Summary
Onyx has been widely adopted for the treatment of arteriovenous malformations (AVMs). However, its control demands operators accumulate a considerable learning curve.
We describe our initial experience using a novel injection method for the embolization of AVMs.
We retrospectively reviewed the data of all 22 patients with brain AVMs (12 men, 10 women; age range, 12-68 years; mean age, 43.2 years) treated by the transarterial coil-augmented Onyx injection technique. The size of the AVMs ranged from 25 mm to 70 mm (average 35.6 mm). The technical feasibility of the procedure, procedure-related complications, angiographic results, and clinical outcome were evaluated.
In every case, endovascular treatment (EVT) was completed. A total of 31 sessions were performed, with a mean injection volume of 6.1 mL (range, 1.5-16.0 mL). An average of 96.7% (range 85%-100%) estimated size reduction was achieved, and 18 AVMs could be completely excluded by EVT alone. The results remained stable on follow-up angiograms. A procedural complication occurred in one patient, with permanent mild neurologic deficit.
Our preliminary series demonstrated that the coil-augmented Onyx injection technique is a valuable adjunct achieving excellent nidal penetration and improving the safety of the procedure.
Key words: embolization, arteriovenous malformation, coil, Onyx, endovascular treatment
Introduction
The treatment of cerebral arteriovenous malformations (AVMs) is challenging and many therapeutic approaches have been proposed, such as neurosurgery, endovascular embolization, radiosurgery, and frequently the association of two or even three of these techniques 1. Current EVT involves intranidal injection of permanent liquid embolic agents with the intent of completely excluding the AVM circulation or, when that is impossible, achieving the largest possible embolized amount of the AVM, allowing a complementary treatment by neurosurgery or radiosurgery.
Recently, packing with Onyx (ev3, Irvine, CA, USA) has demonstrated favorable outcomes with minimal complications 2. However, its control demands operators accumulate a considerable learning curve. Recent reports have employed a balloon-assisted technique for embolization of dural arteriovenous fistulas and AVMs. Transarterial balloon-induced flow arrest allows for better nidal penetration without reflux 3-6. Nevertheless, the deliverability of balloon catheters limits its widespread use. This article describes a novel method, the coil-augmented Onyx injection technique (CAIT), used successfully to treat 22 consecutive patients. The efficacy, safety and possible disadvantages of this technique are described. To our knowledge, this is the first experience with this method reported in the literature.
Patients and Methods
Patients and General Information
Between February 2010 and September 2012, 22 patients (12 men, 10 women; age range, 12-68 years; mean age, 43.2 years) with cerebral AVMs were selected for treatment with CAIT at our institution. Informed consent from the patients and institutional review board approval were obtained. Clinical data on the patients are summarized in Table 1. Their symptoms included intracranial hemorrhage (50.0%, epileptic seizures (36.4%) and incidental diagnosis (13.6%).
Table 1.
Characteristics of the patients
| Characteristics | No (%) |
| Symptoms | |
| Hemorrhage | 11 (50.0) |
| Seizures | 8 (36.4) |
| Incidental | 3 (13.6) |
| Location | |
| Hemisphere | 15 (68.2) |
| Corpus callosum | 3 (13.6) |
| Basal ganglia or thalamus | 2 (9.1) |
| Cerebellum | 2 (9.1) |
| Spetzler-Martin grade | |
| I | 3 (13.6) |
| II | 6 (27.3) |
| III | 8 (36.4) |
| IV | 5 (22.7) |
Of the 22 AVMs treated, nine (40.9%) were located in the right cerebral hemisphere, six (27.3%) in the left cerebral hemisphere, five (22.7%) in the deep brain (three in the corpus callosum, and two in the basal ganglia or thalamus), and two (9.1%) in the cerebellum. Sixteen AVMs were plexiform and six were mixed (plexiform-fistula). The dimensions, measured along the longest axis of the AVM, ranged from 25 mm to 70 mm (mean size 35.6 mm). Deep venous drainage was present in eight AVMs. According to the classification of Spetzler-Martin, three AVMs were classified as grade I; six as grade II; eight as grade III, and five as grade IV.
