascular techniques have allowed the treatment of many aneurysms previously considered extremely high-risk or inoperable, there remains a significant percentage of aneurysms for which any treatment, endovascular or surgical, carries substantial risks 1,2.
The treatment of very small ruptured aneurysms is a problem that often has no optimal solution. We describe eight cases of such aneurysms which were successfully treated with sole stenting using one or two large cell intracranial stents, and evaluate the possibility and safety of stent monotherapy without coiling in the period of subarachnoid hemorrhage (SAH).
Materials and Methods
Patients and evaluation
We performed a retrospective review of eight cases of sole stenting for very small intracranial aneurysms that had ruptured at our institution by July 2012 for the evaluation of the angiographic and clinical effectiveness. Ruptures that occurred from procedures unrelated to stenting were excluded from this study. All the aneurysms were of very small size, less than 3 mm on digital subtraction angiography (DSA), and were in various locations of the cerebral circulation. The neck to dome ratio was over one in all cases. The aneurysms were too small and shallow to allow coil embolization or neck clipping to be done adequately. The aneurysm size and location, stents used, procedural complications, initial and follow-up angiographic classification, clinical status by the Hunt and Hess grading system and final outcome were recorded (Table 1). The O'Kelly-Marotta (OKM) grading scale 3 was used for the evaluation of the angiographic results.
Table 1.
Summary of cases
| Case |
Age/ Sex |
H-H grade |
Size (mm) |
Location |
Kind of stent (technique) |
Immediate and last f/u angiographic results (OKM) |
Clinical outcome |
| 1 | 70/F | 2 | 1.3×1.1 | MCA | 2 Neuroform(Y) | B3→D (22 mos) | No deficit |
| 2 | 67/M | 2 | 2.2×1.8 | ICA | 1 Neuroform | A1→B1 (13 mos) | No deficit |
| 3 | 71/F | 3 | 2.0×2.0 | PCA | 1 Neuroform | A1→C2 (6 mos) | TIA |
| 4 | 51/F | 2 | 1.4×1.3 | MCA(M1) | 1 Enterprise | A1→A1 (6 mos) | No deficit |
| 5 | 52/M | 2 | 2.1×2.0 | BT | 1 Neuroform 1 Enterprise(O) |
B1→D (18 mos) | No deficit |
| 6 | 49/M | 2 | 1.5×1.2 | ICA | 2 Neuroform(O) | A1→D (12 mos) | No deficit |
| 7 | 67/F | 2 | 2.8×2.5 | ICA | 1 Neuroform | A1→B1 (18 mos) | No deficit |
| 8 | 84/M | 1 | 1.8×1.6 | AcomA | 1 Enterprise | A1→A1 (34 mos) | No deficit |
|
H-H grade: Hunt and Hess grade; MCA: middle cerebral artery; ICA: internal carotid artery; PCA: posterior cerebellar artery; BT: basilar trunk; AcomA: anterior communicating artery; Y: Y-configured stenting technique; O: overlapping stent technique; OKM: O'Kelly-Marotta grading scale; TIA: transient ischemic attack. | |||||||
The aneurysms were deemed at the time of the initial treatment to be too small to safely retain a coil inside, even after placement of the stents. Therefore, the patients were treated with endovascular stent placement, using one or two large cell stents such as the Neuroform stent (Boston Scientific, Natick, MA, USA) and/or the Enterprise stent (Cordis Endovascular, Miami Lakes, FL, USA). All patients were recommended to undergo short-term follow-up angiography within two to four weeks after the procedure. Then, our protocol was to perform angiographic follow-up at intervals of six months to one year at least three times. If the morphology of the aneurysm changed or the size increased, we planned to place an additional stent. If the morphology of aneurysm was unchanged and the size reduced on follow-up angiography over at least three times, we considered cessation of follow-up.
