SUMMARY
Katydid receivers face the problem of detecting behaviourally relevant predatory cues from echolocating bats in the same frequency domain as their own conspecific mating signals. We therefore tested the hypothesis that katydids are able to detect the presence of insectivorous bats in spike discharges at early stages of nervous processing in the auditory pathway by using the temporal details characteristic for responses to echolocation sequences. Spike activity was recorded from an identified nerve cell (omega neuron) under both laboratory and field conditions. In the laboratory, the preparation was stimulated with sequences of bat calls at different repetition rates typical for the guild of insectivorous bats, in the presence of background noise. The omega cell fired brief high-frequency bursts of action potentials in response to each bat sound pulse. Repetition rates of 18 and 24 Hz of these pulses resulted in a suppression of activity resulting from background noise, thus facilitating the detection of bat calls. The spike activity typical for responses to bat echolocation contrasts to responses to background noise, producing different distributions of inter-spike intervals. This allowed development of a ‘neuronal bat detector’ algorithm, optimized to detect responses to bats in afferent spike trains. The algorithm was applied to more than 24 hours of outdoor omega-recordings performed either at a rainforest clearing with high bat activity or in rainforest understory, where bat activity was low. In 95% of cases, the algorithm detected a bat reliably, even under high background noise, and correctly rejected responses when an electronic bat detector showed no response.
Keywords: predation, hearing, background noise, katydid
INTRODUCTION
In at least seven taxonomic groups, insects have evolved an amazing variety of hearing organs (Hoy and Robert, 1996; Yager, 1999). The tympanate ear, the most common type of insect hearing organ, has evolved at least 19 times independently on almost all parts of the body, such as wings, mouthparts, thorax, abdomen and legs (Fullard and Yack, 1993; Hoy and Robert, 1996). Their functional significance is given in the two behavioural contexts of identification and localisation of mates or rivals (intraspecific communication) and the detection and localization of predators and hosts (Hoy, 1992; Greenfield, 2002; Stumpner and von Helversen, 2001; Gerhardt and Huber, 2002). The major goal of our study is related to the second task by analyzing the detectability of predator cues under the disturbed conditions of masking noise of the nocturnal tropical rainforest.
Research in the past decade on a number of different taxa emphasized the fact that animals usually communicate and perceive signals in environments with high levels of background noise (reviewed in Brumm and Slabbekoorn, 2005). This is true for any modality, with the consequence being the masking of relevant signals and a reduction of both the probability for signal detection and the correct discrimination between different signals. Applications of game theory (Johnstone, 1994; Johnstone, 1998; Johnstone and Earn, 1999) as well as signal detection theory (Wiley, 1994; Wiley, 2000) demonstrated the importance of errors as a result of noise for the evolution of communication systems.
Although this is now well recognized for intraspecific communication systems, little is known for the same context and the task of predator detection (but see Waters, 1996). The problem is particularly severe for katydids to detect the echolocation calls of insectivorous bats. On the one hand, their high hearing sensitivity in the ultrasonic range is an advantage because it increases the detection range for echolocation calls [for an experimental approach outdoors, see Schul and colleagues (Schul et al., 2000)]. This gives katydids more degrees of freedom to evolve bat-avoidance behaviours compared with insects possessing less-sensitive hearing. However, as katydids cannot categorically distinguish conspecific signals from echolocation calls of bats based on frequency, as in the case of crickets (Wyttenbach et al., 1996), there is a trade-off between increased sensitivity of a sensory system and the potential for masking by noise and increasing the number of errors in detection and discrimination. Acoustic noise measurements in a neotropical rainforest in Panama at night have demonstrated a sound pressure level (SPL) as high as 70 dB (Lang et al., 2005), and, although spectral analysis revealed that most acoustic energy is concentrated in the frequency band below 10 kHz, there is considerable high-frequency noise, with the potential of confounding bat-related events with conspecific calls.
Here, we attempt to demonstrate that the ultimately highly important information about the presence of a predator is still represented in the afferent sensory pathway of katydids and can be read out from the central nervous system despite considerable ambient background noise. We do this by investigating the neural response of the omega cell (ON1 neuron) of tropical katydids to playbacks of an echolocation call of a neotropical bat species. Our results allowed the development of a ‘neuronal bat detector’ algorithm that was optimized for reliable detection of bat-associated bursts in the presence of noise-associated bursts. The algorithm was successfully applied to outdoor recordings of the omega cell activity obtained in the nocturnal tropical rainforest.
MATERIALS AND METHODS
Animals
Neurophysiological experiments were performed using either the katydid Mecopoda elongata (Orthoptera, Tettigoniidae; Mecopodinae) or the Pseudophylline katydids Docidocercus gigliotosi and Cocconotus wheeleri. Mecopoda elongata were reared in crowded colonies at a temperature of 27°C, 70% relative humidity, on a 12 h:12 h light:dark cycle. They were fed ad libitum with fish food, oat flakes and fresh lettuce. Males and females of the other two species (total 14 individuals) were collected at lights in the rainforest on Barro Colorado Island (Panama) and used the following night for experiments outdoors (see below).
Neurophysiology
Two kinds of neurophysiological experiments were performed, both using the same, identified auditory interneuron (omega neuron) as a monitor for signal detection and discrimination. In the laboratory, sequences of echolocation calls were broadcast to insect preparations in combination with rainforest noise. In a rainforest field study, a portable omega cell recording was exposed to natural acoustic scenes, including background noise of insect and anuran calls, as well as echolocation calls of bats.
Our choice was the so-called omega neuron, a local interneuron in the prothoracic ganglion of all katydid species investigated so far. The reason for choosing this neuron was not its potential function in a neuronal network for eliciting a predator-avoidance behaviour but the fact that its activity reflects the input of almost all sensory receptor neurons in the ear (except those tuned to very low frequencies) (Römer, 1985; Stumpner and Molina, 2006). As the neuron fires tonically in response to all kinds of stimuli within its range of frequency tuning, and follows even high repetition rates of bat echolocation calls, it is ideally suited to examine whether information about the presence of bats is represented at a very early level within the auditory pathway and can be extracted from the spike trains even under realistic environmental noise levels.
The methods for the preparation and the recording of the extracellular action potential (AP) activity in portable outdoor preparations have been described in detail by Rheinlaender and Römer (Rheinlaender and Römer, 1986) and Römer and Lewald (Römer and Lewald, 1992). In short, the prothoracic ganglion was surgically exposed in a preparation ventral side up, and an extracellular tungsten electrode was placed close to the anterior omega-tract, where the segments of the two bilaterally homologous cells cross the ganglionic midline. For laboratory experiments (N=10), the insect preparation was placed in an anechoic chamber, 50 cm equidistant from two loudspeakers at a position 90° laterally on one side.