Description of the coil-augmented Onyx injection technique
CAIT using Onyx was applied in cases of selected AVMs, which was favorable for a curative treatment. The goal of the treatment was to completely excluded the nidus or reduce the nidus size to the level (< 3 cm) where radiosurgery would be possible in one or more sessions. All endovascular procedures were performed using the femoral route with general anesthesia. Heparin was administered in the flushing solution (12500 IU/L).
In all patients, the larger feeding arteries from branches of the AchoA, ACA, MCA and PCA, were catheterized for transarterial Onyx injection. A 6-French guiding catheter was placed according to the location of the feeding arteries of the AVMs. Through the guiding catheter, a DMSO-compatible Marathon microcatheter (MTI-ev3, Irvine, CA, USA) was used to catheterize the supplier with the aid of a 0.008-inch Mirage or 0.010-inch Silverspeed microguidewire (MTI-ev3, Irvine, CA, USA). After the Marathon microcatheter was navigated to its intranidal position, a relatively flexible Echelon-10 microcatheter (MTI-ev3, Irvine, CA, USA) used for coil infusion was advanced along the same artery, and positioned proximal to the tip of the Marathon catheter, but past the origins of some key branches. Before injection of the Onyx, a coil was placed to embolize the feeder through the Echelon microcatheter, in order to increase proximal resistance and aid plug formation, resulting in better control of reflux and enhanced distal penetration.
Alternatively, arterial coil embolization was used in high-flow fistulas to prevent distal Onyx migration.
Onyx embolization was carried out as follows: 1) to obtain a trace of the microcatheter trajectory, a nonsubtracted single image of the microcatheter with the guidewire was obtained; 2) the microcatheter was slowly flushed with 0.25 mL of dimethyl sulfoxide (DMSO) over 40 seconds; 3) Onyx-18 was then slowly injected under blank roadmap guidance; 4) the Onyx-18 reflux within the feeder was precisely monitored, and the reflux-hold-rejection technique was used in this period 7-9; 5) when complete obliteration of the nidus was achieved, or when all forward Onyx led into venous spaces, the injection was ended.
After Onyx embolization, the coil was retrieved (if possible) or detached, and two microcatheters were withdrawn. If necessary, a different feeding artery was catheterized and the procedure was repeated.
Post-procedural management
All patients underwent CT scanning within six hours after the procedure. After the procedure, patients were moved to the neurosurgery intensive care unit for strict blood pressure control. Corticosteroids were administrated for five days after the procedure.
Evaluation of outcomes
During the hospital stays, physicians performed neurological examinations of the patients once each day. After discharge, clinical follow-up data were collected by clinic visitation, follow-up angiography, or telephone interview. Clinical outcome was graded according to the modified Rankin score (mRS).
For patients whose nidus was completely excluded by endovascular embolization, follow-up angiogram was recommended six months after the treatment. For patients who underwent radiosurgery, two-year MRI and angiogram were recommended. When the AVM was not obliterated after two years, the angiogram was repeated one year later. If after three years the AVM was still not obliterated, repeated radiosurgery was considered.
Results
Anatomical results
All 22 patients completed the series of endovascular treatments. We performed one to three sessions per patient (mean, 1.4) (Table 2). The mean volume of Onyx injected per AVM was 6.1 mL (1.5-16.0 mL). When we analyzed only the CAIT, the mean volume of Onyx injected was 4.2 mL (1.5-12.7 mL).
Table 2.
Treatment results
| Results | No (%) |
| No of sessions | |
| 1 | 16 (72. 7) |
| 2 | 3 (13.6) |
| 3 | 3 (13.6) |
| Angiographic results | |
| Complete | 18 (81.8) |
| Subtotal + radiosurgery | 4 (18.2) |
Of the 22 AVMs treated, an average of 96.7% (range 85%-100%) estimated size reduction was achieved, and 18 AVMs could be completely excluded by EVT alone.
Another four patients underwent radiosurgery, and they are waiting for two-year post-radiosurgery follow-up results. The results remained stable in all patients on follow-up angiograms.
