Summary
Three patients are described with unruptured large partially thrombosed aneurysms with a peculiar donut-shaped remaining lumen.
Observations suggest that the flow geometry of the aneurysm and parent vessels induces a preferential circular laminar flow inside the aneurysm followed by partial intraluminal thrombosis leaving a donut-shaped lumen to accommodate the circular flow. This flow mechanism of thrombus formation inside aneurysms is different from the more common repeated intramural dissections and hemorrhages that cause growth in most large and giant partially thrombosed aneurysms.
Key words: intracranial aneurysm, partial thrombosis, 3D rotational angiography
Introduction
Partially thrombosed aneurysms are a diverse collection of mostly large and giant aneurysms characterized by organized intraluminal thrombus.
The pathogenesis of intraluminal thrombus in these aneurysms is thought to be related to subacute or chronic wall dissections and repeated intramural hematomas, proliferating vasa vasorum and triggering of inflammatory mechanisms 1-4.
We recently encountered three patients with partially thrombosed large aneurysms with a peculiar donut-shaped remaining lumen where flow conditions probably played a major role in pathogenesis of the intraluminal thrombus.
Case 1
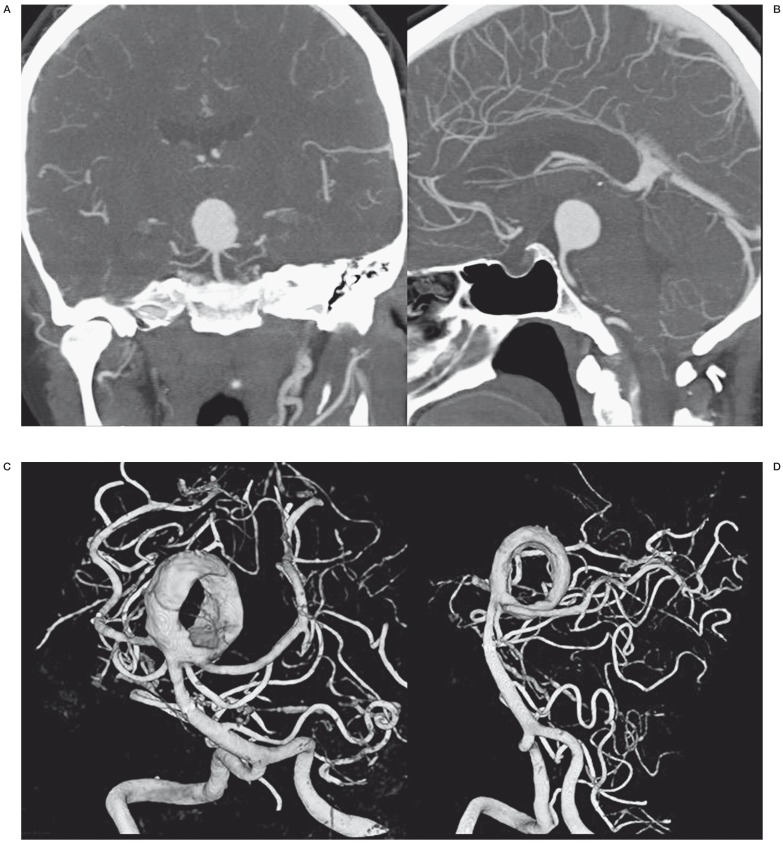
A 50-year-old woman was referred for a native CT scan in another hospital because of intermittent dizziness. An aneurysm was suspected on this CT scan and on subsequent CT angiography a large (20 mm) wide-necked aneurysm on the basilar tip was evident (Figure 1A,B). It was decided to coil this aneurysm after Y-stenting in both posterior cerebral arteries and the patient was preloaded with double antiplatelet medication. However, after placement of the first stent in the right posterior cerebral artery, it was technically impossible to place the second stent in the left posterior cerebral artery. Ten days later, the patient was referred to our hospital. On repeat angiography with 3D rotational angiography (3DRA), the aneurysm was now largely thrombosed leaving a donut-shaped lumen with circular laminar flow (Figure 1C,D). This lumen was occluded with coils without additional stent placement. Apparently, the thrombus was soft since the coils easily went into the thrombosed parts of the aneurysm. Follow-up MRA six months later showed reopening of the lumen with partial resolution of the intraluminal thrombus and the aneurysm was additionally coiled (not shown). Further MRA follow-up is scheduled.
Figure 1.
Incidental large basilar tip aneurysm in a 50-year-old woman. A,B) CT angiography demonstrates large spherical basilar tip aneurysm. C,D) 3DRA of the same aneurysm 10 days after stent placement in the right posterior cerebral artery. The aneurysm is now largely thrombosed with a donut-shaped remaining lumen.
