SUMMARY
Because of call frequency overlap and masking interference, the airborne sound channel represents a limited resource for communication in a species-rich cricket community like the tropical rainforest. Here we studied the frequency tuning of an auditory neuron mediating phonotaxis in the rainforest cricket Paroecanthus podagrosus, suffering from strong competition, in comparison with the same homologous neuron in two species of European field crickets, where such competition does not exist. As predicted, the rainforest species exhibited a more selective tuning compared with the European counterparts. The filter reduced background nocturnal noise levels by 26 dB, compared with only 16 and 10 dB in the two European species. We also quantified the performance of the sensory filter under the different filter regimes by examining the representation of the species-specific amplitude modulation of the male calling song, when embedded in background noise. Again, the filter of the rainforest cricket performed significantly better in terms of representing this important signal parameter. The neuronal representation of the calling song pattern within receivers was maintained for a wide range of signal-to-noise ratios because of the more sharply tuned sensory system and selective attention mechanisms. Finally, the rainforest cricket also showed an almost perfect match between the filter for sensitivity and the peripheral filter for directional hearing, in contrast to its European counterparts. We discuss the consequences of these adaptations for intraspecific acoustic communication and reproductive isolation between species.
Keywords: acoustic communication, cricket, competition, matched filters, directional hearing
INTRODUCTION
The role of competition for limited resources in natural selection has been recognized since the classical work of the Russian ecologist G. F. Gause on different species of Paramecium (Gause, 1934). Ecologists soon recognized that competition for resources between species is an important factor affecting the abundance and distribution of species (Connell, 1983). Competition for a communication channel has, however, only rarely been considered in this context [but see Greenfield (Greenfield, 1983) and Greenfield and Karandinos (Greenfield and Karandinos, 1979) for chemical communication, and Nelson and Marler (Nelson and Marler, 1990) for a discussion of birdsong signal space], although it should be evident that as the number of species using the same channel in an ecosystem increases, the chances of successful communication will decrease.
This is also true for the airborne sound channel, as for every additional species that vocalizes at the same time and location, the background noise level increases, the signal-to-noise level decreases and signal detection and/or discrimination is severely impaired. Impressive examples are mixed-species choruses of birds (Catchpole and Slater, 1995), anurans (Narins and Zelick, 1988; Narins, 1992) and insects (Gogala and Riede, 1995; Sueur, 2002). Even when only two species utilize spectrally similar signals, this can result in complete suppression of calling activity of one species by the other, or a shift in the diurnal calling activity of one species (Schwarz and Wells, 1983; Greenfield, 1988; Römer et al., 1989; Wong et al., 2009). To reduce acoustic competition and the resulting masking interference, species employ a wide variety of behavioral adaptations, including temporal and spatial partitioning of habitats, antiphonal signaling (alternation) and a switch to alternative mate-acquisition tactics (Gerhardt and Huber, 2002).
Habitats differ strongly in species richness, and the degree of acoustic competition and background noise varies accordingly. Tropical rainforests are among those habitats with highest species diversity (Erwin, 1982). Acoustic noise measurements in a neotropical rainforest of Panama at night have demonstrated sound pressure levels (SPLs) as high as 70 dB (Lang et al., 2005), and spectral analysis of sounds from distinct rainforest sites revealed that a great proportion is due to the signaling activity of insects (Ellinger and Hödl, 2003; Lang et al., 2005; Diwakar and Balakrishnan, 2006). Of these, the calling activity of crickets constitutes the main audio frequency band between approximately 2 and 9 kHz where most acoustic energy is concentrated, thus indirectly confirming the high species richness of crickets in tropical rainforests (Riede, 1993). These studies, together with our own preliminary measurements (see below), indicate that calling song carrier frequencies of sympatric species sometimes do not differ by more than 200 Hz. Current models of peripheral and central nervous processing in crickets cannot explain how signal detection, discrimination and localization is achieved under these masking noise conditions. However, the fact that so many species still communicate under these conditions means that they can deal with these environmental conditions to an extent that the use of acoustic signals in fitness-related tasks is still a likely evolutionary outcome of natural selection (Brumm and Slabberkoorn, 2005). We consider a sharpening of peripheral filters as an important sensory adaptation to improve signal-to-noise (S:N) ratio in a complex acoustic environment. Evidence for such a scenario comes from work on the dendrobatid frog Allobates femoralis, where the occurrence of only one additional frog species, calling in an overlapping frequency range, significantly predicted narrower and asymmetric frequency response curves for phonotactic behaviour (Amézquita et al., 2006).
Crickets are ideally suited for this study for several reasons. Phonotaxis of females towards the conspecific male calling song is the first step in mate choice (Gerhardt and Huber, 2002). Usually, the calling song is a rather pure tone, with higher harmonics at lower intensity. The calling song is produced with a species-specific temporal pattern (reviewed in Pollack, 1998). Carrier frequencies vary between different cricket species from approximately 1.5 kHz in tree and mole crickets up to approximately 9 kHz in scaly crickets (subfamily Mogoplistinae) (Bennet-Clark, 1998). Consequently, in habitats with a high species diversity of crickets, one would expect to find this range of frequencies occupied. This has been confirmed in many tropical rainforests (Ellinger and Hödl, 2003; Lang et al., 2005; Diwakar and Balakrishnan, 2006).
As a result of environmental selection on the acoustic communication channel, one would expect to find a correlation between the sound spectrum produced by the sender and the tuning properties of receivers (Endler, 1992; Ryan and Keddy-Hector, 1992; Meyer and Elsner, 1997), but few studies have considered both background noise spectra and receiver tuning (Römer and Bailey, 1998; Witte et al., 2005; Amézquita et al., 2006). The sensory adaptation we consider is a narrowing of the tuning of the ear around the species-specific calling frequency. Thus, any sound outside the sensitivity range of the filter will play a reduced role in masking of the signals, depending on the sharpness of the tuning (the matched filter hypothesis) (Capranica and Moffat, 1983; Wehner, 1989). This was already demonstrated in Drosophila montana, where noise outside the frequency band of the courtship song has no effect on song detection and recognition by females, whereas with inband noise this was severely impaired below S:N ratios of −6 dB (Samarra et al., 2009).
However, there is a second important step in phonotaxis, consisting of the localization of the sender. In crickets, the evolution of adaptations in a sensitivity filter is constrained by the biophysical basis of sound localization. Different from humans or larger mammals, who can exploit interaural time and intensity differences (IIDs), the latter caused by diffraction of sound around the head, crickets cannot rely on these mechanisms when using frequencies with larger wavelengths because of their small size. Instead, the necessary IIDs result from a pressure difference receiver with a functional three-input system for the sound through the tympanum and two tracheal openings (Hill and Boyan, 1976; Wendler and Löhe, 1993; Michelsen and Löhe, 1995; Michelsen, 1998). This mechanism is frequency dependent, resulting in a sharply tuned directionality, so that in the receiver a second ‘matched filter’ exists for directional hearing. By examining both matched filters in the same individuals, we have shown that in three of four species of crickets from temperate and subtropical regions examined the best frequencies of these two filters are poorly matched (Kostarakos et al., 2009). Only in one species, the Australian Teleogryllus commodus, which occurs sympatrically with T. oceanicus and uses a neighboring carrier frequency in the calling song (4.0 and 4.8 kHz), was the match between both filters high. Thus it appears that competition for carrier frequency may also result in an adaptive process that enables to match two filters which are important for the task to detect and to localize mating signals. For the rainforest cricket species examined here we would thus expect, in addition to the increased selectivity in the sensitivity filter, a better match between the two filters compared with European species without acoustic competition.