Endovascular treatment
The neurosurgeon and interventionalist at our institution discussed the appropriate treatment of these aneurysms. We suggested two treatment options to patients and their relatives who gave informed consent for each treatment. For some reason such as poor medical condition of patients or aversion to surgery, they chose the endovascular treatment. All procedures were performed within 48 hours after confirming the diagnosis of ruptured aneurysms under monitored intravenous anesthesia with Propofol (30-70 μg/kg/min) and Remifentanyl (0.02 μg/kg/min). The Neuroform and Enterprise stents were used. Microcell stents like PED and Silk flow-diverting stents were not available at that time in our institute. Single stenting was used in five patients, the telescopic in-stent stenting technique was used in two, and Y-configured stenting was used in one. When using the telescopic in-stent stenting technique, the shorter stent was deployed first covering the aneurysm neck, and next the longer stent with the same or larger diameter was deployed within the shorter stent covering both ends. The open cell designed stent (Neuroform) was used preferentially. In cases when navigation was expected to be difficult, the closed cell stent (Enterprise) was used.
Peri- and post-procedural antiplatelets and anticoagulation protocols
All procedures were performed with the patients under systemic heparinization (the initial heparin bolus was 3,000-5,000 IU, but in some cases where the procedural time was over one hour, an additional bolus of heparin 1,000-2,000 IU was injected, which was usually started just after the placement of the first stent spanning the aneurysm neck, together with an IV injection of aspirin 900 mg). Preloading with aspirin and clopidogrel was not undertaken. Even if the procedure had been scheduled for the next day, the one-day preoperative dual antiplatelet medication was not given because of the rebleeding risk. The patients were continued on IV systemic heparinization for the next 24 hours to maintain an activated clotting time that was twice their baseline, and both aspirin 100 mg and clopidogrel 75 mg were taken for more than six weeks after the procedure. Then after this, aspirin 100 mg was continued for six months. In cases when ischemic symptoms developed during the dual antiplatelet treatment period, the aspirin dose was doubled and statin derivatives were added for another six months. In the single aspirin period, dual antiplatelet treatment with clopidogrel was then restarted. Then the next regimen was decided according to the follow-up angiographic findings and the symptoms that developed, on a case-by-case basis.
Angiographic evaluation
The O'Kelly-Marotta (OKM) grading scale was used for evaluation of the angiographic results 3. Angiography running into the venous phase is required to determine the appropriate grade. Two parameters were assessed: the volume of contrast filling and the degree of stasis of the contrast material. Aneurysm filling was graded as: A: complete (>95%), B: incomplete (5-95%), C: neck remnant (<5%), or D: no filling (0%). The stasis grade was determined by the timing of contrast clearance from the aneurysm sac as defined by the phases of the angiogram: 1: no stasis within the arterial phase, 2: moderate stasis of contrast (clearance of contrast prior to the venous phase), 3: constant stasis of contrast in aneurysm in the venous phase and beyond. This grading defines a total of ten possible grades, with the stasis grades coupled to each of the filling grades with the exception of Grade D, no filling (A1, A2, A3, B1, B2, B3,C1, C2, C3) 3. This retrospective review presents a series of eight patients who received sole stenting with large cell stents for the treatment of very small aneurysms, less than 3 mm in size, that were a challenge to treat, and we discuss the feasibility of these stents, using the follow-up angiographic results graded according to the O'Kelly-Marotta (OKM) grading scale.
Results
The demographic data are summarized in Table 1. There were eight patients of whom four were men and four were women. The mean age was 63.9 years and the age ranged from 49 to 84 years. The locations of the aneurysms were as follows: three aneurysms were located in the internal carotid artery (ICA), two were in the middle cerebral artery (MCA), and three were located each in the posterior cerebral artery (PCA), basilar trunk (BT) and anterior communicating artery (AcomA) respectively. All eight patients presented with subarachnoid hemorrhage, as diagnosed by unenhanced head computed tomography (CT). Each patient underwent CT angiography (CTA) followed by digital subtraction angiography (DSA). Among the eight aneurysms, seven (87.5%) were detected on the CTA only, and all were identified with DSA. The procedure was performed within 48 hours of the onset of symptoms. Sole stenting without coiling was performed: five were single stentings, two were in-stent stentings and one was a Y-configured stenting. The last follow-up DSA was performed from six to 34 months after the procedure.