Case 2
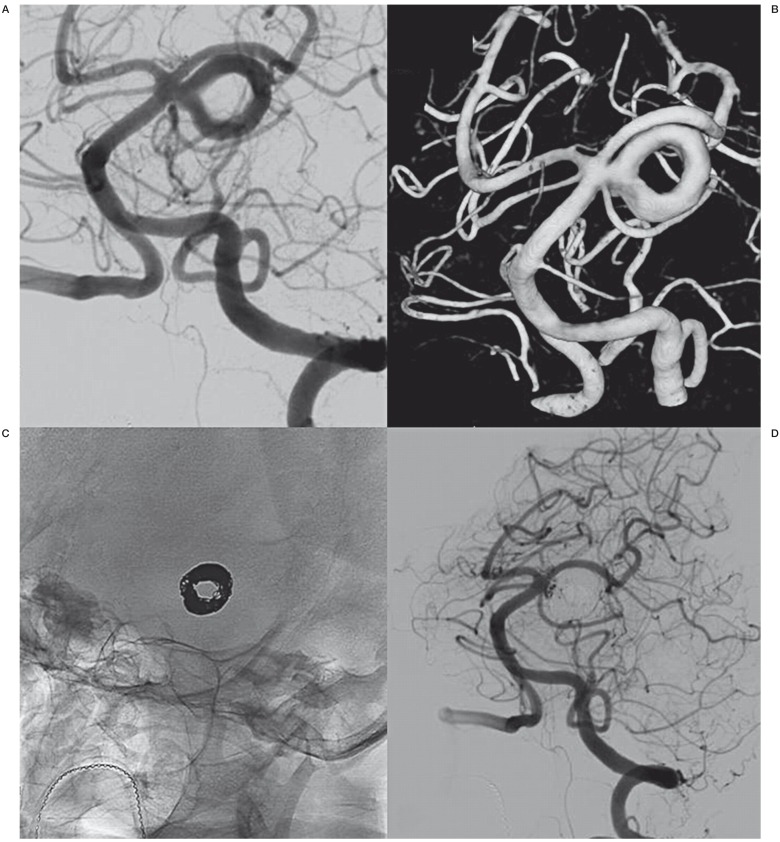
A 70-year-old woman with TIAs was referred for MRI and a large partially thrombosed basilar aneurysm was found. She was referred to our hospital for endovascular treatment. Angiography with 3DRA revealed an 18mm partially thrombosed aneurysm on the left superior cerebellar artery with a donut shaped lumen (Figure 2A,B). The flow was circular and laminar with distal inflow and proximal outflow. The donut-shaped lumen could be completely occluded with coils (Figure 2C,D). The coils did not penetrate into the thrombus. Follow up MRA is scheduled.
Figure 2.
Incidental superior cerebellar artery aneurysm in a 70-year-old woman. A,B) 2D angiography (A) and 3DRA (B) show the large left superior cerebellar artery aneurysm with donut–shaped lumen. C) Circular deposition of coils in the donut-shaped lumen. D) Almost complete occlusion after coiling.
Case 3
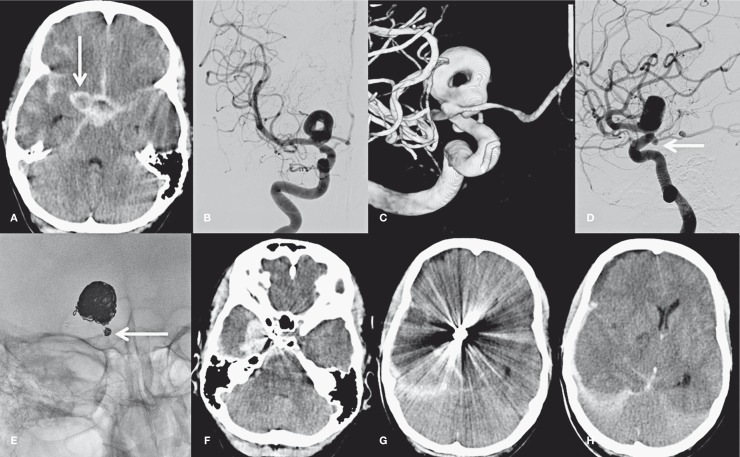
A 46-year-old man was admitted with poor grade subarachnoid hemorrhage. CT showed diffuse subarachnoid blood (Figure 3A) with a large aneurysm in the region of the right carotid tip (arrow in Figure 3A). Angiography and 3DRA revealed a 17 mm partially thrombosed carotid tip aneurysm with a donut-shaped lumen. There was an additional small aneurysm on the right posterior communicating artery (PcomA) (Figure 3D, arrow). Because both aneurysms had a wide neck, a stent was positioned in the M1 and supraclinoid carotid artery that covered both aneurysms. Subsequently both aneurysms were coiled with rather loose packing in the small PcomA aneurysm (Figure 3E, arrow). Before stent placement, 500 mg intravenous aspirin were administered and after coiling the patient was loaded with double anti-platelet medication. The patient gradually recovered but on day 10 after coiling there was a sudden clinical deterioration. CT scan (Figure 3 F-H) showed a recurrent subarachnoid hemorrhage with a large subdural component causing mass effect and midline shift. Since the hemorrhage was mostly subdural, the most probable cause was a rebleed from the small PcomA aneurysm and not from the large carotid tip aneurysm. The patient died the same evening.