MATERIALS AND METHODS
Study area and animals
The present study was conducted on Barro Colorado Island (9°10′N, 79°51′W, Panama) during the dry season from January to April 2009 and additional sound recordings were collected from May to June 2010. Adult crickets (Eneopterinae: Paroecanthus podagrosus Saussure 1897) of either sex were exclusively collected on the island. Animals were kept in small plastic boxes and used within a maximum of 3 days for neurophysiological experiments.
Taxonomy
Paroecanthus podagrosus was well described and illustrated by Saussure (Saussure et al., 1897). The species was also described by Chopard (Chopard, 1912) as P. vicinus from French Guyana but synonymised subsequently by the same author [see p. 250 in Chopard (Chopard, 1931)]. His re-description and picture coincide well with our specimens at hand, indicating a much wider distribution than previously thought. It should be noted, however, that the genus Paroecanthus seems to be much more species rich, as shown by recent descriptions of six new species from Costa Rica (Otte, 2006). This shows that the taxonomy of the genus is difficult and still in flux. Therefore, voucher specimens for eventual taxonomic re-examination have been deposited at the Museum Koenig, Bonn, Germany.
Sound recordings
Calling songs of 29 males were recorded in the laboratory at an ambient temperature of 24°C. Songs were recorded from isolated males with an automated setup using electret microphones (frequency range 50–16,000 Hz; LMLM-09, Hama, Monheim, Germany) placed near the animal and a PowerLab for digitalization (sampling rate 40 kHz; series 4/25, ADInstruments, Sydney, Australia). Sound analysis was performed using audio software CoolEdit Pro 2.0 (Adobe Systems, San Jose, CA, USA) and graphic representation of spectrogram and waveforms in Fig. 1 were carried out using the Seewave package in R (Sueur et al., 2008).
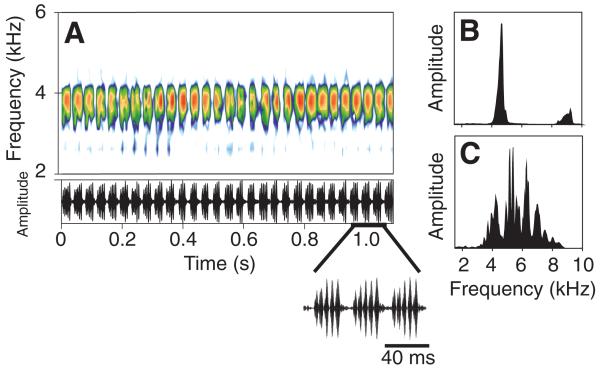
Fig. 1.
Calling song characteristics of Paroecanthus podagrosus. (A) Sonogram (top) and oscillogram (bottom); (B) spectrogram of the field recording of the calling song of Gryllus campestris; and (C) spectrogram of a recording of background noise in the nocturnal rainforest of Panama. Note the different maxima in the background noise due to the carrier frequencies of calling songs of different cricket species.
Outdoor recordings of the acoustic background were taken at nine different locations, including primary and secondary forest, starting at 19:00 h local time until approximately midnight. Recordings were made using a Telinga microphone (Pro7W, Tobo, Sweden) and digitized with a PDM670 Marantz recorder (D&M Holdings Inc., Kanagawa, Japan) at a sampling rate of 44.1 kHz.
Neurophysiology
To examine auditory filter properties, we used extracellularly recorded responses of ascending interneuron 1 (AN1), as its neuronal activity encodes behaviorally relevant information about the male calling song and is essential for positive phonotaxis (Schildberger and Hörner, 1988). In addition, summed action potential (AP) activity of auditory receptor fibers in the leg nerve were used to test the hypothesis that an increased sharpness in frequency tuning is either of peripheral or central nervous origin. The method for recording AP activity of the AN1 neurons with electrochemically sharpened tungsten hook electrodes has been described in detail by Stabel et al. (Stabel et al., 1989) and Kostarakos et al. (Kostarakos et al., 2008). In addition, we recorded the responses of auditory receptor fibers, which run through the front leg nerve into the prothoracic ganglion. For this preparation, the ganglion and the adjoining part of the nerve were exposed up to the coxa, and the nerve was hooked onto electrolytically sharpened hook electrodes. After cutting the nerve near the entrance at the prothoracic ganglion, the preparation was sealed with Vaseline® to prevent desiccation.
AP activity was amplified using a custom-made amplifier (Topview Electronic, Weiz, Austria) and digitized at a sampling rate of 20 kHz (PowerLab 4/25, ADInstruments) for online visualization. Additionally, neuronal activity was monitored through headphones. The response threshold for each stimulus was defined as the SPL that elicited at least one AP in each syllable in at least three out of five stimulations. All experiments were carried out in an acoustically isolated Faraday cage where the background noise level was below 28 dB SPL at a frequency range from 2 to 10 kHz. Ambient temperature during neurophysiological recordings was 24–25°C.
Stimuli used to determine the threshold responses of AN1 and receptor fibers consisted of a standard temporal pattern of four consecutive pulses (duration 23 ms, pulse interval 16 ms), which were followed by a silent interval of 750 ms before the stimulus was repeated. The carrier frequency was varied from 2.3 to 5.5 kHz in steps of 0.1 kHz. Stimuli were generated using CoolEdit Pro 2.0, broadcast using an external audio interface (Edirol FA-101, Roland, Hamamatsu, Japan), amplified (stereo power amplifier SA1, Tucker-Davis Technologies, Alachua, FL, USA) and attenuated (programmable attenuator PA5, Tucker-Davis Technologies) in steps of 1 dB. The tuning was measured with ipsilateral stimulation, with the speaker (FF1, Tucker-Davis Technologies, flat frequency response from 1 to 50 kHz) placed ipsilaterally at 90 deg off the longitudinal body axis at a distance of 25 cm (acoustic free field). In order to measure only the peripheral directionality provided by the anatomical arrangement of the acoustic tracheae in the periphery, inhibitory central nervous interactions were eliminated by cutting the contralateral leg nerve. IIDs were calculated by measuring the thresholds of AN1 for all carrier frequencies with ipsilateral and contralateral stimulation at an angular position of 90 deg. The threshold differences between ipsilateral and contralateral stimulation represent the IID for a given frequency. Because the opening status of the prothoracic spiracle is important for the peripheral directionality (Michelsen, 1998), in the neurophysiological experiments presented here this status was controlled carefully and kept partially open, as observed in previous behavioral trials with Gryllus bimaculatus females (Kostarakos et al., 2009). For comparison, the filter data for the Gryllus species (see below) were taken from Kostarakos et al. (Kostarakos et al., 2008; Kostarakos et al., 2009); ambient temperature in these experiments varied between 21 and 23°C.