Complete aneurysm obliteration on the follow-up angiography was observed in three cases (37.5%), and size reduction or stable angiographic findings were seen in five (62.5%). There was no procedure-related rupture or growth of the aneurysm on follow-up angiography in any of the cases. Angiographic changes were evaluated by the OKM grading scale, used in the assessment of intracranial aneurysms treated with flow-diverting stents. Absence of flow change in the aneurysm and in the parent artery immediately after stenting, compared to the flow observed in the diagnostic angiography, was found in six patients (A1); aneurysmal flow reduction in the arterial phase without aneurysmal flow stagnation was detected in one patient (B1); and aneurysmal flow stagnation was seen in one patient (B3). Complete obliteration of the aneurysm with an intact parent artery (D) was detected in three patients in the follow-up angiography 12-22 months post-procedure; all of these three patients had received overlapping stents including Y-configured stents. There was no increase in the size of the aneurysm seen in any of the cases, even in the single stent cases, and there was one case of asymptomatic in-stent stenosis (case 1) during the follow-up period. There were no permanent symptomatic complications. However, transient ischemic attack occurred in one patient (12.5%) several days after the dual antiplatelet medication was changed to single aspirin six weeks after the procedure; dual antiplatelet treatment was restarted in this patient (case 3) and statin derivatives were added to the treatment regimen.
Illustrative cases
Case 1
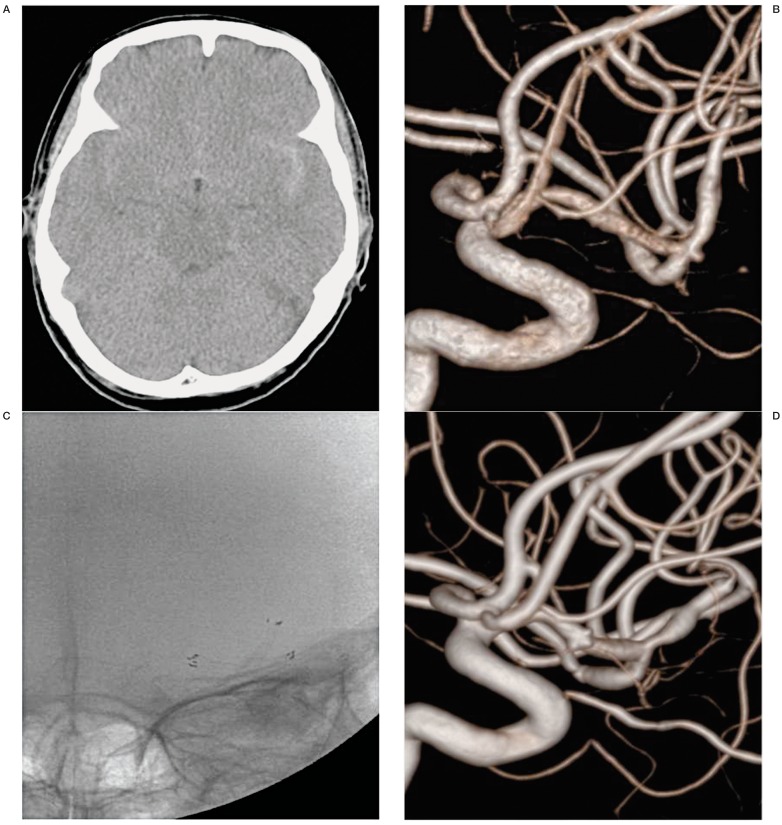
A 70-year-old woman presented with severe headache. Brain CT scan revealed subarachnoid hemorrhage (SAH) in the basal cistern, predominantly in the left sylvian cistern (Figure 1A). Three-dimensional rotational angiography (3DRA) showed a 1.3 × 1.1 mm sized left middle cerebral artery (MCA) aneurysm (Figure 1B). After reviewing the surgical and endovascular options, her relatives selected endovascular treatment because of her age and poor medical condition. Subsequently, Y-configured stenting was chosen as the treatment. A 3.0 mm × 20 mm Neuroform stent was deployed from the superior branch of M2 to M1, followed by a second 3.0 mm × 20 mm Neuroform stent that was deployed from the inferior temporal branch of M2 to M1 to reconstruct the MCA aneurysm base (Figure 1C). After the procedure, the patient was taken to the intensive care unit and was maintained on oral antiplatelet agents according to our protocol. She was discharged without any neurological defects on the 30th day of hospitalization. A follow-up angiogram 22 months later demonstrated the disappearance of the aneurysm (OKM grade D, Figure 1D) and the presence of asymptomatic post-stent stenosis at both M2 segments.