Sound stimulation
As a standard stimulus for a bat echolocation call, we used the search calls of the bat Saccopteryx bilineata (Emballonuridae), an aerial-hunting bat species abundant in Central America. These calls consist of short pulses (duration 8.5 ms) that are emitted at regular periods of 40–140 ms (7–25 Hz) at a carrier frequency alternating between 45 and 48 kHz (Jung et al., 2007; Fig. 1B). A digital recording of one of these pulses at a sampling rate of 250 kHz was used to create search calls at three different pulse repetition rates (PRRs) of 10, 18 and 24 Hz, covering the range of PRRs reported for search calls of various insectivorous bat species [Saccopteryx bilineata: average search call rate 13.7 Hz (Jung et al., 2007); Eptesicus fuscus: 1~20 call/s (Kick and Simmons, 1984); substrate gleaning Myotis evotis: ~20Hz (Faure and Barclay, 1994)]. This is also the rate of PRRs recorded at the edge of a large clearing on Barro Colorado Island (Fig. 1C), where some of the outdoor neurophysiological experiments were performed. In the playback experiment, one sequence of these search calls lasted for 2 s and was repeated after an interval of 5 s before the next sequence started. Each sequence was repeated 60–160 times to establish peri-stimulus-time histograms (PSTHs) of the omega cell responses.
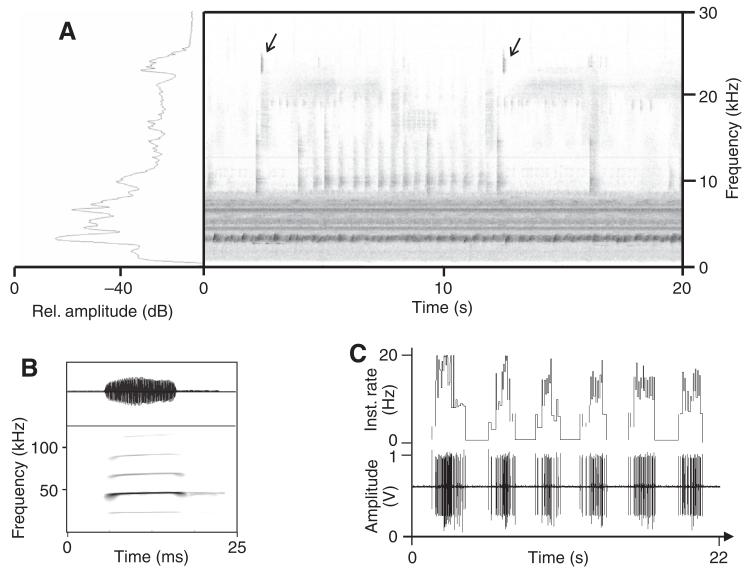
Fig. 1.
(A) Sonogram and spectrum of background noise recorded in the nocturnal rainforest on Barro Colorado Island. Note the continuous-frequency band between 3 and 9 kHz mainly occupied by crickets and frogs, compared with the high-frequency and ultrasonic events caused mainly by katydids. Arrows: short, low-redundant calls of the katydid Docidocercus gigliotosi. (B) Sonogram and oscillogram of the echolocation call of Saccopteryx bilineata, with a main frequency component at 45 kHz, used as a playback stimulus (fast Fourier transformation size: 1024). (C) Recording of echolocation pulses with an electronic bat detector at the edge of a rainforest clearing. The sequences last for ~2 s and have pulse repetition rates (PRRs) between 7 and 20 Hz (shown above).
For playbacks of the echolocation sequences, a custom-written Spike2 script driving the D/A output of a Power 1401 (Cambridge electronic design) was used. The echolocation signal passed an attenuator (Heinecken attenuator) and was amplified 10 fold (Heinecken sound amplifier) for broadcast through a string tweeter (LEAF Tweeter EAS-10TH400A). The source level of many aerial insectivorous bats is around 130 dB (Holderied and von Helversen, 2003). The average source level for search calls of Saccopteryx bilineata is 121.8±7.3 dB SPL (Surlykke and Kalko, 2008). The SPL at the insect preparation was calibrated to 65 dB SPL peak, equivalent to a 10 kHz continuous pure tone. This approximately corresponds to a bat at a target distance of ~11 m (see also Schulze and Schul, 2001), taking into account a spreading loss of −40 dB and an atmospheric absorption of 1.4 dB/m (45 kHz, 28°C and 75% relative humidity).
Sequences of this search call were broadcast either in the presence or absence of natural background noise. The background had been recorded at four different locations in the understory of the nocturnal rainforest of Barro Colorado Island (Drayton: 18 min, Lutz tower: 22 min, Gross trail: 5 min, Miller trail: 6 min), using a sound-level meter (CEL 414 plus attached CEL-296 digital filter; settings: linear; fast time-constant) with a condenser microphone (LD 2540, Type 4133, range 4 Hz to 45 kHz). The set-up was protected from humidity and rainfall and heated to 2°C above ambient temperature with an infrared bulb to prevent fogging of the microphone membrane. Recordings were sampled at a rate of 96 kHz. A sonogram of a section of 20 s of such a recording is shown in Fig. 1A. Most of the acoustic energy is concentrated within an almost continuous-frequency band between 3 and 8 kHz (mainly due to the calling of crickets and some frogs), whereas high sonic or ultrasonic events occur more discontinuously and result from the calling of katydids.
The sound file selected for noise playbacks had a total length of 51 min. An external fire-wire sound card (FA-101 Edirol) with a D/A conversion rate of 96 kHz was used for playbacks of background noise. After passing an attenuator (PA-5 Tucker Davis), the signal was amplified 10 fold (Heinecken sound amplifier) and broadcast to the ipsilateral side of the preparation via a dome speaker with a rather flat response characteristic up to 50 kHz (Universal Supertweeter, Tannoy). Calibration of background noise was performed using a calibrated ¼″ microphone (Microtech Gefell MK301 SN: 8633) connected to a sound-level meter (CEL 414). The noise level was adjusted to 65 dB SPL (rel. 20 μPa; slow reading) at the location of the insect preparation as this was the average noise level regularly measured in the first half of new-moon nights (Lang et al., 2005).
The stimuli representing echolocation sequences of bats at different PRRs and background noise was broadcast to omega cell preparations from the ipsilateral side, either in isolation or in combination. The playback of noise started at a random point in the sequence for each preparation. Additionally, auditory threshold curves (Fig. 2A) were obtained from five individuals of Mecopoda elongata using pure-tone sound pulses (20 ms in duration) that were generated by means of sound-editing software (Cool edit 2000, Syntrillium) and broadcast using a DA-card that supports sampling rates up to 250 kHz.
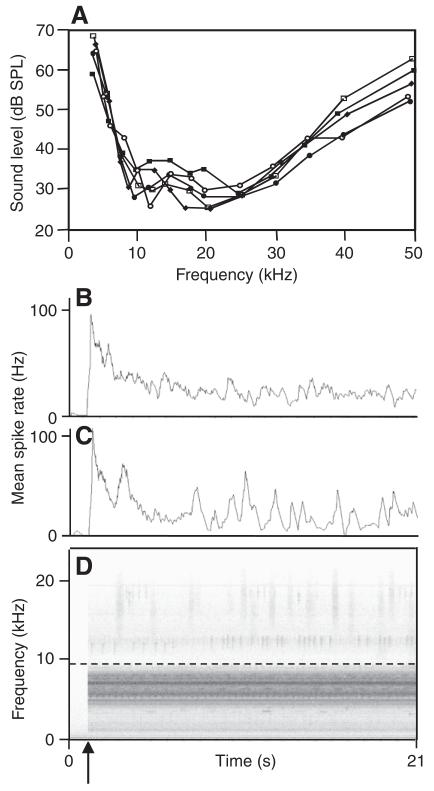
Fig. 2.