The strong detrimental effect of background noise for insect communication is caused by the masking of the species-specific amplitude modulation. We therefore evaluated the effect of the filter in P. podagrosus relative to those of the two European cricket species, G. bimaculatus and G. campestris, in the task of preserving this amplitude modulation in background noise. For this purpose we used their standardized AN1 tuning curves to create species-specific audio filters with the software CoolEdit. The best frequency of all filters was set to 3.8 kHz, which is the mean carrier frequency of male calling songs. Outdoor sound recordings of background noise from nine different locations were selected based on their maximum power in the frequency range between 2 and 9 kHz. Samples of 30 s were then digitally mixed with a 30 s sound recording of P. podagrosus recorded under laboratory conditions, so that the root mean square (r.m.s.) amplitude (integration time 50 ms) of the background noise and the P. podagrosus song was the same. These mixed recordings were then filtered using one of the filter functions obtained from the three cricket species. The filtered sound recordings were analyzed with respect to the similarity in the amplitude modulation of the filtered sound relative to the amplitude modulation of the unmasked calling song. This was done by calculating the composed sound envelopes (Hilbert transformation) and by computation of the correlation coefficients between the filtered signal plus noise envelopes relative to the isolated signal, using built-in functions in MATLAB (R2008b, The MathWorks Inc., Natick, MA, USA).
In a second approach, we used the same filter functions obtained from the three cricket species in the CoolEdit software to filter the same background noise recordings without the P. podagrosus signal to examine the possible adaptive effect of the sharpness of a sensory filter. The decrease in the r.m.s. amplitude of the background noise after filtering is a quantitative measure of the ability of the filter to reduce the masking effect of background noise for a receiver.
Finally, we investigated the effect of the increased sharpness of the auditory filter on signal perception using neurophysiological methods. Preparations with recordings of AN1 AP activity were stimulated with conspecific calling song models of P. podagrosus, and in addition with playbacks of rainforest background noise at various S:N ratios ranging from +3 to −9 dB. Conspecific calling songs and background noise were broadcast through different speakers (Type FF1 magnetic speaker, Tucker-Davis Technologies), both placed at 90 deg ipsilateral. AP activity was amplified using a custom-made amplifier (Topview Electronic, Weiz, Austria) and digitized at a sampling rate of 40 kHz (PowerLab 4/25, ADInstruments) for offline analysis.
Mean values are presented ±s.d. unless otherwise indicated.
RESULTS
The calling song of male P. podagrosus consists of repetitive series of chirps, each composed of four to six pulses with a mean carrier frequency of 3.8±0.2 kHz and a pulse rate of 205±17 Hz (Fig. 1A). The total duration of the chirp series was highly variable, lasting from a few seconds up to 2 min. The perception of the song is complicated by other cricket species calling at the same time and location in the nocturnal tropical rainforest. In the spectral analysis of such background noise (Fig. 1C), the multiple peaks in the spectrum indicate the different carrier frequencies occupied by these sympatric species. By contrast, the task of detecting the calling song of a conspecific male in a European field cricket (G. campestis) is rather easy because there is no acoustic energy within the relevant frequency range other than that of its calling song (Fig. 1B).
Sensitivity tuning
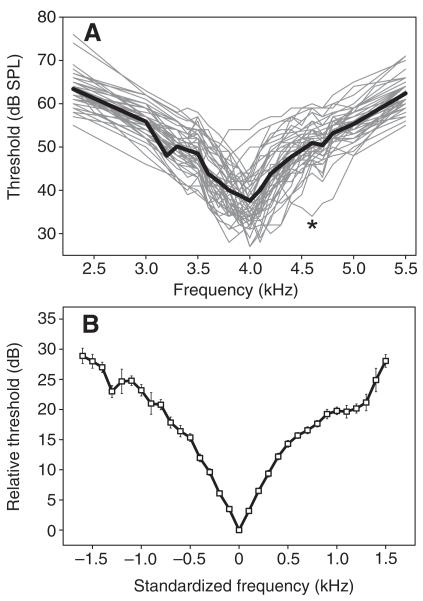
We determined the frequency tuning of AN1 in 47 male and female P. podagrosus crickets (Fig. 2A). Individual tuning curves varied in their best frequency (frequency of lowest threshold) from 3.4 to 4.2 kHz, with one outlier at 4.6 kHz. The mean sensitivity at the best frequency was 34.3±4.0 dB SPL, with the lowest thresholds ranging between 27 and 42 dB SPL. The mean tuning curve calculated from all 47 preparations is given in Fig. 2A as the bold line, indicating a rather symmetrical roll-off (~20 dB/2 kHz) towards higher and lower frequencies. However, because of the interindividual variability of the receivers’ best frequency and their absolute sensitivity, the mean tuning curve underestimates the frequency selectivity of individual P. podagrosus receivers. Therefore, we standardized the tuning by defining the best frequency as 0 kHz and arranging higher thresholds on both sides of the frequency axis relative to this standard (Fig. 2B). This analysis reveals a remarkable selective frequency tuning for P. podagrosus, with a steep roll-off (23 dB/1 kHz and 20 dB/1 kHz) to lower and higher frequencies, respectively. The mean of individual best frequencies was 3.9 kHz. As a quantitative value for the sharpness of frequency tuning we calculated the width of the tuning curve 5 dB above threshold at the best frequency. This value was 366±115 Hz on average.
Fig. 2.
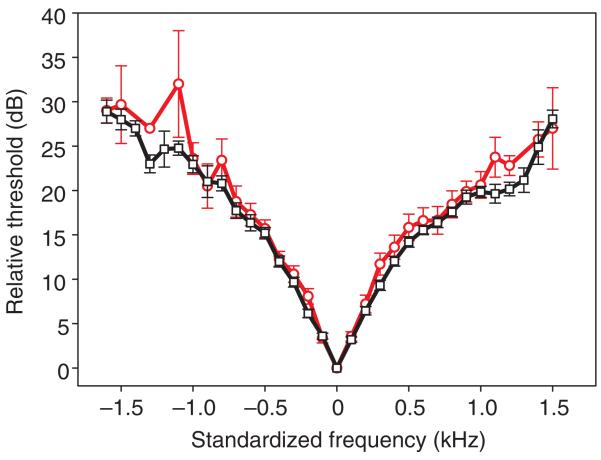
(A) Sensitivity tuning of ascending neuron 1 (AN1) in P. podagrosus (N=47). The mean tuning curve, representing the population mean sensitivity of receivers, is shown as the bold line. The asterisk indicates an outlier at a best frequency of 4.6 kHz. (B) Standardized mean (±s.e.m.) tuning of AN1. The best frequency in each individual was set at 0 Hz, and the higher thresholds to lower and higher frequencies were averaged accordingly.
Directional tuning
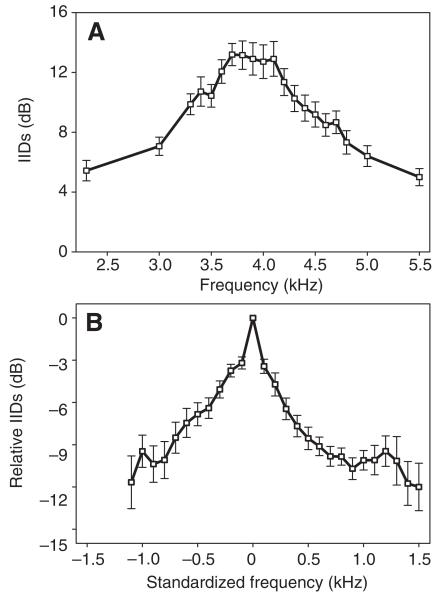
In a total of 32 crickets we managed to determine both the frequency tuning of AN1 and the peripheral directionality in the same individuals. The mean directional selectivity of these preparations is shown in Fig. 3A, demonstrating a second directional tuning with the highest values of IIDs at frequencies between 3.8 and 4.1 kHz. Individual maximum IID values varied, ranging from 11 to 26 dB with a mean of 17.1±4.4 dB. Again, inter-individual variation in the amount of IIDs and the best frequency of directional tuning underestimates the sharpness of directional tuning in the averaged curve of Fig. 3A. We therefore standardized the directional tuning as well by setting the frequency with the highest IID to zero and arranging the lower and higher frequency values accordingly. The standardized directional tuning is shown in Fig. 3B, demonstrating that peripheral directionality is also sharply tuned to one particular frequency, and that a deviation from this frequency by only 500 Hz to either side would decrease the directionality of the system by approximately 7 to 9 dB.