Clinical results and complications
According to the mRS, all patients were functionally independent at the last follow-up (mRS 0-2). Twenty-one patients showed improvement or the same clinical state at the time of discharge and the last follow-up and no hemorrhage or re-hemorrhage occurred in these patients. Of those, two patients with seizures were symptom-free and antiepileptic drugs were discontinued. Only one patient had neurologic worsening after the procedure because of technical complications, resulting in permanent neurologic deficit.
One complication occurred in a 32-year-old woman harboring an unruptured cerebellar AVM treated in the first session. 95% occlusion was achieved, and a small amount of circulation was still identified supplied by the branch of the right posterior inferior cerebellar artery (PICA). She suffered from a sudden severe headache about an hour after the procedure. We postulated the reason of bleeding was hemodynamic changes, which caused inflow-outflow imbalance. She was treated conservatively. Fortunately, she recovered well with a mild neurological deficit at 15-month follow-up.
Illustrative Cases
Case 1
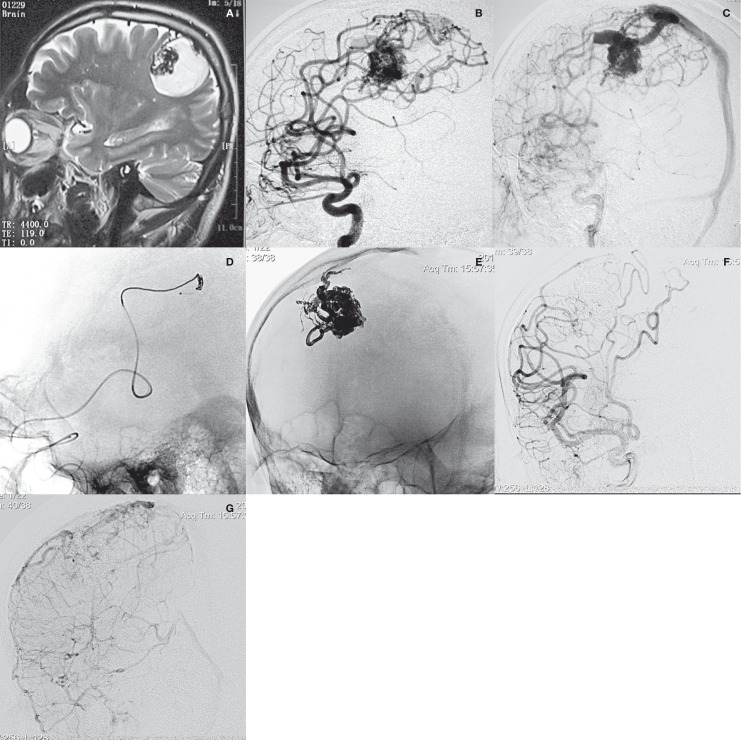
A 55-year-old man presented with intraparenchymal hemorrhage. On admission, physical examination revealed left hemiparalysis. The left-sided Babinski's response was positive. MRI scan demonstrated abnormal vascular structures at the right central area. Angiography showed a fronto-parietal AVM supplied by two branches of the fronto-parietal ascending arteries and drained by a superficial vein into the superior sagittal sinus. Onyx embolization by CAIT was performed. Complete obliteration was achieved by delivering 4.3 mL of Onyx-18 from one feeder with adequate penetration. Three days after the EVT, the patient was discharged in a stable neurological condition. At follow-up, he had recovered well with mild left paraesthesia (Figure 1).
Figure 1.
Patient 2. A) MRI scan demonstrated abnormal vascular structures at the right central area. B,C) Angiogram showed a fronto-parietal AVM, supplied by two branches of the fronto-parietal ascending arteries and drained by a superficial vein into the superior sagittal sinus. D) Onyx embolization by CAIT was performed. E) Plain radiography showed the final cast of Onyx. F,G) Post-procedural angiogram demonstrated complete exclusion of the AVM.