Response of an omega cell of a katydid to rainforest noise. (A) Tuning curves of five omega cells of the katydid M. elongata. (B,C) Spike-frequency adaptation in response to playbacks of low-pass-filtered rainforest noise (cut-off frequency 9 kHz; B) and the full spectrum (C), and the respective 20 s sequence of the noise in (D). The arrow indicates the time of playback onset. The dashed horizontal line in D indicates the cut-off frequency of the low-pass filter. Curves in B and C represent the mean spike rate of omega cell firing calculated in time-windows of duration 300 ms.
Outdoor neurophysiology
In addition to laboratory experiments, we also used a method previously described as the ‘biological microphone’ technique to record the action-potential activity of the omega cell in the natural environment of the insect (Rheinlaender and Römer, 1986; Römer and Lewald, 1992). Recordings were made on Barro Colorado Island, located in central Panama (0°09′N, 79°51′W) within Gatún Lake, part of the Panama Canal. Data collection took place in February and March 2005. The activity of the omega cell was recorded at night outdoors at two different locations, differing in the amount of echolocation activity of aerial-hunting bats. One location was a large clearing, where many bats were flying, as evident from the strong activity of echolocation calls monitored through an electronic bat-detector (Pettersson Electronic). This is commercially available equipment that allows hearing ultrasonic sound by digitally scaling the frequency down into the range of human hearing. A typical recording of such bat echolocation activity is shown in Fig. 1B, where such sequences last ~2 s, with PRRs ranging between 7 and 20 (see also Jung et al., 2007). The second location was within the understory of the rainforest, where systematic monitoring with such a bat-detector indicated only little bat activity. The omega cell preparation was placed at a height of 1 m above ground level. The extracellular action-potential discharges of the neuron as a result of background noise were amplified by a custom-made amplifier and recorded onto one channel of an AD-converter (Powerlab 4/25, model: ML845, AD-Instruments, Germany; sampling rate 10 kHz). The bat-detector was placed at a distance of 10 cm from the preparation, with the microphone pointing in the mid-canopy direction, and its output was recorded on a separate channel of the Powerlab. This allowed correlating the bursting activity of the omega cell with either background noise or the presence of bats. The spike recordings were converted into the software ‘Spike 2’ (Cambridge Electronic Design, UK) for offline analysis. A total of 14 preparations, covering a total recording time of ~24 h were analyzed in these outdoor recordings.
Development of a ‘neuronal bat-detector algorithm’
For the generation of parameterization data, we used field recordings that consisted of a simultaneous recording of the omega cell activity of a katydid and the output of the electronic bat detector (Pettersson, Sweden, D1000, divide-by-10). For positive parameterisation data (total duration: 600 s), recordings performed at the large clearing with high echolocation activity of aerial-hunting bats were used, in contrast to negative parameterization data (total duration: 4390 s), for which we used recordings in the rainforest understory with low bat activity. These recordings were used for the development of a rule-based neuronal bat-detector algorithm that scans brief time-segments of 0.5 s (‘scanning window’) for the occurrence of bat-related spike intervals.
Using a non-linear optimisation method based on a generalised reduction gradient (Microsoft Excel-Solver, Leon Lasdon, University of Texas/Austin, TX, USA, and Allan Waren, Cleveland State University, OH, USA), a rule was found that maximised the number of detected bat echolocation calls in positive parameterisation data and simultaneously minimised the number of falsely detected bat echolocation calls in negative parameterisation data. An optimum performance was achieved when: (1) a minimum of five inter-spike intervals (ISIs) lasted for less than 6 ms and (2) at least three spike intervals were present between 50 ms and 140 ms. The first ISI detects bursts and is very similar to the ISI used to discriminate bursts from single spikes in the AN2 of crickets (Marsat and Pollack, 2006). The second ISI corresponds to the repetition period of echolocation calls of bats in search for prey (7–20 Hz). For a maximum performance of the neuronal bat-detector algorithm, a further criterion was implemented: the number of spike intervals lasting for less than 6 ms needed to be higher than the number of spike intervals counted between 50 and 140 ms. These rules comprise a neuronal bat-detector algorithm that scanned omega cell recordings for bat-related bursts in validation data. The neuronal bat-detector algorithm processed omega cell recordings off-line by highlighting all segments, including spike intervals that fulfilled the above detection criteria with a red tick (custom-written Spike2 script; Cambridge Electronic Design, UK). Scanning for bat-related bursts was carried out in ‘scanning windows’ showing 50% overlap. This procedure increased the chance for detecting bat-associated bursts in the activity of the omega cell. The total duration of recordings used for the validation of the neuronal bat-detector algorithm was 2.52 h and 24.25 h for the locations with many and few bats, respectively.
RESULTS
Aerial-hunting bats emit echolocation signals in search for prey at regular intervals of about 40–140 ms [7–25 Hz (Jung et al., 2007)]. We monitored the activity of aerial-hunting insectivorous bats by using the electronic bat-detector in forest clearings. They reveal a typical temporal pattern with a series of search pulses lasting ~2 s in duration interrupted by pauses of varying duration (Fig. 1C). An example of a 45 kHz search signal of Saccopteryx bileneata, a bat species abundant in Central America, is shown in Fig. 1B. We used this pulse as a standard for playbacks of echolocation sequences in laboratory experiments.
Characteristics of rainforest background noise
Fig. 1A presents a sonogram of background noise in the nocturnal rainforest recorded ~1 h after sunset. The acoustic background is characterised by a strong, almost continuous sonic component in the range between 3 and ~9 kHz and, in addition, high sonic and ultrasonic components with a clear temporal structure. The low-frequency channel of this background is mainly due to calling of different cricket and some frog species, which share this narrow frequency range (Riede, 1997; Ellinger and Hödl, 2003). By contrast, most of the noise in the high-frequency channel starting at ~10 kHz is represented by calling songs of katydids, which use broadband carriers and may well extend into the high-ultrasonic range.
The tuning curves of five omega cell preparations of a katydid (Mecopoda elongata) are shown in Fig. 2A. Rather similar tuning and absolute sensitivities have been reported for the homologous cell in a number of other katydid species (Stumpner and Molina, 2006). The cell is most sensitive at frequencies between 10 and 30 kHz, with a steep roll-off towards frequencies in the low-frequency channel, and still rather high sensitivity between 40 and 60 dB SPL for higher ultrasonic frequencies. Not surprisingly, katydids are most sensitive for frequencies where most of the high-frequency background noise in the nocturnal rainforest is concentrated because most of these events result from the calling songs of katydids.