Fig. 3.
(A) Mean directional tuning in P. podagrosus, with the highest values of interaural intensity differences (IIDs) at frequencies between 3.7 and 4.1 kHz (N=32). For further explanation see Results, Directional tuning. (B) Standardized mean directional tuning. The optimum frequency in each individual was set at 0 Hz, and the IIDs to lower and higher frequencies were averaged accordingly. Means are presented ±s.e.m.
Match of both tuned filters
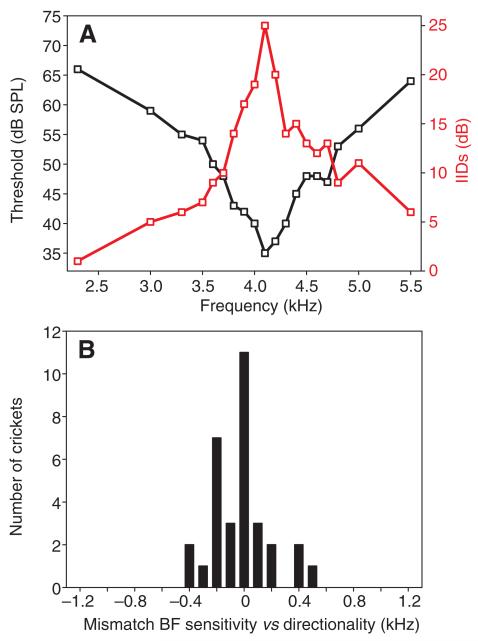
We have shown previously (Kostarakos et al., 2008; Kostarakos et al., 2009) that, in three of four species of field cricket investigated, a considerable mismatch exists between the best frequency of sensitivity and directionality in individual receivers (e.g. mean mismatch for T. oceanicus 645±370 Hz; maximum mismatch 1.2 kHz). An increased selectivity in frequency tuning would be of little value if the best frequency of directional hearing is strongly mismatched to the best frequency of sensitivity, under conditions of high competition in the acoustic communication channel. We therefore analyzed the match of the tuned filters in all crickets (N=32) where both had been established. Fig. 4A shows an example of a perfect match, i.e. the best frequency of sensitivity and directionality was the same; this was found for one-third of all crickets. However, even in the remaining individuals the mismatch was rather small (Fig. 4B). In 81% of all individuals, the maximum mismatch was only ±200 Hz (mean mismatch ±150 Hz). Thus there was no statistical difference between the best frequencies of the optima (paired t-test, t=−1.065, P=0.295, N=32).
Fig. 4.
Analysis of the match in the best frequencies of the filters for sensitivity and directionality in P. podagrosus. (A) Comparison of both filters (black, sensitivity; red, directionality) in one P. podagrosus individual. Note the perfect match of the best frequency in both filters. (B) Quantification of the mismatch between the best frequency of the two filters in P. podagrosus. Negative values indicate that the best frequency in directionality was below the best frequency in sensitivity (N=32).
Origin of increased frequency selectivity
Because we observed an increased selectivity in AN1 tuning in P. podagrosus compared with species of field crickets with little acoustic competition, the mechanism for this selectivity could be either of peripheral or central nervous origin. We therefore compared the frequency tuning of afferent receptor fibers with the tuning of AN1 in 15 individuals (Fig. 5). The standardized averaged tuning curves are almost identical within the complete frequency range, indicating that the increased frequency selectivity is not caused by central nervous processes; it is already present at the level of the receptors, and AN1 collects receptor information without further affecting the frequency range.
Fig. 5.
Comparison of the standardized mean (±s.e.m.) sensitivity tuning of AN1 (black squares) with the tuning of auditory receptors recorded in the foreleg of the same P. podagrosus individuals (N=15, red circles). For each tuning curve, the best frequency in each individual was set at 0 Hz, and the higher thresholds to lower and higher frequencies were averaged accordingly.
The adaptive significance of the filter in P. podagrosus for hearing in noise
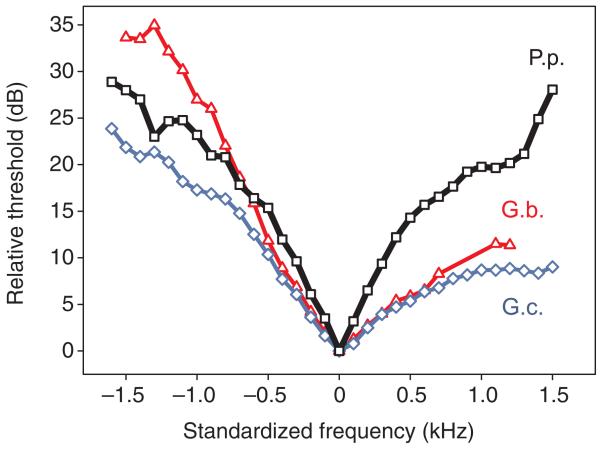
A comparison of the standardized tuning curve of AN1 in P. podagrosus with the same, homologous neuron in the two European field cricket species without acoustic competition is shown in Fig. 6 [data from Kostarakos et al. (Kostarakos et al., 2009)]. The increased steepness of the P. podagrosus tuning curve is particularly pronounced towards high frequencies. To quantify the selectivity of tuning we calculated the mean width of the tuning curve 5 dB above the threshold at the best frequency. These values were 366±115 Hz for P. podagrosus, 757±262 Hz for G. campestris and 660±291 Hz for G. bimaculatus. In addition, we normalized the respective filters to the best frequency (width at 5 dB above threshold/best frequency), so that filters can be compared across taxa. These values were 0.093±0.03 for P. podagrosus, 0.16±0.05 for G. campestris and 0.14±0.06 for G. bimaculatus.
Fig. 6.
Comparison of the standardized mean sensitivity tuning of AN1 in P. podagrosus (P.p.) with the tuning of the same homologous neuron in two species of field crickets G. bimaculatus (G.b.) and G. campestris (G.c.) where acoustic competition in neighbouring frequency bands does not exist. Data for the Gryllus species are taken from Kostarakos et al. (Kostarakos et al., 2009). These tuning curves are implemented as filter functions in Audio software to document their filter performance in background noise. For further explanations see text.