Figure 1.
A 70-year-old woman (Case 1) with a ruptured MCA aneurysm. A) Subarachnoid hemorrhage in basal cistern and predominantly left sylvian cistern on the brain CT scan. B) Very small aneurysm (1.3 × 1.1mm) at the MCA bifurcation on 3-dimensional rotational angiogram. C) Y-configured stenting for aneurysm of MCA bifurcation on the fluoroscopy. D) A follow-up angiogram 22 months later demonstrated the disappearance of the aneurysm and the presence of asymptomatic post-stent stenosis at both M2 segments.
Case 5
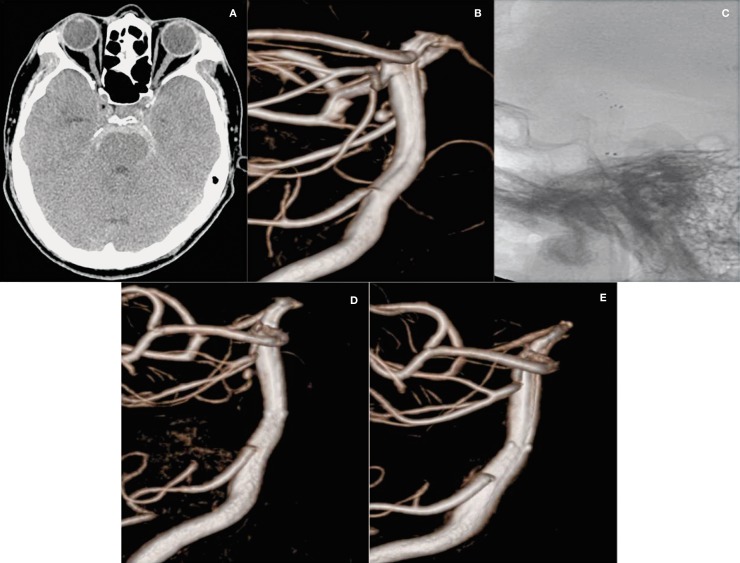
A 52-year-old man was admitted to our hospital with severe headache, nausea and vomiting (H-H grade 2). Brain CT scan showed SAH in the prepontine cistern (Figure 2A). A dorsal wall 2.1 × 2.0 mm aneurysm of the basilar trunk was identified on the 3DRA (Figure 2B). We decided to perform stent monotherapy using the overlapping stent technique because the aneurysm was too small and the patient had an unsuitable dome-to-neck ratio to allow treatment with simple coiling. Furthermore, the location of the aneurysm made clipping too difficult. A 4.0 mm × 20 mm Neuroform3 stent was deployed from the distal to the proximal basilar artery and this fully covered the aneurysm. This was followed by a 4.0 mm × 22 mm Enterprise stent that was deployed from the right posterior cerebellar artery to the basilar artery within the prior stent (Figure 2C). Postoperative immediate 3DRA showed decreased contrast filling within the aneurysm (OKM grade B1, Figure 2D). The patient was discharged without neurological deficit, and a follow-up angiogram 18 months later demonstrated the disappearance of the aneurysm (OKM grade D, Figure 2E).