Case 2
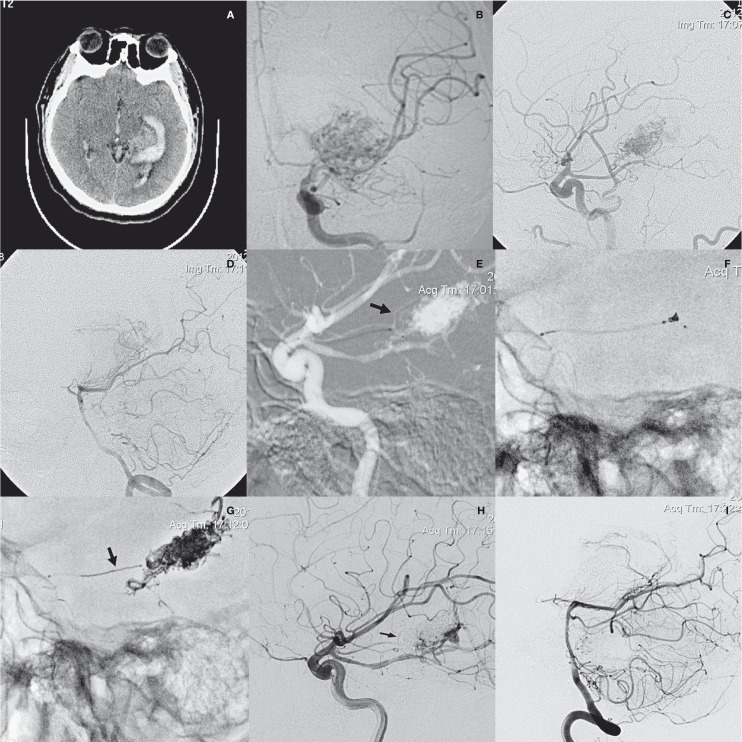
A 26-year-old man presented with sudden headache and right hemiplegia. CT scan demonstrated hematoma at the left basal ganglia area and ventricle. Angiography showed an AVM at left the parahippocampal gyrus and basal ganglia, supplied by a terminal branch of the anterior choroidal artery and several small branches of the fetal posterior communicating artery (PCOMA), and drained by Rosenthal's vein. Because the fetal PCOMA was an “en passager” artery with several feeding branches, and the anterior choroidal artery was a terminal feeder, we chose the latter for catheterization. Though it is slim, navigation was not difficult. Onyx embolization by CAIT was performed. The tip of the coil-infusion microcatheter was navigated to the origin of a penetrating branch and a coil was placed distal to those penetrating branches. Obliteration of about 90% of the nidus was achieved by delivering 3.0 mL of Onyx-18 from that feeder with excellent penetration and minimal reflux. One week after the EVT, the patient underwent gamma knife therapy. At six-month follow-up, he had recovered well with mild left paralysis (Figure 2).
Figure 2.
Patient 9. A) CT scan demonstrated hematoma at the left basal ganglia area and ventricle. B-D) Angiography showed an AVM at left the parahippocampal gyrus and basal ganglia, supplied by a terminal branch of the anterior choroidal artery and several small branches of the posterior choroidal arteries, and drained by Rosenthal's vein. E) Roadmap image showed that the tip of the coil-infusion microcatheter had been navigated to the origin of a penetrating branch (arrow). F) A 2 mm×2 cm coil was placed distal to those penetrating branches. G) Plain radiography showed the final cast of Onyx and the retrieved coil (arrow). H,I) Post-procedural angiogram demonstrated sub-complete exclusion of the AVM with the penetrating branch patent.
Discussion
The successful treatment of cerebral AVMs represents a major challenge. Thanks to the improvement of endovascular materials and experience, embolization has become increasingly important in the management of cerebral AVMs. The main goals of AVM embolization are nidus reduction before surgery or radiosurgery, and curative embolization. Nidus reduction aims to minimize the operative difficulty and risk during surgical removal, which is characterized by the constraint of not pursuing as much of the occlusion as possible 10. Curative embolization aims to achieve total occlusion of the AVMs 11, and in cases of subtotal occlusion, embolization can reduce the volume of the nidus to make it amenable to radiosurgery. Similarly, the risk of hemorrhage may be reduced by embolizing the feeding artery or intranidal aneurysms while awaiting the delayed occlusion achieved by radiosurgery. All AVMs in this series were embolized with a curative intent. Four cases of post-embolization AVMs with a maximum diameter smaller than 2.5 cm and not amenable to re-embolization underwent stereotactic radiosurgery. The optimal time between prior embolization and radiosurgery is not yet known, but generally waiting for a period of several weeks is considered beneficial. At our institution, if the likelihood of vascular ischemic complications or residual cerebral edema associated with embolization is low in patients with ruptured AVMs, early radiosurgery are recommended.