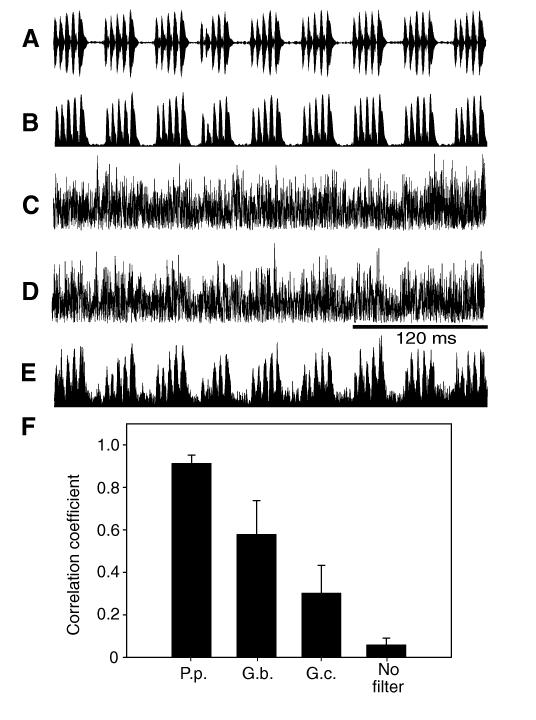
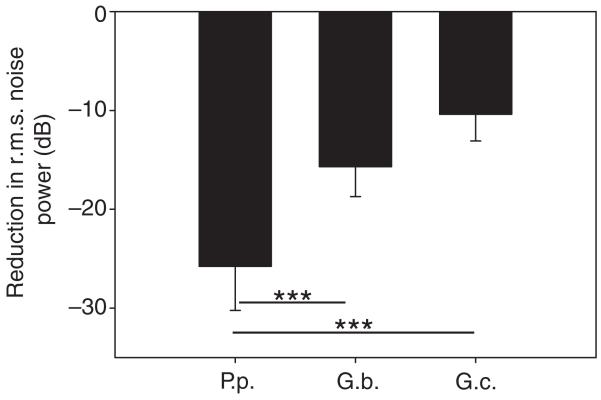
The performance of these sensitivity filters in P. podagrosus and the two Gryllus species for detecting the conspecific song in the presence of background noise was analyzed in two ways. First, we quantified the degree to which the amplitude modulation of the P. podagrosus song is represented in the receiver in the presence of noise, as this is the information most important for species recognition. Fig. 7C demonstrates that the amplitude modulation of the song is completely masked by background noise if no filter is used. Correlating the amplitude modulation (AM) of the signal plus unfiltered noise with the AM of the signal results in a correlation of less than 0.1 (Fig. 7F). However, after filtering with the AN1 filter of P. podagrosus (Fig. 7E) there is a clear AM pattern detectable in the sound signal despite the background noise, which is also expressed in the high value of the correlation coefficient of 0.91. Remarkably, the performance of the filters in the two European cricket species G. bimaculatus and G. campestris is significantly reduced compared with that of P. podagrosus, yielding correlation coefficients of only 0.58 (Mann–Whitney U-test; U=80, P=<0.001, N=9) and 0.30 (Mann–Whitney U-test; U=81, P=<0.001, N=9; see also the sound recording in Fig. 7D for the filter performance in G. campestris), respectively. For the second analysis, we filtered the same background noise recordings used above, but without the digitally mixed P. podagrosus signal, and examined the decrease in r.m.s. amplitude of the background noise after filtering. These results are summarized in Fig. 8. Compared with the unfiltered situation, the P. podagrosus filter reduced the r.m.s. power in the background on average by 25.8 dB, a reduction which is significantly higher compared with the 15.7 and 10.4 dB reductions resulting from the filters in G. bimaculatus (t-test; t=−5.605, P<0.001, N=9) and G. campestris (t-test; t=−8.850, P<0.001, N=9), respectively.
Fig. 7.
The effect of the different filter functions in detecting the specific amplitude modulation (AM) of the P. podagrosus calling song embedded in background noise. (A) Oscillogram and (B) AM of P. podagrosus calling song. (C) AM of P. podagrosus calling song embedded in background noise without any filtering, (D) with the filter of G. campestris and (E) with the filter of P. podagrosus. Note the increase in the quality of representation of the AM of conspecific song by using the more selective filter. (F) Correlation of the AM of P. podagrosus calling song with the AM of the same calling song embedded in background noise (N=9) under the filter regimes of the three cricket species, and without any filter.
Fig. 8.
Mean (±s.d.) change in r.m.s. power in the noise spectra from nine different locations and times, after filtering with the sensitivity filters of P. podagrosus, G. bimaculatus and G. campestris.
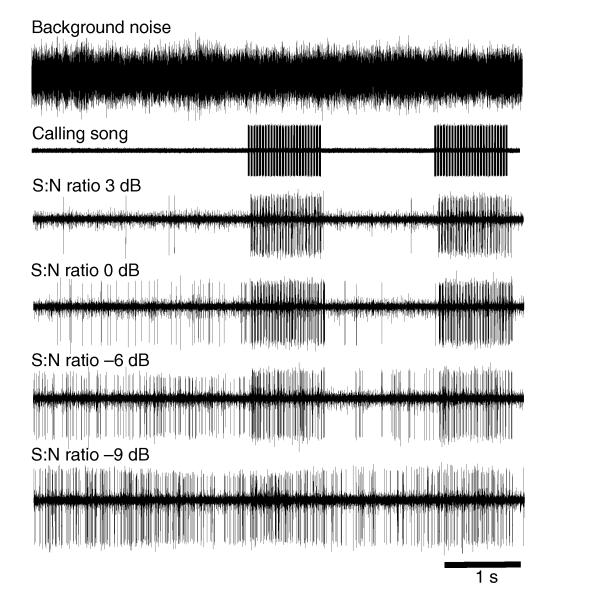
The performance of the P. podagrosus AN1 filter in reducing the amount of background noise shown above should result in a clear neuronal representation of the conspecific signal even under unfavorable S:N conditions. We tested this prediction by recording AN1 activity in response to conspecific calling song under various S:N ratios. Fig. 9 shows one example for such an experiment, for S:N ratios from 3 to −9 dB. Clearly, in the noise-only situation before the conspecific stimulus started, the background noise at an S:N ratio of 3 dB elicited almost no activity at all, in contrast to the signal, which was only 3 dB higher compared to the background. At lower S:N ratios of 0 and −6 dB, the background noise induced an increase in AN1 activity, but the temporal pattern of the calling song was still preserved in the firing pattern. Notably, during the time between the two series of calling songs, the response to the background noise alone was strongly reduced compared with the time before the song started. Almost complete masking occurred in this preparation at an S:N ratio of −9 dB.
Fig. 9.
Performance of the P. podagrosus AN1 filter for the representation of conspecific signals in background noise. One example of AN1 activity in response to conspecific calling song and background noise is shown under various signal-to-noise (S:N) ratios from 3 to −9 dB. Note the lack of activity in the noise-only situation before the conspecific stimulus started (S:N ratio 3 dB) and the increasing activity with decreasing S:N ratios of 0 and −6 dB. Yet, the temporal pattern of the calling song is still preserved in the firing pattern. Note also the suppression of activity during the time in between the two series of calling songs, at the same S:N ratios. Complete masking occurs at an S:N ratio of −9 dB.
DISCUSSION
Detecting the calling songs of mates is severely impaired for female cricket receivers in the nocturnal rainforest because of the simultaneous calling activity of other cricket species within a narrow range of frequencies between 2 and 9 kHz. The sonic background noise is dominated by this frequency band, and SPLs of up to 70 dB have been reported, varying with the lunar cycle (Lang et al., 2005). This is different to field crickets in temperate habitats, where such competition for carrier frequencies does not exist (Fig. 1). We therefore expected to find not only a correlation between the sound spectrum produced by the sender and the tuning properties of receivers (Endler, 1992; Ryan and Keddy-Hector, 1992; Meyer and Elsner, 1997), but also specific adaptations as a result of background noise.