Neuronal responses to bat sound and background noise
For the performance of signal detection and discrimination, it is important to consider that receivers are permanently exposed to background noise, and it can be expected that the afferent nervous system exhibits some kind of adaptation in response to this noise (Gollisch and Herz, 2004; Herz et al., 2005). We examined the degree of adaptation in response to a noise level of 70 dB SPL in playbacks, where the pre-recorded noise either contained the full spectrum between 3 and 35 kHz or was low-pass filtered (cut-off frequency <9 kHz). An example of one experiment is shown in Fig. 2B,C, representing the average spike rate calculated in time-windows of 300 ms. After the full-spectrum rainforest noise was switched on at 70 dB SPL, there was a strong initial increase of the omega cell firing rate up to peak values of ~110 APs/s, followed by a decrease in firing rate, with a time-constant of 106±64 ms (average of six individuals). However, the firing rate in response to the full-spectrum background noise never reached a constant steady-state value – rather, it fluctuated on top of a given adaptation value owing to the bursting activity in response to high-frequency events in the background (Fig. 2C). The same experiment performed with low-pass-filtered background noise (Fig. 2B,D) revealed approximately the same increase, followed by a decrease after onset (decay constant 52±40 ms; mean of six individuals); however, the firing rate reached a value of ~30 APs/s, which remained constant throughout the time of recording, with only minor fluctuations. These fluctuations can be expressed as the standard deviation of the average spike rate calculated in a time period of 10 s after the firing reached a steady state (10 s after stimulus onset). This results in a mean standard deviation of 15.8 Hz for full-spectrum noise and 5.8 Hz for low-pass-filtered noise (N=6 preparations).
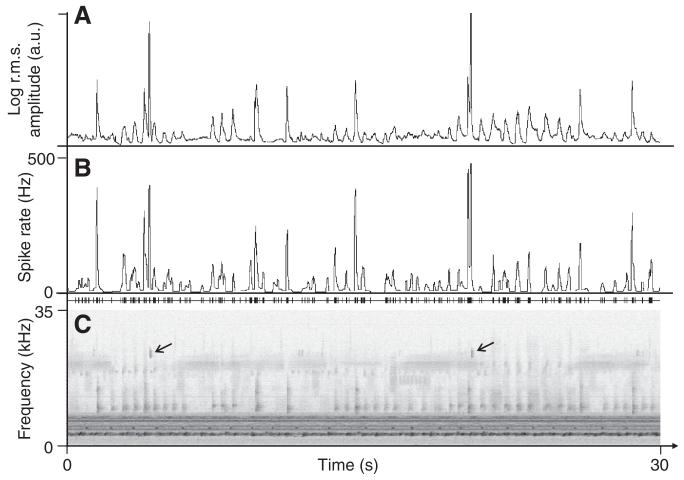
As both the tuning curves and the results of the adaptation experiments in Fig. 2 suggest that the steady-state spiking response of the omega cell favours the encoding of high-frequency events present in the rainforest background noise, we compared the log-transformed r.m.s. amplitude of all sound signals above 9 kHz and the instantaneous spike rate of the katydid omega cell (smoothing window size for both curves: 40 ms). The section lasting for 30 s in the example of Fig. 3 demonstrates the striking similarity of the ‘r.m.s. signal’ (upper trace) and the smoothed instantaneous spike rate (middle trace), with a resulting cross-correlation coefficient (CC) of 0.8. Calculating the CC in 1-min segments resulted in an average CC of 0.77±0.02 calculated across recordings from different individuals. The same calculation applied to omega cell recordings obtained from other katydid species exposed to nocturnal background noise yielded similar quantitative values (Euceraia atrix: 0.78±0.04, Phillophyllia ingens: 0.75±0.02). The spiking response of the omega cell is thus biased towards brief high-frequency events.
Fig. 3.
Omega cell firing in response to background noise. Mean spike rate of the omega cell (B) in response to playback of background noise (C) calculated in a time-window of duration 40 ms, compared with the root mean square (r.m.s.) envelope of the high-pass-filtered and log-transformed rainforest noise (A). The sonogram in C is a section of 30 s of noise, with the two arrows pointing to the short chirps of D. gigliotosi, which result in the strong increase in the spike rate of the cell. a.u., arbitrary units.
Suppressed responses to background noise favours the detection of bat-like sound
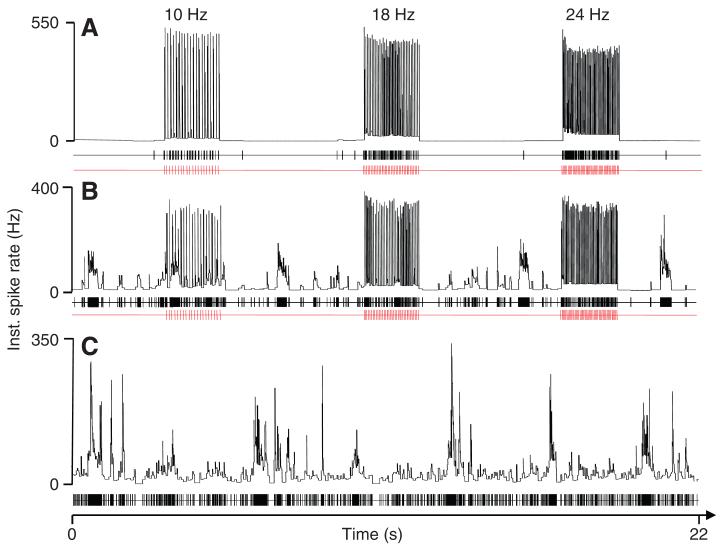
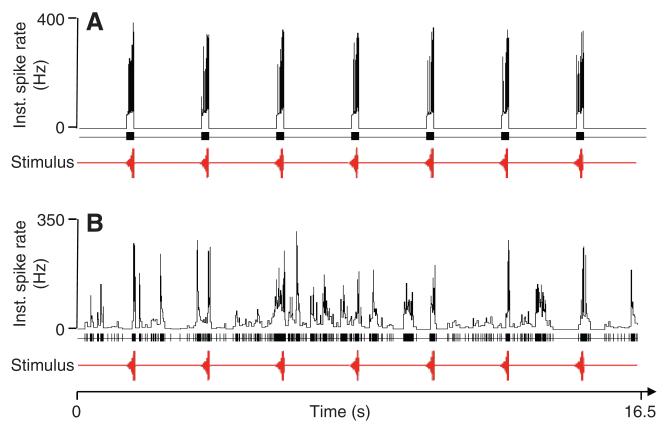
The results of the experiments presented in Fig. 3 demonstrate that omega cell preparations exposed to nocturnal noise conditions preferentially respond to events in the high-frequency channel, as predicted from the tuning of katydid ears and the spectral analysis of background noise (Fig. 2A). Ultrasonic echolocation calls of bats are also within this sensitive range of katydid receivers. We therefore investigated in a series of laboratory experiments how the representation of bat echolocation calls is affected by background noise. Fig. 4A shows a representative example of an omega cell response to bat calls at three repetition rates of 10, 18 and 24 Hz. The omega cell of the katydid typically responds to playbacks of sequences of this echolocation signal with a high bursting activity, firing a brief burst in response to each echolocation sound pulse. Without background noise, the instantaneous spike rate (inverse of ISI) in response to these stimuli reaches values of ~500 Hz, and there is nearly no activity in the time-periods between the stimuli. When the same stimuli were presented at a constant background noise level of 65 dB SPL (Fig. 4B), the instantaneous spike rate in response to the bat stimuli was reduced to values of ~350 Hz. In addition, in the time-periods without stimuli, there was also irregular burst activity of the cell in response to background noise, with instantaneous spike rates reaching peak values of 200 Hz. However, when the same constant background noise level was presented alone (Fig. 4C), the bursting activity of the cell in response to the background was much stronger, with instantaneous spike rates reaching peak values between 300 and 350 Hz. On average, the response of the omega cell to background noise was significantly reduced under playbacks of bat echolocation calls, both in terms of the number of spikes produced and the mean instantaneous spike rate. Playback of rainforest sound in the absence of bat signals resulted in an average steady-state spike frequency of 33.0±9.81 Hz (N=10). This is twice the average spike rate that was measured in the intervals lasting for two seconds that follow the presentation of 10 Hz echolocation sequences (15.9±7.9 Hz).