Onyx is a new embolic material that has been gradually adopted for AVM embolization during the past decade, and some authors have already reported their initial experiences in the treatment of AVMs with Onyx 12-14. As mentioned in these articles, Onyx is a permanent, non-adhesive polymer, liquid embolic agent, which has shown more successful results in obliterating larger parts of the AVM. The overall initial complete obliteration rate of intracranial AVMs with Onyx is relatively high compared with that of other embolic agents 15,16. The reason for this success lies in the nature of Onyx itself, which allows for longer injections, better control, and deeper nidus penetration creating a solid cast. During AVM embolization, the intranidal progression of Onyx relies on a pressure gradient. First, the Onyx reaches the nidus in its liquid state. Once a small portion of the nidus is occluded and Onyx begins solidify, it tends to flow back along the afferent artery. As this reflux occurs, the arterial access is occluded by solidification of Onyx enclosing the microcatheter. At that point, the resistance to Onyx flow becomes lower within the nidus, as opposed to the resistance around the solidified plug in the afferent artery, and a larger second entry occurs 17.
There several reports of transarterial balloon-assisted techniques providing flow control during Onyx embolization of dural arteriovenous fistulas and cerebral AVMs5,6,18. Inflation of a balloon creates a “plug”, allows for enhanced distal penetration and better control of reflux. However, current cerebrovascular balloons do not usually have enough flexibility for navigation in the tortuous and slim feeding arteries of cerebral AVMs. Inflation may also cause injuries to the vessel wall. In our opinion, CAIT provides a new adjunctive method for the transarterial Onyx–based embolization of complex AVMs. We found that the technique is helpful in the treatment of such cases, and we do not believe this technique has a higher risk of procedure-related complications. As our series shows, we obtained complete angiographic exclusion in 81.8% of cases (18 of 22 patients). No procedural complications occurred due to the coil infusion. This result is better than that in other reports using Onyx in the treatment of brain AVMs. Series of AVMs embolized with curative intent showed results as high as 23.5% 19 to 53.9% 20 of total occlusion, albeit with the onus of higher rates of complications, especially bleeding. However, it may not be directly comparable because our analysis was based on the results of a specific group of patients considered potentially curable by EVT.
According to our experience, CAIT has several advantages. First, the navigability of the microcatheter used for coil infusion is much better than the balloon. Second, the coil can provide enough proximal resistance, which can aid plug formation and achieve better distal penetration, thereby increasing the amount of Onyx injected from one feeding pedicle and reducing procedural time and radiation exposure. In our series, an average of 96.7% (range 85%-100%) estimated size reduction with a mean of 1.4 sessions per patient was achieved. We did not observe hair loss in any of our patients. Third, coil mass produces better reflux control to avoid inadvertent embolization of key branches. As in illustrated case 2, the coil was used to protect the penetrating branches from AchoA. After the procedure, the coil had to be retrieved to keep the branches patent. However, the coil that may become embedded in the Onyx and thus is potentially dangerous. If the reflux reaches the catheter tip, there is a high risk of causing the coil to unravel or pulling the Onyx back. Lastly, the coil can decrease the rapid flow within the fistulous connections, thus minimizing the risk of distal migration of the embolic material into the venous or pulmonary circulation caused by high-flow shunting 21,22. In addition, because the coil mass avoids the formation of a peri-catheter “plug”, the Marathon catheter is unlikely to be trapped by the reflux of Onyx, which allows the procedure to be carried out more safely since there is sufficient time for angiographic checks. We acknowledge that the simplest approach to tackle a retained microcatheter is by using the Sonic (BALT, Montmorency, France), which has a detachable tip intended to remain in place within the Onyx cast following embolization. However, this catheter is not available in our region. In each procedure when CAIT was used, the microcatheter could be retrieved without any difficulty, even when the feeding pedicle represented a tortuous and distal loop.