Figure 2.
A 52-year-old man (Case 5) with a ruptured aneurysm on the dorsal wall of the basilar trunk. A) Subarachnoid hemorrhage in the prepontine cistern on the brain CT scan. B) Very small aneurysm (2.1 × 2.0 mm) of the basilar trunk on the dorsal wall on 3-dimensional rotational angiogram. C) Double stents using the stent-within-stent technique on fluoroscopy. D) Decreased contrast filling within the aneurysm on the immediate post-operation angiography. E) Follow-up ICA angiogram at 18 months post-operation shows the disappearance of the aneurysm.
Discussion
Rationale for sole stenting
Very small aneurysms are difficult and dangerous to treat with surgical clipping or endovascular coiling because they have fragile walls and an indistinct neck. Surgical clipping and wrapping have been considered to be risky for these aneurysms because of the risk of intraoperative and postoperative rebleeding 1,4. Although trapping with or without a bypass has been recommended as the appropriate treatment 1, and the use of an encircling clip graft as a rescue technique 5, this sophisticated surgery may not be always possible, especially in cases when the aneurysm has ruptured. Endovascular trapping or parent artery occlusion (PAO) of the affected arterial segment after a successful balloon test occlusion (BTO) has been suggested as a safe treatment producing good collateral circulation 6. However, even if a patient has good collateral flow with BTO, performing PAO in ruptured cases might have the potential of producing cerebral ischemia in the short and long term, particularly in the situation of cerebral vasospasm 7. Along with the development of stent material and stenting techniques, a new concept has recently evolved of using “endovascular bypass” or “endovascular reconstruction” techniques which enable the affected parent artery and perforators to be preserved, and the aneurysm to be safely occluded 7-10. Compared to the deconstructive methods of trapping or PAO, the reconstructive methods may be more definitive and the ideal treatment physiologically. Sole stenting is one of the reconstructive methods used and has been reported as an alternative treatment for complex aneurysms in which it is extremely difficult and dangerous to deploy a coil 7-10. Their treatment mechanism involves hemodynamic change and a thrombotic phenomenon. The implanted stent alters the flow pattern of the affected artery and reduces flow into the aneurysmal sac, and finally results in aneurysmal thrombosis and occlusion 11. Additionally, neointimal tissue covers the luminal surface of the stent and the affected artery is reconstructed 12.