Our results show a significant change in auditory tuning properties of the same homologous AN1 in the rainforest cricket P. podagrosus compared with their European counterparts, and we have argued that this is the result of an evolutionary adaptation due to high background noise levels. However, we cannot exclude the possibility that the differences between P. podagrosus and the two European species are the outcome of phylogenetic constraints. Although so far we do not have comparative data from other cricket species with the same statistical power as for P. podagrosus, we obtained neurophysiological data of three additional species in three different genera of the subfamilies Eneopterinae and Podoscirtinae in Panama. Following the methodology and analysis for P. podagrosus, we determined the tuning curves of the same homologous AN1 and calculated the width of tuning 5 dB above threshold for three individuals of each species. Values ranged from 180 to 280 Hz for Amblyrethus sp. (Eneopterinae), from 360 to 460 Hz for Diatrypa sp. (Eneopterinae) and from 220 to 680 Hz for Aphonomorphus sp. (Podoscirtinae), and are thus mostly well below those of the European cricket species (Gryllinae), indicating a general tendency for narrow tuning, which might be a characteristic feature at least of these tropical subfamilies. Clearly, more comparative data from more cricket species and taxa are needed, or data from Paroecanthus populations suffering from different levels of background noise, to finally confirm the adaptive hypothesis of selective tuning. The same argument also holds for the extent of the mismatch in tuning between sensitivity and directionality (see below).
Alternatively, the broader tuning in the European species could be maintained by selection due to temperature variation during signaling times, if song carrier frequency varies with ambient temperature (Walker, 1962). Such temperature effects on song carrier frequency occur in a few cricket species but have not been observed in G. bimaculatus (Doherty, 1985) or G. campestris (Kutsch, 1969).
The increased selectivity of the established filter is adaptive in two ways. First, the rejection of sound outside the sensitivity range of the filter reduces the amount of background noise relevant for masking of the calling song by 26 dB, a reduction which is significantly higher than the corresponding filters in the two European field cricket species (Fig. 8). The P. podagrosus filter also performs much better compared with one found in the cricket frog Acris crepitans, which reduces habitat noise by 16.5–18.5 dB (Witte et al., 2005). Second, by limiting the perception to those frequencies inside the more selective filter, it strongly enhances the perception of the signal parameter most relevant for discrimination between conspecific and heterospecific signals, i.e. its AM. Whereas in the masked signal without filtering the AM is not detectable at all, the performance of the P. podagrosus filter was excellent, yielding mean correlation coefficients of 0.91 (Fig. 7). Because this is only an acoustic measure of the signal in noise, and does not indicate how well the signal is represented within the afferent sensory system, we analyzed this representation using AN1 activity under various S:N ratios. Our neurophysiological results confirmed that a receiver will be able to perceive the conspecific AM for a wide range of S:N ratios (Fig. 9). The observed threshold S:N ratio at which the signal suffers complete masking is between −6 and −9 dB, and thus rather similar to the one found in Drosophila behavior (Samarra et al., 2009). Two proximate mechanisms appear to contribute to this representation: apart from the enhanced filter function, the selective attention mechanism first reported for crickets (Pollack, 1988) and homologous neurons in katydids (Römer and Krusch, 2000) further reduces the activity in AN1 as a result of background noise. This mechanism is based on a membrane hyperpolarization with a slow time constant in the order of 5 s, which renders most of the excitatory synaptic activity of a neuron subthreshold. Although we did not perform intracellular recordings of AN1 activity, the suppression of AP activity is evident after series of stimulation with conspecific signals (Fig. 9; S:N ratios of 0 and −6 dB). We must emphasize, however, that such a mechanism can be effective in species such as P. podagrosus and others with high signal duty cycles, but would be maladaptive in species with short, low redundant signals, because it would suppress the representation of conspecific signals and favor background noise. This leaves an interesting field for future comparative studies on a range of cricket species differing in signal duty cycle, with the prediction that selection due to acoustic competition modifies the degree with which the selective attention mechanism is established in crickets.
A comparison of the three filters standardized for their best frequency (Fig. 6) indicates that the increased filter performance of the P. podagrosus AN1 is mainly due to the increased steepness of the slope towards higher frequencies, whereas the slope towards lower frequencies is rather similar. If the filter with its best frequency at 4 kHz has been shaped by natural selection to avoid masking interference, selection should indeed have modified the slope towards higher frequencies, because there is more masking potential in the noise spectrum at higher frequencies compared with frequencies below 4 kHz (see typical noise spectrum in Fig. 1C). A rather similar situation has been reported for the two sympatric cricket species Teleogryllus oceanicus and T. commodus (with calling song frequencies of 4.8 and 4.0 kHz, respectively); the AN1 filter of T. commodus exhibits a steeper slope towards higher frequencies compared with other field crickets, which could aid in separating the two calling songs in receivers (Kostarakos et al., 2009). The strong effect of a single competing species on the formof the frequency response curve in receivers was also documented for populations of the frog Allobates femoralis, which, when compared with A. trivittatus, show more selective and asymmetric tuning (Amezquita et al., 2006). Such adaptations in receivers have not been observed in populations of A. femoralis without the species A. trivittatus competing for neighbouring carrier frequencies.
Of course, in addition to the sensory adaptation described here, other physiological and behavioral adaptations might contribute to acoustic niche segregation. Interference could be avoided by temporal separation of singing males [but see Diwarkar and Balakrishnan (Diwarkar and Balakrishnan, 2006) for a negative result with respect to temporal segregation], or the structural complexity of a tropical forest could allow species to stratify their microhabitats for acoustic communication in the vertical dimension (Diwarkar and Balakrishnan, 2007). Other physiological mechanisms apart from selective attention, which could enhance the detectability of signals in masking noise, are comodulation masking release (Klump et al., 1995; Verhey et al., 2003) and the temporal filter properties of neuronal elements in the auditory pathway (Wohlgemuth and Ronacher, 2007; Weschke and Ronacher, 2008). However, despite all these potential mechanisms it is not surprising that matched selective filtering as demonstrated in our data is probably the most important mechanism as it operates effectively at the very beginning of sensory processing and frees the central nervous system from computational strain (Wehner, 1989; Capranica and Moffat, 1967). Our finding that the increased tuning of AN1 is identical to the tuning of auditory receptors in the ear of P. podagrosus (Fig. 5) indicates that it is not the result of central nervous inhibition (through inhibitory side-bands acting on the excitatory response); rather, it is a property of the periphery, due to either mechanical vibration properties of the tympanum or intrinsic properties of the population of receptors tuned to low frequencies (Pollack, 2000).
The match of the filters for sensitivity and directionality
One of the specific properties of the hearing system in crickets is the way they achieve reasonable directionality via a pressure difference receiver (Hill and Boyan, 1976; Michelsen, 1998; Wendler and Löhe, 1993; Michelsen and Löhe, 1995). The phase delay mechanism for contralateral sound through the system of connecting trachea between both forelegs is inherently and sharply tuned to a particular frequency, so that two frequency filters exist in crickets: one for sensitivity and one for directionality. Surprisingly, when these two filters were examined in the same individual receivers of three species of field crickets, a considerable mismatch was observed in their best frequency [see Kostarakos et al. (Kostarakos et al., 2009) for a discussion of evolutionary trends and constraints explaining the mismatch]. The highly tuned directional filter also exist, in P. podagrosus, providing IIDs of 17 dB at the best frequency, with a decrease of 7–9 dB at frequencies ±500 Hz from the best frequency (Fig. 3).