While useful in these cases, CAIT is not without limitation. For example, navigation of rather large Echelon microcatheters in distal territories may be difficult, because these microcatheters are more rigid and might be too short for the distal AVM territories. In addition, there may still be theoretically increased vessel rupture risk with this technique during the procedure, particularly in a small and tortuous vessel. Additionally, another potential disadvantage is that proximal occlusion may cause Onyx influx into distal indirect invisible collaterals, some of which may be dangerous anastomoses. Although this disadvantage remains theoretical at this early stage, meticulous fluoroscopic monitoring of Onyx deposition and a good understanding of cerebrovascular and the AVM angioarchitecture are critical to minimize the possibility of its occurrence.
Conclusions
The coil-augmented approach to induce flow arrest becomes a valuable adjunct, allows for excellent nidal penetration, and improves the safety of the embolization procedure. Nevertheless, additional large series with long-term follow-up are necessary to determine the efficacy and safety of this novel method.
References
- 1.Richling B, Killer M, Al-Schameri AR, et al. Therapy of brain arteriovenous malformations: multimodality treatment from a balanced standpoint. Neurosurgery. 2006;59(5) Suppl 3:S148–157. doi: 10.1227/01.NEU.0000237408.95785.64. doi: 10.1227/01.NEU.0000237408.95785.64. [DOI] [PubMed] [Google Scholar]
- 2.van Beijnum J, van der Worp HB, Buis DR, et al. Treatment of brain arteriovenous malformations: a systematic review and meta-analysis. JAMA. 2011;306(18):2011–2019. doi: 10.1001/jama.2011.1632. doi: 10.1001/jama.2011.1632. [DOI] [PubMed] [Google Scholar]
- 3.Andreou A, Ioannidis I, Nasis N. Transarterial balloon-assisted glue embolization of high-flow arteriovenous fistulas. Neuroradiology. 2008;50(3):267–272. doi: 10.1007/s00234-007-0322-1. doi: 10.1007/s00234-007-0322-1. [DOI] [PubMed] [Google Scholar]
- 4.Newman CB, Hu YC, McDougall CG, et al. Balloon-assisted Onyx embolization of cerebral single-channel pial arteriovenous fistulas. J Neurosurg Pediatr. 2011;7:637–642. doi: 10.3171/2011.4.PEDS10577. doi: 10.3171/2011.4.PEDS10577. [DOI] [PubMed] [Google Scholar]
- 5.Shi ZS, Loh Y, Duckwiler GR, et al. Balloon-assisted transarterial embolization of intracranial dural arteriovenous fistulas. J Neurosurg. 2009;110(5):921–928. doi: 10.3171/2008.10.JNS08119. doi: 10.3171/2008.10.JNS08119. [DOI] [PubMed] [Google Scholar]
- 6.Orozco LD, Luzardo GD, Buciuc RF. Transarterial balloon assisted Onyx embolization of peri-callosal arteriovenous malformations. J Neurointerv Surg. 2013;5(4):e18. doi: 10.1136/neurintsurg-2012-010388. doi: 10.1136/neurintsurg-2012-010388. [DOI] [PubMed] [Google Scholar]
- 7.Mounayer C, Hammami N, Piotin M, et al. Nidal embolization of brain arteriovenous malformations using Onyx in 94 patients. Am J Neuroradiol. 2007;28(3):518–523. [PMC free article] [PubMed] [Google Scholar]
- 8.van Rooij WJ, Sluzewski M, Beute GN. Brain AVM embolization with Onyx. Am J Neuroradiol. 2007;28(1):172–177. [PMC free article] [PubMed] [Google Scholar]
- 9.Weber W, Kis B, Siekmann R, et al. Endovascular treatment of intracranial arteriovenous malformations with onyx: technical aspects. Am J Neuroradiol. 2007;28(2):371–377. [PMC free article] [PubMed] [Google Scholar]
- 10.Weber W, Kis B, Siekmann R, et al. Preoperative embolization of intracranial arteriovenous malformations with Onyx. Neurosurgery. 2007;61(2):252–254. doi: 10.1227/01.NEU.0000255473.60505.84. doi: 10.1227/01.NEU.0000255473.60505.84. [DOI] [PubMed] [Google Scholar]