Limitation of flow-diverters
Flow-diverting stents offer a new alternative for the treatment of aneurysms with difficult morphologies, including giant, wide-necked, fusiform, and blister types. The Pipeline Embolization Device (PED, ev3 Endovascular, Plymouth, MN, USA) and Silk flow-diverting stent (Balt Extrusion, Montmorency, France) are porous endoluminal sleeves designed to cover approximately 30% to 35% of the neck surface area, roughly five times that of the area covered by the Neuroform and Enterprise stents 13,14. These microcell stents are purported to immediately reduce the inflow and outflow jets from the aneurysm and virtually eliminate shear stress on the aneurysm wall, while still allowing blood flow to branch arteries and perforators covered by the device 13-16. Since microcell stents were introduced, various complications have been reported, and they are difficult to use in the distal portion of the ICA bifurcation because of the obstruction of perforators or the unfeasible navigation of the tortuous vasculature. The use of a microcell stent is associated with a risk of rebleeding, especially in cases where the aneurysm has ruptured, because of vessel injury and the high dosage of antiplatelet medication administered 17,18. Recent studies reported that 11 patients with ruptured aneurysms were treated by PED and among them two patients died from rebleeding during the six-month follow-up 18. In addition, thromboembolic complications have occurred because the microcell stent has a dense stent surface and navigation can be poor 19. The exact duration of treatment and the best cocktail of antiplatelets and anticoagulants that should be given are not yet known, but lifetime administration may be necessary in these situations 19,20. Clopidogrel is discontinued after six months, whereas aspirin therapy remains lifelong if the six-month follow-up angiogram shows a complete occlusion of the aneurysm 19. If the microstent is deployed distally over the bifurcation of the internal carotid artery, more long-term dual antiplatelet use is recommended because of the risk of thromboembolic events in the stent-covered parent artery 15. The authors consider that although the microcell stent might be useful in cases when a blister-like aneurysm is located in the distal ICA, the large cell stent might be more useful in cases where other arteries are affected and the artery has a small caliber and fine perforators such as the middle cerebral artery, posterior cerebral artery or anterior cerebral artery. In ruptured cases, the use of potent antiplatelet drugs and a microstent might be less appropriate than stenting with a large cell stent because of unexpected hemorrhagic complications such as aneurysmal rebleeding and intraparenchymal hemorrhage.
Results of sole stenting
In the sole stenting technique for very small ruptured aneurysms, the optimal antiplatelet or anticoagulation regimen remains uncertain. Many surgeons have a fear of rebleeding or thromboembolism occurring in the patient and because of this, may hesitate to use the stent monotherapy technique. In fact, these complications have developed in several cases. In Zenteno et al.'s series, one of 20 patients (5%) who had stent monotherapy for dissecting and non-dissecting aneurysms of the posterior circulation had a thromboembolic event and one patient (5%) experienced rebleeding at two weeks post-procedure 9. Their series was composed of 11 ruptured cases, which had no procedural and thromboembolic complications except for one case of delayed rebleeding. In another series of stent monotherapy for ruptured pseudoaneurysm, two of ten patients (20%) had thromboembolic events, of whom one had a partial visual field defect and the other was asymptomatic, and no rebleeding was encountered 10.
Considering the characteristics of the presenting diseases, their results might be relatively acceptable. However, Gaughen et al. reported that three of six cases treated by the overlapping technique for blood blister aneurysms had aneurysms which were continuously growing at the follow-up angiography and were additionally treated by coil embolization 7. Although there were no growing cases in our series, short-term follow-up angiography would be necessary. Another author's antiplatelet protocol in cases of sole stenting was similar to ours. In our institute, preloading with aspirin and clopidogrel was not undertaken.
Even if the procedure was scheduled for the next day, one-day preoperative dual antiplatelet regimen was not given because of the risk of rebleeding. One patient had transient ischemic attack for several days after the dual antiplatelet treatment was changed to single aspirin at six weeks after the procedure, and dual antiplatelet drugs were restarted along with statin derivatives in this patient. The patient has not experienced neurologic symptoms since the change in medication. One patient in one study experienced rebleeding during chemical angioplasty for vasospasm and expired. In our cases, a growing aneurysm was not detected and additional coiling or surgical clipping was not needed.
Overlapping stenting vs single stenting
It is uncertain whether an overlapping stent produces better angiographic results than single stenting. In experimental studies, Kim et al. reported that the hemodynamic influence varied with the number of stents, and that the double stent model was associated with more significant changes 21. By contrast, Rhee et al. suggested that there was no significant effect related to the number of stents (porosity) 22. However, a recent clinical series showed that the overlapping stent technique was more effective than single stenting because there was decreased porosity 6,7. The results of using microcell stents such as PED or Silk flow-diverting stents are in line with this theory. In our series, three cases treated by overlapping or Y-configured stent technique had better occlusive results (OKM grade D) on follow-up angiography than cases treated by single stent deployment. We agree that the overlapping stenting technique might be a more effective method. However, unexpected technical problems such as vessel injury, stent malposition and increasing thrombogenicity related to the additional deployment of a stent within a prior stent should be overcome.