Fig. 4.
Response of the omega cell to bat echolocation calls. Instantaneous spike rates in response to different pulse repetition rates of 10 Hz, 18 Hz and 24 Hz reach values of 550 Hz in the undisturbed situation without background noise (A) but are reduced to ~350 Hz under conditions of masking noise (B). However, compared with the response to the background alone (C), the activity of the cell to the background is reduced owing to the response to the bat calls. Lower panels in A–C indicate stimulus pulses (in red) and spike activity (black). The SPL for background noise and bat calls was 65 dB.
In order to compare the representation of echolocation pulses in the omega cell with the representation of conspecific signals, we conducted a series of rather similar experiments using a conspecific stimulus of a katydid without noise and under the same background noise scene as shown in Fig. 4B. Males of M. elongata produce chirps of duration 250–300 ms at regular intervals of ~2 s (Hartbauer et al., 2005; Hartbauer et al., 2006). Without background noise, these chirps produce bursts of APs in female receivers, with instantaneous spike rates reaching 400 Hz (Fig. 5A). However, background noise presented at the same SPL as the conspecific chirp resulted in episodes of almost complete masking of stimulus-related bursts owing to the irregular bursting activity in response to the background noise (Fig. 5B). In contrast to the results with playbacks of echolocation calls (Fig. 4B), the stimulus-related activity did not alter the amount of spike activity in response to the background, either in terms of instantaneous spike rates or spike number.
Fig. 5.
Response of the omega cell to conspecific calls. (A) Instantaneous spike rates in response to regularly repeated chirps of M. elongata without noise (A) and under simultaneously presented background noise (B). Note the strong masking of the responses to conspecific chirps as a result of burst responses owing to the background. Lower panels are conspecific chirps (in red) and spike activity (black). The SPL for background noise and conspecific chirps was 65 dB.
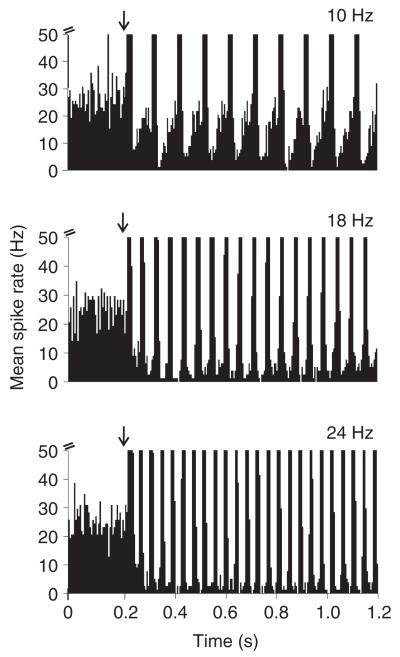
The suppressed response of the omega cell to rainforest noise during the playback of echolocation pulses becomes obvious by calculating PSTHs. In such PSTHs, one can observe a suppression of responses to the background already at the beginning of an echolocation sequence, in particular for higher repetition rates of 18 and 24 Hz. Fig. 6 shows one example for a PRR of 10, 18 and 24 Hz, where the background noise activity in the time between the repetitive pulses decays with time-constants in the range of 120–200 ms (for 18 and 24 Hz PRRs). A quantitative comparison of the average spike rate measured in intervals between bat-associated bursts with the spike rate measured 1 s before the echolocation signal had started (i.e. the response to the background alone) revealed a significant suppression for PRRs of 18 and 24 Hz (PRR 18 Hz: 4.4±4.5 Hz between bursts and 13.8±7.9 during noise; PRR 24 Hz: 5.1±5.9 Hz between bursts and 12.4±8.3 Hz during noise; P<0.05, Student’s t-test, N=10).
Fig. 6.
Suppression of background noise responses during responses to bat calls. Peri-stimulus-time histograms (PSTHs) of omega cell responses to stimulation with echolocation PRRs of 10, 18 and 24 Hz in the presence of rainforest background noise. Note the suppressed response to background noise shortly after the onset of the bat sequence. Bin-size in PSTHs=5 ms. Arrows indicate onset of stimulation.
These results suggest that there is a neuronal mechanism that, when the cell is strongly activated by the repetitive echolocation stimuli, reduces the amount of background activity both within the repetitive search pulses and even for some time in the bat-free intervals. In order to demonstrate that this mechanism should also be relevant for katydid receivers in their natural habitats, and to show how the magnitude of the suppression depends on the activity of the omega cell, we conducted a series of outdoor experiments and recorded the activity of the cell without stimulation and in response to stimuli differing in duration.
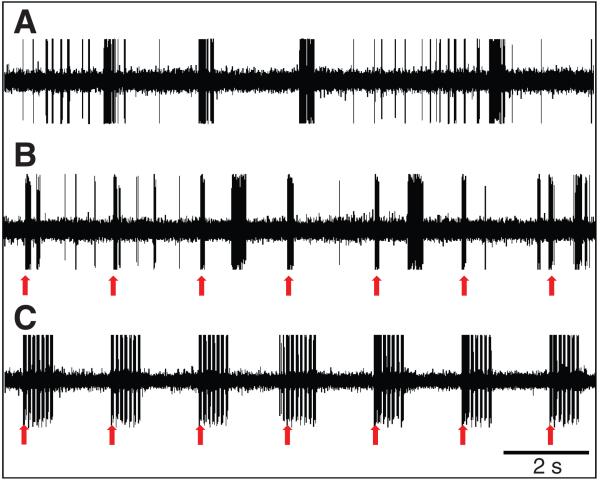
Fig. 7 demonstrates one example of an experiment where the omega cell preparation was placed in the rainforest understory at approximately 19.15 h. Without any given stimulus, the cell fires bursts of action potentials differing in duration and spike number and only a few isolated spikes (upper trace, no stimulus). The bursting activity is due to the various high-frequency and/or ultrasonic sounds created within the hearing range of this preparation. When the same preparation was stimulated with single sound-pulses of 20 kHz and a duration of 70 ms (rate 0.5/s; 20 dB above the threshold), the cell responded with regular bursts of action potentials, but the likelihood of bursting activity due to background noise remained unchanged (Fig. 7, middle trace; stimulus presentation indicated by arrows). However, when repetitive sequences of seven sound pulses at a repetition rate of 10 Hz were broadcast to the preparation, so that the cell fired with a high instantaneous spike frequency, these sequences were well represented in the spike discharge of the cell, but, in addition, the bursting activity in response to background noise was almost completely suppressed (Fig. 7, lower trace).