- 11.Katsaridis V, Papagiannaki C, Aimar E. Curative embolization of cerebral arteriovenous malformations (AVMs) with Onyx in 101 patients. Neuroradiology. 2008;50(7):589–597. doi: 10.1007/s00234-008-0382-x. doi: 10.1007/s00234-008-0382-x. [DOI] [PubMed] [Google Scholar]
- 12.Taylor CL, Dutton K, Rappard G, et al. Complications of preoperative embolization of cerebral arteriovenous malformations. J Neurosurg. 2004;100(5):810–812. doi: 10.3171/jns.2004.100.5.0810. doi: 10.3171/jns.2004.100.5.0810. [DOI] [PubMed] [Google Scholar]
- 13.Jahan R, Murayama Y, Gobin YP, et al. Embolization of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients. Neurosurgery. 2001;48(5):984–995. doi: 10.1097/00006123-200105000-00003. doi: 10.1097/00006123-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Jayaraman MV, Marcellus ML, Do HM, et al. Neurologic complications of arteriovenous malformation embolization using liquid embolic agents. Am J Neuroradiol. 2008;29(2):242–246. doi: 10.3174/ajnr.A0793. doi: 10.3174/ajnr.A0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velat GJ, Reavey-Cantwell JF, Sistrom C, et al. Comparison of N-butyl cyanoacrylate and onyx for the embolization of intracranial arteriovenous malformations: analysis of fluoroscopy and procedure times. Neurosurgery. 2008;63(1) Suppl 1:ONS 73–78. doi: 10.1227/01.neu.0000335015.83616.12. doi: 10.1227/01.NEU.0000320136.05677.91. [DOI] [PubMed] [Google Scholar]
- 16.Wallace RC, Flom RA, Khayata MH, et al. The safety and effectiveness of brain arteriovenous malformation embolization using acrylic and particles: The experiences of a single institution. Neurosurgery. 1995;37(4):606–618. doi: 10.1227/00006123-199510000-00002. doi: 10.1227/00006123-199510000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Santillan A, Zink W, Knopman J, et al. Balloon-assisted technique for trapped microcatheter retrieval following onyx embolization. A case report. Interv Neuroradiol. 2009;15(4):453–455. doi: 10.1177/159101990901500414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang G, Gao X, Li Z, et al. Endovascular treatment for dural arteriovenous fistula at the foramen magnum: report of five consecutive patients and experience with balloon-augmented transarterial Onyx injection. J Neuroradiol. 2013;40(2):134–139. doi: 10.1016/j.neurad.2012.09.001. doi: 10.1016/j.neurad.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Pierot L, Cognard C, Herbreteau D, et al. Endovascular treatment of brain arteriovenous malformations using a liquid embolic agent: results of a prospective, multicentre study (BRAVO) Eur Radiol. 2013;23(10):2838–2845. doi: 10.1007/s00330-013-2870-6. doi: 10.1007/s00330-013-2870-6. [DOI] [PubMed] [Google Scholar]
- 20.Katsaridis V, Papagiannaki C, Aimar E. Curative embolization of cerebral arteriovenous malformation (AVMs) with Onyx in 101 patients. Neuroradiology. 2008;50(7):589–597. doi: 10.1007/s00234-008-0382-x. doi: 10.1007/s00234-008-0382-x. [DOI] [PubMed] [Google Scholar]
- 21.Kjellin IB, Boechat MI, Viñuela F, et al. Pulmonary emboli following therapeutic embolization of cerebral arteriovenous malformations in children. Pediatr Radiol. 2000;30(4):279–283. doi: 10.1007/s002470050741. doi: 10.1007/s002470050741. [DOI] [PubMed] [Google Scholar]
- 22.Pelz DM, Lownie SP, Fox AJ, et al. Symptomatic pulmonary complications from liquid acrylate embolization of brain arteriovenous malformations. Am J Neuroradiol. 1995;16(1):19–26. [PMC free article] [PubMed] [Google Scholar]