Conclusions
Stent monotherapy with large cell stents is an incomplete treatment unless there is isolation of the aneurysm from the intracranial circulation with or without mechanical obliteration of the aneurysm neck.
However it can have a positive role in keeping the aneurysm size stable even in ruptured cases. However it is essential to be alert to the possibility of regrowth of the aneurysm or rebleeding which might cause a catastrophic condition. Early follow-up angiography and careful control of the dosage of antiplatelets given should be considered.
Acknowledgments
The present research was conducted under a research fund from Dankook University in 2012.
References
- 1.Kawashima A, Okada Y, Kawamata T, et al. Successful treatment of a blood blister-like aneurysm of the internal carotid artery by trapping with a high-flow bypass. J Clin Neurosci. 2008;15:797–800. doi: 10.1016/j.jocn.2007.03.012. doi: 10.1016/j.jocn.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Kim BM, Chung EC, Park SI, et al. Treatment of blood blister-like aneurysm of the internal carotid artery with stent-assisted coil embolization followed by stent-within-a-stent technique. Case report. J Neurosurg. 2007;107:1211–1213. doi: 10.3171/JNS-07/12/1211. doi: 10.3171/JNS-07/12/1211. [DOI] [PubMed] [Google Scholar]
- 3.O'Kelly CJ, Krings T, Fiorella D, et al. A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol. 2010;16:133–137. doi: 10.1177/159101991001600204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogawa A, Suzuki M, Ogasawara K. Aneurysms at nonbranching sites in the supraclinoid portion of the internal carotid artery: internal carotid artery trunk aneurysms. Neurosurgery. 2000;47:578–583. doi: 10.1097/00006123-200009000-00008. discussion 583-576. doi: 10.1227/00006123-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Sekula RF, Jr, Cohen DB, Quigley MR, et al. Primary treatment of a blister-like aneurysm with an encircling clip graft: technical case report. Neurosurgery. 2006;59(Supp 1):ONSE168. doi: 10.1227/01.neu.0000220058.17532.b5. discussion ONSE168. doi: 10.1227/01.NEU.0000220058.17532.B5. [DOI] [PubMed] [Google Scholar]
- 6.Kim YW, Park IS, Baik MW, et al. Endovascular treatment of blood blister-like aneurysms using multiple self-expanding stents. J Korean Neurosurg Soc. 2011;49:116–119. doi: 10.3340/jkns.2011.49.2.116. doi: 10.3340/jkns.2011.49.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaughen JR, Jr, Hasan D, Dumont AS, et al. The efficacy of endovascular stenting in the treatment of supraclinoid internal carotid artery blister aneurysms using a stent-in-stent technique. Am J Neuroradiol. 2010;31:1132–1138. doi: 10.3174/ajnr.A2016. doi: 10.3174/ajnr.A201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YJ. Sole stenting technique for treatment of complex aneurysms. J Korean Neurosurg Soc. 2009;46:545–551. doi: 10.3340/jkns.2009.46.6.545. doi: 10.3340/jkns.2009.46.6.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zenteno MA, Santos-Franco JA, Freitas-Modenesi JM, et al. Use of the sole stenting technique for the management of aneurysms in the posterior circulation in a prospective series of 20 patients. J Neurosurg. 2008;108:1104–1118. doi: 10.3171/JNS/2008/108/6/1104. doi: 10.3171/JNS/2008/108/6/1104. [DOI] [PubMed] [Google Scholar]