Figure 3.
A 46-year-old man with poor grade subarachnoid hemorrhage. A) CT scan shows SAH and aneurysm (arrow). B-D) 2D angiography (B,D) and 3DRA (C) demonstrate a donut-shaped large carotid tip aneurysm and a second aneurysm on the PcomA (arrow in D). E) Coil meshes after coiling of the carotid tip aneurysm and the small PcomA aneurysm (arrow). F-H) CT scan 10 days after coiling shows recurrent hemorrhage from the PcomA aneurysm with a large subdural component and mass effect.
Discussion
We observed three patients with partially thrombosed intracranial aneurysms with a donut-shaped remaining lumen. All three aneurysms were large and unruptured, were spherical in shape and had circular and laminar flow with a distal inflow zone and proximal outflow zone. Two aneurysms were located on a bifurcation and one was a sidewall. It is likely that aneurysm and parent vessel geometry played an important role in the pathogenesis of the intraluminal thrombus. In patient 1 the intraluminal thrombus in the basilar tip aneurysm emerged in a time course of several days after stent placement in the right posterior cerebral artery. Apparently, the flow alteration caused by the stent induced a preference of circular flow in the spherical aneurysm with very slow flow or even cessation of flow in the remaining parts of the spherical aneurysm. This lack of flow in large parts of the aneurysm in turn caused thrombosis leaving a donut-shaped lumen. During coiling, the coils easily penetrated the thrombus in patients 1 and 3 suggesting relatively fresh and soft intraluminal thrombus. In patient 2 the thrombus was too hard for the coils to penetrate suggesting hard and longstanding thrombus.
Our observations strongly suggest that the formation of intraluminal thrombus in some aneurysms is related to flow conditions and may be a dynamic phenomenon. When aneurysm and parent vessel geometry favor a particular circular laminar flow pattern, flow in the remaining part of the aneurysm may slow down or even stop thereby inducing spontaneous thrombosis with a remaining donut shaped lumen to accommodate the flow. The thrombus in these aneurysms is located in the aneurysm lumen itself and is not intramural.
The pathogenesis of intraluminal thrombus in these type of aneurysms differs from most other partially thrombosed aneurysms. Most partially thrombosed aneurysms are characterized by recurrent subacute intramural dissections with repeated subadventitial bleeding and progressive enlargement of the aneurysm. On MRI, the repeated wall dissections may cause a so-called onion-skin appearance. The process of growth by dissections and intramural hemorrhage from the vasa vasorum may induce peri-aneurysmal edema and rim enhancement of the aneurysm wall. The location of the thrombus in these aneurysms is mainly in the aneurysm wall (intramural) and not in the aneurysm lumen 1-3. This same mechanism of growth is also suggested for serpentine aneurysms that develop from fusiform aneurysms 5.
Our three cases demonstrate that besides the more common intramural location of thrombus in partially thrombosed aneurysms caused by repeated wall dissections there is a subset of partially thrombosed aneurysms with intraluminal location of the thrombus induced by flow conditions inside the aneurysms.
References
- 1.Krings T, Alvarez H, Reinacher P, et al. Growth and rupture mechanism of partially thrombosed aneurysms. Interv Neuroradiol. 2007;13:117–126. doi: 10.1177/159101990701300201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin AJ, Hetts SW, Dillon WP, et al. MR imaging of partially thrombosed cerebral aneurysms: characteristics and evolution. Am J Neuroradiol. 2011;32:346–351. doi: 10.3174/ajnr.A2298. doi: 10.3174/ajnr.A2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roccatagliata L, Guédin P, Condette-Auliac S, et al. Partially thrombosed intracranial aneurysms: symptoms, evolution, and therapeutic management. Acta Neurochir (Wien) 2010;152:2133–2142. doi: 10.1007/s00701-010-0772-9. doi: 10.1007/s00701-010-0772-9. [DOI] [PubMed] [Google Scholar]
- 4.Ferns SP, van Rooij WJ, Sluzewski M, et al. Partially thrombosed intracranial aneurysms presenting with mass effect: long-term clinical and imaging follow-up after endovascular treatment. Am J Neuroradiol. 2010;31:1197–1205. doi: 10.3174/ajnr.A2057. doi: 10.3174/ajnr.A2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Rooij WJ, Sluzewski M, Beute GN. Endovascular treatment of giant serpentine aneurysms. Am J Neuroradiol. 2008;29:1418–1419. doi: 10.3174/ajnr.A1071. doi: 10.3174/ajnr.A1071. [DOI] [PMC free article] [PubMed] [Google Scholar]