Fig. 7.
Selective attention and sensitivity to background noise in the field. Omega cell firing in an outdoor experiment performed approximately 1 h after sunset. The cell fires bursts of action potentials in response to the acoustic background (A, no stimulus). Short sound pulses (70 ms, 20 kHz carrier frequency; rate 0.5/s; 20 dB above the threshold) elicit brief bursts of APs (arrows), but the cell also fires with irregular bursts in response to the background (B). After stimulation with seven repetitive sound pulses (70 ms, pulse interval 30 ms; 20 kHz carrier frequency; rate 0.5/s; 20 dB above the threshold), the cell does respond only to these stimuli (arrows), whereas responses to the background are almost completely suppressed (C).
Omega bursts associated with repetitive echolocation pulses exhibited characteristic ISIs (see Fig. 4). A calculation of interval histograms of the activity of the omega cell revealed two maxima, one below 6 ms, corresponding to the high-frequency bursting of the cell to the search pulses, whereas the second maximum is at an interval corresponding to the echolocation pulse-repetition rate (Fig. 8A, examples for PRRs of 10 Hz and 18 Hz). For 10 Hz and 18 Hz PRRs, the relevant maxima are approximately 95 ms and 48 ms, respectively. By contrast, the interval histogram revealed a different pattern when intervals were counted in 10 omega cell recordings exposed to rainforest background noise alone (Fig. 8A, left). Here, a characteristic broad maximum with a peak at 18 ms was found, with only a few spike intervals counted below 6 ms. The difference between these spike interval distributions in the afferent spike trains in response to bat calls relative to background noise opens the possibility that the afferent nervous system might detect the presence of aerial-hunting bats in a noisy acoustic background by evaluating details in spike timing of afferent discharges.
Fig. 8.
Performance of the neuronal bat-detector algorithm. (A) Spike-interval histograms in omega cell recordings in response to playbacks of background noise (average ± s.d. of 10 individuals) alone or in combination with echolocation sequences with PRRs of 10 Hz and 18 Hz. (B–D) Simultaneous outdoor recordings of the activity of the omega cell of D. gigliotosi (upper trace) and an electronic bat detector (lower trace). Vertical ticks in B and D (red) indicate time segments of 0.5 s in which the neuronal algorithm suggested bat activity. (B,D) Results obtained with outdoor recordings in a forest clearing on Barro Colorado Island, where bat activity was usually high, or in the forest understory that possessed little or no bat activity (C). Note the detection performance of the neuronal algorithm even in the presence of strong action-potential activity due to background noise in (D). Traces labeled as ‘electronic bat detector’ in B–D show the echolocation pulses of bats recorded by the electronic bat detector.
A neuronal bat-detector algorithm
We tested this hypothesis by developing a ‘neuronal bat-detector algorithm’ based on the analysis of spike intervals. The performance of this rule-based algorithm was rather impressive with respect to the two types of positive decisions: (1) the algorithm detected a bat when the electronic detector indicated a bat (‘hit’), and (2) the algorithm did not respond when the electronic bat detector indicated no bat (‘correct rejection’). In 94.4% of cases, the algorithm detected a bat reliably, even under very high background noise (see Fig. 8B) and correctly rejected a bat when the electronic bat detector showed no response, even when the spiking activity due to background was high (Fig. 8C) (a recording period of 2.52 h was used for detector validation). There are two types of errors that are possible in the detection task: (1) the algorithm detected the presence of bats where there was no bat signal in the electronic bat detector (‘false alarm’), and (2) the algorithm did not respond when the electronic bat detector indicated a bat (‘missed signal’). The probability of these two errors was surprisingly small. In more than 24 h of recordings of omega cells in the rainforest understory, the rate of ‘false alarms’ was about one every 13.5 min, and similarly small was the probability of a ‘missed signal’ (5.6%, total number of bursts: 1053).
The high sensitivity of the algorithm for spike timings characteristic of responses to bat search calls can be seen in the example of Fig. 8D (see arrowhead), in a situation where high background noise induced strong activity in the omega cell, but nevertheless the algorithm detected sparse bat activity that was superimposed on the background noise activity.
Nevertheless, the rule-based algorithm for detecting bat echolocation signals in the omega cell activity is susceptible to false alarms if the background noise exhibits temporal elements that result in significant spike intervals indicative of bat activity (see Fig. 8A). In a rare case of an outdoor recording of the activity of the omega cell in a forest clearing, there was an unknown katydid producing repetitive sound pulses at a distance of ~10 m. This acoustic background was suprathreshold for the preparation, and the algorithm created false alarms and suggested bat activity (Fig. 9; vertical ticks in upper trace).
Fig. 9.
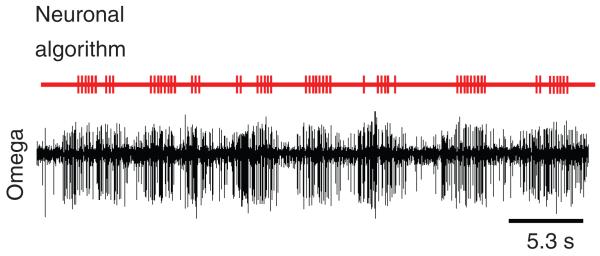
False alarms of the neuronal bat-detector algorithm. Outdoor recording of the activity of the omega cell of D. gigliotosi in a forest clearing when an unknown katydid produced repetitive sound pulses at a distance of ~10 m. In this rare case, the neuronal algorithm created false alarms and suggested responses to bat echolocation signals in the omega cell recording (red vertical ticks in upper trace).