- 10.Fiorella D, Albuquerque FC, Deshmukh VR, et al. Endovascular reconstruction with the Neuroform stent as monotherapy for the treatment of uncoilable intradural pseudoaneurysms. Neurosurgery. 2006;59:291–300. doi: 10.1227/01.NEU.0000223650.11954.6C. discussion 291-300. doi: 10.1227/01.NEU.0000223650.11954.6C. [DOI] [PubMed] [Google Scholar]
- 11.Lieber BB, Gounis MJ. The physics of endoluminal stenting in the treatment of cerebrovascular aneurysms. Neurol Res. 2002;24(Suppl 1):S33–S42. doi: 10.1179/016164102101200014. doi: 10.1179/016164102101200014. [DOI] [PubMed] [Google Scholar]
- 12.Lopes D, Sani S. Histological postmortem study of an internal carotid artery aneurysm treated with the Neuroform stent. Neurosurgery. 2005;56:E416. doi: 10.1227/01.neu.0000147977.07736.66. discussion E416. doi: 10.1227/01.NEU.0000147977.07736.66. [DOI] [PubMed] [Google Scholar]
- 13.Kallmes DF, Ding YH, Dai D, et al. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007;38:2346–2352. doi: 10.1161/STROKEAHA.106.479576. doi: 10.1161/STROKEAHA.106.479576. [DOI] [PubMed] [Google Scholar]
- 14.Kallmes DF, Ding YH, Dai D, et al. A second-generation, endoluminal, flow-disrupting device for treatment of saccular aneurysms. Am J Neuroradiol. 2009;30:1153–1158. doi: 10.3174/ajnr.A1530. doi: 10.3174/ajnr.A1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulcsar Z, Wetzel SG, Augsburger L, et al. Effect of flow diversion treatment on very small ruptured aneurysms. Neurosurgery. 2010;67:789–793. doi: 10.1227/01.NEU.0000372920.39101.55. doi: 10.1227/01.NEU.0000372920.39101.55. [DOI] [PubMed] [Google Scholar]
- 16.Sadasivan C, Cesar L, Seong J, et al. An original flow diversion device for the treatment of intracranial aneurysms: evaluation in the rabbit elastase-induced model. Stroke. 2009;40:952–958. doi: 10.1161/STROKEAHA.108.533760. doi: 10.1161/STROKEAHA.108.533760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung GK, Tsang AC, Lui WM. Pipeline embolization device for intracranial aneurysm: a systematic review. Clin Neuroradiol. 2012;22:295–303. doi: 10.1007/s00062-012-0178-6. doi: 10.1007/s00062-012-0178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAuliffe W, Wenderoth JD. Immediate and midterm results following treatment of recently ruptured intracranial aneurysms with the Pipeline embolization device. Am J Neuroradiol. 2012;33:487–493. doi: 10.3174/ajnr.A2797. doi: 10.3174/ajnr.A2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chitale R, Gonzalez LF, Randazzo C, et al. Single center experience with pipeline stent: feasibility, technique, and complications. Neurosurgery. 2012;71:679–691. doi: 10.1227/NEU.0b013e318260fe86. discussion 691. doi: 10.1227/NEU.0b013e318260fe86. [DOI] [PubMed] [Google Scholar]
- 20.Klisch J, Turk A, Turner R, et al. Very late thrombosis of flow-diverting constructs after the treatment of large fusiform posterior circulation aneurysms. Am J Neuroradiol. 2011;32:627–632. doi: 10.3174/ajnr.A2571. doi: 10.3174/ajnr.A2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim M, Levy EI, Meng H, et al. Quantification of hemodynamic changes induced by virtual placement of multiple stents across a wide-necked basilar trunk aneurysm. Neurosurgery. 2007;61:1305–1312. doi: 10.1227/01.NEU.0000280168.25968.49. discussion 1312-1303. doi: 10.1227/01.neu.0000306110.55174.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhee K, Han MH, Cha SH. Changes of flow characteristics by stenting in aneurysm models: influence of aneurysm geometry and stent porosity. Ann Biomed Eng. 2002;30:894–904. doi: 10.1114/1.1500406. doi: 10.1114/1.1500406. [DOI] [PubMed] [Google Scholar]