Given the highly tuned sensitivity filter, it would be strongly maladaptive if a similar mismatch between both filters were to exist, as observed in the European field crickets. Indeed, our results confirm that in most receivers the match is close to perfect (Fig. 4), and on average the deviation of the respective best frequencies is only 150 Hz, which is significantly less compared with the deviation observed in G. campestris and G. bimaculatus (442 and 365 Hz, respectively). Again, normalizing the mean mismatch to the best frequency of the sensitivity filter results in values of 0.004 for P. podagrosus, compared with 0.098 in G. campestris and 0.074 in G. bimaculatus. These data suggest that independent evolution of both filters is possible, and that during evolution the task of signal detection and localization may have been driven by independent constraints (Kostarakos et al., 2009). Interestingly, in the sympatric situation of two cricket species competing for neighboring frequency channels, one species (T. commodus) demonstrated a close to perfect match between sensitivity and directionality filter, like P. podagrosus, whereas T. oceanicus had the strongest mismatch of both filters. Also consistent with the P. podagrosus data is the increased selectivity of the sensitivity filter of T. commodus, in particular at the high frequency side of the tuning, where it should select against the calling song frequency of 4.8 kHz of the competing species (Kostarakos et al., 2009). Thus it appears that, under strong selection for carrier frequencies on the transmission channel by either one or several competing species, the match between two independent traits is possible.
Does selection for increased tuning reduce the potential for sexual selection on calling song carrier frequency?
As a result of sharper tuning of receivers, strong selection would act on signalers to call exactly at the carrier frequency to which receivers are tuned, because if they do not follow the female bias in tuning they would be unable to adequately stimulate the females’ hearing system. A previous study on G. bimaculatus has demonstrated a strong preference of females for the best frequency in AN1-tuning in two-choice trials, and male carrier frequency has been shown to vary in a population by approximately 1 kHz (Kostarakos et al., 2008). Female P. podagrosus vary only little with respect to the best frequency in sensitivity (Fig. 2A), so given this bias in female selectivity, we would expect a reduction in variance of the calling song carrier frequency compared with those species where the selectivity in tuning is reduced. Ongoing studies on the whole assemblage of nocturnal rainforest crickets are trying to establish this tendency based on a multi-species comparisons with European species without song interference, and preliminary data indicate that, for the majority of cricket species, the variance in carrier frequency of the male calling song is reduced compared with the two European cricket species.
A reduced variance in this important song trait will in turn reduce the potential for female preference for the trait. To compensate for this loss of information in one signal parameter for mate choice, females could rely more on other parameters such as call rate rather than carrier frequency, or even other song types such as courtship song.
ACKNOWLEDGEMENTS
We are grateful to the Smithsonian Tropical Research Institute (STRI) and the National Authority for the Environment (ANAM) for logistical support, research and export permits SEX/A-115-08 and SEX/A-143-08, which ensured that all work was conducted in compliance with current Panamanian laws. We also thank Anne Einhäupl and Stefan Hirtenlehner for their work as field assistants on the project, which was supported by a grant from the Austrian Science Foundation (project P20882-B09 to H.R.), and two anonymous referees for valuable comments.
REFERENCES
- Amézquita A, Hödl W, Lima AP, Castellanos L, Erdtmann L, de Araulo MC. Masking interference and the evolution of the acoustic communication system in the Amazonian dendrobatid frog Allobates femoralis. Evolution. 2006;60:1874–1887. [PubMed] [Google Scholar]
- Bennet-Clark HC. Size and scale effects as constraints in insect sound communication. Philos. Trans. R. Soc. Lond. B. 1998;353:407–419. [Google Scholar]
- Brumm H, Slabberkoorn H. Acoustic communication in noise. In: Slater PJB, Snowdon CT, Roper TJ, Brockmann HJ, Naguib M, editors. Advances in the Study of Behavior. Vol. 35. Academic Press; San Diego, CA: 2005. pp. 151–209. [Google Scholar]
- Capranica RR, Moffat AJM. Neurobehavioral correlates of sound communication in anurans. In: Ewert JP, Capranica RR, Ingle D, editors. Advances in Vertebrate Neuroethology. Plenum; New York: 1983. pp. 701–730. [Google Scholar]
- Catchpole CK, Slater PJB. Bird Song: Biological Themes and Variations. Cambridge University Press; Cambridge: 1995. [Google Scholar]
- Chopard L. Contribution a l’étude des orthoptères de la Guyane française (2e Memoire: Gryllidae) Ann. Soc. Entomol. France. 1912;81:401–432. [Google Scholar]
- Chopard L. Ergebnisse einer zoologischen Sammelreise nach Brasilien, insbesondere in das Amazonasgebiet, ausgeführt von Dr H. Zerny. VIII. Teil. Orthoptera: Gryllodea. Ann. Naturhistorisch. Mus. Wien. 1931;46243 [Google Scholar]
- Connell JH. On the prevalence and relative importance of interspecific competition: evidence from field experiments. Am. Nat. 1983;122:661–696. [Google Scholar]
- Diwakar S, Balakrishnan R. The assemblage of acoustically communicating crickets of a tropical evergreen forest in southern India: call diversity and diel calling patterns. Bioacoustics Int. J. Anim. Sound Rec. 2006;16:1–23. [Google Scholar]
- Diwakar S, Balakrishnan R. Vertical stratification in an acoustically communicating ensiferan assemblage of a tropical evergreen forest in southern India. J. Trop. Ecol. 2007;23:479–486. [Google Scholar]
- Doherty JA. Temperature coupling and “trade-off” phenomena in the acoustic communication system of the cricket, Gryllus bimaculatus De Geer (Gryllidae) J. Exp. Biol. 1985;114:17–35. [Google Scholar]
- Ellinger N, Hödl W. Habitat acoustics of a neotropical lowland forest. Bioacoustics. 2003;13:297–321. [Google Scholar]
- Endler JA. Signals, signal conditions, and the direction of evolution. Am. Nat. 1992;139:125–153. [Google Scholar]
- Erwin TL. Tropical forests: their richness in Coleoptera and other arthropod species. Coleopt. Bull. 1982;36:74–75. [Google Scholar]
- Gause GF. The Struggle for Existence. Williams and Wilkins Co; Baltimore, MD: 1934. [Google Scholar]
- Gerhardt HC, Huber F. Acoustic Communication in Insects and Anurans. University of Chicago Press; Chicago, IL: 2002. [Google Scholar]
- Gogala M, Riede K. Time sharing of song activity by cicadas in Temengor Forest Reserve, Hulu Perak, and Sabah, Malaysia. Malayan Nat. J. 1995;49:48–54. [Google Scholar]
- Greenfield MD. Reproductive isolation in clearwing moths (Lepidoptera: Sesiidae): a tropical-temperate comparison. Ecology. 1983;64:362–375. [Google Scholar]