DISCUSSION
Sensory and behavioural mechanisms have evolved in nocturnal flying insects, allowing early detection of, and escape from, aerial-hunting insectivorous bats [moths (Roeder, 1967; Miller and Surlykke, 2001); katydids (Libersat and Hoy, 1991; Faure and Hoy, 2000a; Schulze and Schul, 2001; Fullard et al., 2003); mantids (Triblehorn and Yager, 2001); crickets (Pollack, 1984; Nolen and Hoy, 1986) (for reviews see Hoy, 1992; Wyttenbach et al., 1996; Fullard, 1998)]. Aerial-hunting as well as gleaning bats emit brief ultrasound sonar signals in regular periods. The perception of these more-or-less regular high-frequency echolocation pulses indicates for the prey the presence of bats and has the potential to alert them. However, most of the studies on startle responses to ultrasound were performed in the absence of any relevant acoustic background. Although the importance of background noise for the detection and discrimination of signals in intraspecific communication has attracted increasing attention (Gerhardt and Klump, 1988; Ronacher et al., 2004; Brumm, 2004; Wysocki and Ladich, 2005; Poesel et al., 2007; Arch et al., 2008), comparatively little is known in the context of predator detection (Lohrey et al., 2009), although it should be evident that errors in detection and the discrimination of predator cues from irrelevant ones can have fundamental consequences for fitness.
Discriminating predatory cues from background noise
In contrast to crickets, which can discriminate conspecific signals from echolocation calls of bats on the basis of categorical perception of frequency (Wyttenbach et al., 1996), this is not possible for katydids, where the high-sonic and ultrasonic frequency range is also used for communication. There are two consequences arising from this situation: first, the only reliable information for discrimination appears to be based on temporal properties of the conspecific signals relative to the echolocation calls of bats, as suggested by Schul and colleagues (Schul et al., 2000). And second, if the high-frequency channel is occupied by many species signalling in this range, then the channel is very noisy, which renders the task of discriminating ‘good’ from ‘bad’ even more difficult than merely detecting a signal in a given channel.
We have provided several lines of evidence for the amount of noise in the high-frequency channel. First, in sonograms of sound recordings of the acoustic background in the nocturnal rainforest, there are always multiple sound sources with carrier frequencies ranging from 10 kHz into the ultrasonic range (Fig. 1A) (see also Riede, 1997; Lang et al., 2005; Balakrishnan, 2005; Ellinger and Hodl, 2003). For the interpretation of their amplitudes, it is important to keep in mind that these recordings were obtained in the rainforest understory, and, although the microphone was directed in the mid-canopy direction, the amplitudes of the high-frequency calls of most katydids will be strongly reduced owing to higher excess attenuation characteristics for ultrasound (Römer and Lewald, 1992).
Second, the broadband tuning of katydids and their high sensitivity in the high-sonic and ultrasonic frequency range (Fig. 2A) renders katydid receivers susceptible to this kind of acoustic background. Although we have shown tuning curves for only one of the investigated katydids, comparative analysis of the tuning of the omega cell of six other species by Römer and colleagues (Römer et al., 1989) and by Stumpner and Molina (Stumpner and Molina, 2006) demonstrate only little interspecific variation in bandwidth and/or absolute sensitivity. And finally, when receivers are exposed to this background, either in laboratory playbacks or when placed directly in the natural habitat, the bursting activity of the cell correlated highly with the high-frequency events in the noise (Figs 3 and 7). Interestingly, the sonic component still contributes to the noise response. As the data shown in Fig. 2 demonstrate, both the sonic and ultrasonic components of background noise result in strong spike-rate adaptation after a few seconds of stimulus onset, thus the representation of any signal should only be studied in this adapted state, when receivers are permanently confronted with this noise (or long-lasting series of stimuli) (Givois and Pollack, 2000). The underlying adaptation controlling the firing rate of the omega cell is most likely input driven (at the receptor level), considering the rather fast receptor adaptation in response to constant sound signals (Gollisch and Herz, 2004). Time-constants of ~60 ms were also found in the onset responses of receptor neurons in grasshoppers (Ronacher and Hennig, 2004; Herz et al., 2005). In addition, a calcium-activated potassium current hyperpolarizes the cell membrane and results in a gain-control of the omega cell response in crickets (Pollack, 1988; Sobel and Tank, 1994) and in the homologous omega neuron of katydids (Römer and Krusch, 2000). This long-lasting effect, with time-constants in the range of 5 s, might play a role in the sustained response of the omega cell to rainforest noise (Fig. 2B,C; Fig. 7).
High sensitivity to predatory cues due to the suppressed response to background
The fact that the omega cell of receivers fires bursts of action potentials in response to the high-frequency background even in the adapted state would interfere with the simultaneous detection of bat echolocation sound, and even more so with the discrimination from other acoustic events in the channel. However, our playback experiments with bat echolocation calls at three different PRRs demonstrate high instantaneous firing in response to each bat pulse, although the rate is reduced compared with a situation without background. More importantly, the firing in response to the background is significantly reduced in the short intervals between the burst responses to the bat pulses, in particular for PRRs of 18 and 24 Hz (Fig. 6). This short time-constant of the background noise suppression in the order of a few-hundred milliseconds indicates that the underlying mechanism is different from, and functions in addition to, the one described as ‘selective attention’ in the homologous omega neuron of crickets (Pollack, 1988) and katydids (Römer and Krusch, 2000). The gain control of the excitability of the cell suppresses low-level sound signals (Sobel and Tank, 1994), and this mechanism appears to reduce the average response to the background noise in the intervals between successive sequences of bat pulses as well. The playback experiments performed directly in the rainforest (Fig. 7) further support the notion that the long-lasting effect of noise suppression is identical to the gain-control mechanism described above as it depends on the activity level of the omega cell and is only significant when the cell is strongly activated for more than 500 ms (see also Pollack, 1988).
A reliable ‘neuronal bat-detector’
How can receivers detect the echolocation calls of bats and discriminate them from irrelevant noise and/or conspecific songs? The afferent spike timing differed significantly and resulted in characteristic ISIs, when background noise was broadcast alone or when, simultaneously, bat echolocation calls were presented (Fig. 8A). Given these differences, we asked whether it was possible to find simple rules that could be used by a ‘neuronal bat-detector algorithm’ for such a detection and discrimination task under noisy background conditions. The performance of the suggested ISI-based algorithm located the presence of bat activity with high probability while producing only a low frequency of false alarms.
There are, however, some methodological limitations in these calculations mainly owing to the fact that the electronic bat detector and the insect ear might differ in their sensitivity and/or their directionality to bat sound. Thus, there might have existed cases when the nervous preparation responded to a bat when the electronic bat detector was not sensitive enough or its directional beam was not in line with the bat call. These cases would be considered incorrectly as ‘false alarms’ in the analysis. Alternatively, when the preparation was less sensitive than the electronic bat detector, this would lead to incorrect decisions in terms of ‘missed signals’. In both of these cases, the low number of both types of errors would be reduced even more. However, in many hours of field recordings, it quite often happened that either receiver responded to single bat pulses when the other did not (very often the first or last pulses in the sequence; see example in Fig. 8B), but this rarely happened for complete sequences of search calls. We can thus be quite confident that our analysis of signal detection yielded realistic quantitative values.