- Greenfield MD. Interspecific acoustic interactions among katydids Neoconocephalus: Inhibition-induced shifts in diel activity. Anim. Behav. 1988;36:684–695. [Google Scholar]
- Greenfield MD, Karandinos MG. Resource partitioning of the sex communication channel in clearwing moths (Lepidoptera: Sesiidae) of Wisconsin. Ecol. Monogr. 1979;49:403–426. [Google Scholar]
- Hill KG, Boyan GS. Directional hearing in crickets. Nature. 1976;262:390–391. doi: 10.1038/262390a0. [DOI] [PubMed] [Google Scholar]
- Klump GM, Langemann U. Comodulation masking release in a songbird. Hear. Res. 1995;87:157–164. doi: 10.1016/0378-5955(95)00087-k. [DOI] [PubMed] [Google Scholar]
- Kostarakos K, Hartbauer M, Römer H. Matched filters, mate choice and the evolution of sexually selected traits. PLoS ONE. 3:e3005. doi: 10.1371/journal.pone.0003005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostarakos K, Hennig M, Römer H. Two matched filters and the evolution of mating signals in four species of cricket. Front. Zool. 2009;6:22. doi: 10.1186/1742-9994-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsch W. Neuromuskuläre Aktivität bei verschiedenen Verhaltensweisen yon drei Grillenarten. Z. Vgl. Physiol. 1969;63:335–378. [Google Scholar]
- Lang A, Teppner I, Hartbauer M, Römer H. Predation and noise in communication networks of neotropical katydids. In: McGregor P, editor. Animal Communication Networks. Cambridge University Press; Cambridge: 2005. pp. 152–169. [Google Scholar]
- Meyer J, Elsner N. Can spectral cues contribute to species separation in closely related grasshoppers? J. Comp. Physiol. A. 1997;180:171–180. [Google Scholar]
- Michelsen A. Biophysical basis of sound localization in insects. In: Hoy RR, Popper AN, Fay RR, editors. Comparative Hearing: Insects. Springer; New York: 1998. pp. 18–22. [Google Scholar]
- Michelsen A, Löhe G. Tuned directionality in cricket ears. Nature. 1995;375:639. [Google Scholar]
- Narins PM. Biological constraints on anuran acoustic communication: auditory capabilities of naturally behaving animals. In: Webster DB, Fay RR, Popper AN, editors. The Evolutionary Biology of Hearing. Springer; New York: 1992. pp. 439–454. [Google Scholar]
- Narins PM, Zelick R. The effects of noise on auditory processing and behaviour in amphibians. In: Fritsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W, editors. The Evolution of the Amphibian Auditory System. Wiley; New York: 1988. pp. 511–536. [Google Scholar]
- Nelson DA, Marler P. The perception of birdsong and an ecological concept of signal space. In: Berkley MA, Stebbins WC, editors. Comparative Perception, Vol. 2, Complex Signals. Wiley; New York: 1990. pp. 443–478. [Google Scholar]
- Otte D. Eighty-four new cricket species (Orthoptera: Grylloidea) from La Selva, Costa Rica. Trans. Am. Entomol. Soc. 2006;132:299–418. [Google Scholar]
- Pollack GS. Selective attention in an insect auditory neuron. J. Neurosci. 1988;8:2635–2639. doi: 10.1523/JNEUROSCI.08-07-02635.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack GS. Neural processing of acoustic signals. In: Hoy RR, Popper AN, Fay RR, editors. Comparative Hearing: Insects. Springer; New York: 1998. pp. 139–196. [Google Scholar]
- Pollack GS. Who, what, where? Recognition and localization of acoustic signals by insects. Curr. Opin. Neurobiol. 2000;10:763–767. doi: 10.1016/s0959-4388(00)00161-6. [DOI] [PubMed] [Google Scholar]
- Riede K. Monitoring biodiversity: analysis of Amazonian rainforest sounds. Ambio. 1993;22:546–548. [Google Scholar]
- Römer H, Bailey W. Strategies for hearing in noise: peripheral control over auditory sensitivity in the bushcricket Sciarasaga quadrata (Austrosaginae:Tettigoniidae) J. Exp. Biol. 1998;201:1023–1033. doi: 10.1242/jeb.201.7.1023. [DOI] [PubMed] [Google Scholar]
- Römer H, Krusch M. A gain control mechanism for processing of chorus sounds in the afferent auditory pathway of the bushcricket Tettigonia viridissima (Orthoptera: Tettigoniidae) J. Comp. Physiol. A. 2000;186:181–191. doi: 10.1007/s003590050018. [DOI] [PubMed] [Google Scholar]
- Römer H, Bailey W, Dadour I. Insect hearing in the field. III. Masking by noise. J. Comp. Physiol. A. 1989;164:609–620. [Google Scholar]
- Ryan MJ, Keddy-Hector A. Directional pattern of female mate choice and the role of sensory biases. Am. Nat. 1992;139:4–35. [Google Scholar]
- Samarra FIP, Klappert K, Brumm H, Miller PJO. Background noise constrains communication: acoustic masking of courtship song in the fruit fly Drosophila montana. Behaviour. 2009;146:1635–1648. [Google Scholar]
- Saussure H, Zehntner L, Pictet A, De Bormans C. Insecta. Orthoptera. In: Godman FD, Salvin O, editors. Biologia Centrali-Americana. Vol. 1. R. H. Porter; London: 1897. pp. 1893–1899.pp. 1–285. [Google Scholar]
- Schildberger K, Hörner M. The function of auditory neurons in cricket phonotaxis. I. Effect of hyperpolarization of identified neurons on sound localization. J. Comp. Physiol. A. 1988;163:621–631. [Google Scholar]
- Schwarz JJ, Wells KD. An experimental study of acoustic interference between two species of neotropical treefrogs. Anim. Behav. 1983;31:181–190. [Google Scholar]
- Stabel J, Wendler G, Scharstein H. Cricket phonotaxis: localization depends on recognition of the calling song pattern. J. Comp. Physiol. A. 1989;165:165–177. [Google Scholar]
- Sueur J. Cicada acoustic communication: potential sound partitioning in a multispecies community from Mexico (Hemiptera: Cicadomorpha: Cicadidae) Biol. J. Linn. Soc. 2002;75:379–394. [Google Scholar]
- Sueur J, Aubin T, Simonis C. Seewave: a free modular tool for sound analysis and synthesis. Bioacoustics. 2008;18:213–226. [Google Scholar]
- Verhey JL, Pressnitzer D, Winter IM. The psychophysics and physiology of comodulation masking release. Exp. Brain Res. 2003;153:405–417. doi: 10.1007/s00221-003-1607-1. [DOI] [PubMed] [Google Scholar]
- Walker TJ. Factors responsible for intraspecific variation in the calling songs of crickets. Evolution. 1962;16:407–428. [Google Scholar]
- Wehner R. “Matched filters” - neural models of the external world. J. Comp. Physiol. A. 1989;161:511–531. [Google Scholar]
- Wendler G, Löhe G. The role of the medial septum in the acoustic trachea of the cricket Gryllus. J. Comp. Physiol. A. 1993;173:557–564. [Google Scholar]
- Weschke G, Ronacher B. Influence of sound pressure level on the processing of amplitude modulations by auditory neurons of the locust. J. Comp. Physiol. A. 2008;194:255–265. doi: 10.1007/s00359-007-0303-1. [DOI] [PubMed] [Google Scholar]
- Witte K, Farris HE, Ryan MJ, Wilczynski W. How cricket frog females deal with a noisy world: habitat-related differences in auditory tuning. Behav. Ecol. 2005;16:571–557. [Google Scholar]
- Wohlgemuth S, Ronacher B. Auditory discrimination of amplitude modulations based on metric distances of spike trains. J. Neurophysiol. 2007;97:3082–3092. doi: 10.1152/jn.01235.2006. [DOI] [PubMed] [Google Scholar]
- Wong S, Parada H, Narins PM. Heterospecific acoustic interference: effects on calling in the frog Oophaga pumilio in Nicaragua. Biotropica. 2009;41:74–80. doi: 10.1111/j.1744-7429.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]