The excellent performance of the neuronal bat-detector algorithm is also surprising considering experimental results with humans or animals obtained under various levels of background noise, when the task was not merely the detection of a stimulus but to discriminate between two or more variants. In this situation, the possibilities for errors increase compared with the detection task, and applications of game theory (Johnstone, 1994; Johnstone, 1998; Johnstone and Earn, 1999) as well as signal detection theory (Wiley, 1994; Wiley, 2000) demonstrated the importance of errors as a result of noise for the evolution of communication systems. In humans, error levels increase for tasks that require discrimination compared with those requiring detection only, and subjects failed to discriminate when they correctly detected a signal (Swets et al., 1978). Even under no background noise at all, discrimination performance decreases as the number of choices increases, as evident in the consistency of preferences of female anurans (Gerhardt, 1982; Telford et al., 1989; Márquez and Bosch, 1997). Moderate levels of background noise from a natural chorus altered the ability of females to discriminate between male calls in a neotropical frog (Wollermann and Wiley, 2002), and the preference of females for low-frequency calls was abolished altogether under higher levels of noise.
Another kind of categorical perception in the time domain?
We therefore wonder whether the excellent performance of the presented neuronal bat-detector algorithm might result from the fact that this is not a task that requires discriminating between a certain range of spike intervals from many other spike intervals present in the activity owing to the background. Instead, the task could be based on a kind of categorical perception of brief bursts that need to fall into a certain time-window in order to be indicative for the presence of a bat. Bursts characterised by short ISIs of less than 6 ms are known to reliably signal salient stimulus features and reliably predict behavioural responses (Marsat and Pollack, 2006). The size of the scanning window used by the neuronal bat detector limits the temporal resolution to 0.5 s, and thus it is impossible to analyse exactly at what time within the 0.5 s period the decision about the presence of a bat can be made. However, we should also consider that the algorithm was designed to scan for the search phase of bats, and not the final attack phase. If the prey were able to detect the approaching predator, it would give sufficient time to react appropriately within the 1.5–4 s, as calculated by Schul and colleagues (Schul et al., 2000).
Our data demonstrate that the information about the presence of one of the main predators for katydids is faithfully encoded in afferent spike trains of a first-order interneuron, even under substantial levels of background noise. We do not argue, however, that the omega cell in katydids represents the neuronal bat detector. Rather, we used this cell because it integrates most of the activity of auditory receptors in the ear as an indicator for the information that can be extracted from the timing of spikes in its discharges. In a similar way, spike timing has been used in the context of intraspecific communication of grasshoppers in the context of discrimination between several variants of the same male signal (Machens et al., 2001). Given that neurons of some moth species are capable of coding pulse periods typical for the transition from the search to the attack phase of bats (Boyan and Fullard, 1986; Boyan and Miller, 1991) and that, for some katydids, selective phonotaxis is due to the recognition of conspecific pulse rates (Bush and Schul, 2006), we can be sure that a neuronal calculation of inter-burst intervals in afferent spike trains is a task that can be solved in katydids. The ultimate test will be behavioural experiments using the acoustic startle response of katydids in flight as an indicator for the detection and discrimination from events in the acoustic background. This is part of ongoing research with various species of katydid in the rainforest.
According to Faure and Hoy (Faure and Hoy, 2000a; Faure and Hoy, 2000b) and Schul and colleagues (Schul et al., 2000), one of the best candidate neurons for the representation of bat echolocation signals is TN1. TN1 has a more selective tuning biased to higher frequencies compared with the omega cell but is also inhibited by low frequencies, which dominate the background noise (Zhantiev and Korsunovskaja, 1983). Our preliminary results with outdoor recordings of this cell in rainforest clearings show rather consistent responses to bat echolocation pulses, whereas the same preparations within the rainforest (i.e. under high background noise) show rather irregular spike activity. Thus, it remains to be tested whether this neuron can indeed represent the ‘real’ bat detector for rainforest katydids.
ACKNOWLEDGEMENTS
We thank Annemarie Surlykke for kindly providing a sound recording of the echolocation signal of Saccopteryx bilineata, and Jacob Tougaard for helpful comments on an earlier draft of the manuscript. Funding was provided by the Austrian Science Foundation (FWF), project P17986-B06 to H.R. This article is freely accessible online from the date of publication.
LIST OF ABBREVIATIONS
- SPL
sound pressure level
- PRR
pulse repetition rate
- AP
action potential
- ISI
inter-spike interval
REFERENCES
- Arch VS, Grafe TU, Narins PM. Ultrasonic signalling by a Bornean frog. Biol. Lett. 2008;4:19–22. doi: 10.1098/rsbl.2007.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan R. Neurobiology and behaviour: a network of connections. Curr. Sci. 2005;89:1147–1165. [Google Scholar]
- Boyan GS, Fullard J. Interneurones responding to sound in the tobacco budworm moth Heliothis virescens (Noctuidae): morphological and physiological characteristics. J. Comp. Physiol. A. 1986;158:391–404. [Google Scholar]
- Boyan GS, Miller LA. Parallel processing of afferent input by identified interneurones in the auditory pathway of the noctuid moth Noctua pronuba (L.) J. Comp. Physiol. A. 1991;168:727–738. doi: 10.1007/BF00224361. [DOI] [PubMed] [Google Scholar]
- Brumm H. The impact of environmental noise on song amplitude in a territorial bird. J. Anim. Ecol. 2004;73:434–440. [Google Scholar]
- Brumm H, Slabbekoorn H. Acoustic communication in noise. Adv. Stud. Behav. 2005;35:151–209. [Google Scholar]
- Bush SL, Schul J. Pulse-rate recognition in an insect: evidence of a role for oscillatory neurons. J. Comp. Physiol. A. 2006;192:113–121. doi: 10.1007/s00359-005-0053-x. [DOI] [PubMed] [Google Scholar]
- Ellinger N, Hödl W. Habitat acoustics of a neotropical lowland rainforest. Bioacoustics. 2003;13:297–321. [Google Scholar]
- Faure A, Barclay RMR. Substrate-gleaning versus aerial-hawking: plasticity in the foraging and echolocation behaviour of the long-eared bat, Myotis evotis. J. Comp. Physiol. A. 1994;174:651–660. doi: 10.1007/BF00217386. [DOI] [PubMed] [Google Scholar]
- Faure PA, Hoy RR. Neuroethology of the katydid T-cell. I. Tuning and responses to pure tones. J. Exp. Biol. 2000a;203:3225–3242. doi: 10.1242/jeb.203.21.3225. [DOI] [PubMed] [Google Scholar]
- Faure PA, Hoy RR. Neuroethology of the katydid T-cell. II. Responses to acoustic playback of conspecific and predatory signals. J. Exp. Biol. 2000b;203:3243–3254. doi: 10.1242/jeb.203.21.3243. [DOI] [PubMed] [Google Scholar]
- Fullard JH. Sensory coevolution of moths and bats. In: Hoy RR, Popper AN, Fay RR, editors. Comparative Hearing: Insects. Springer Handbook of Auditory Research, Springer-Verlag; New York: 1998. pp. 279–326. [Google Scholar]
- Fullard JH, Yack JE. The evolutionary biology of insect hearing. Trends Ecol. Evol. 1993;8:248–252. doi: 10.1016/0169-5347(93)90200-9. [DOI] [PubMed] [Google Scholar]
- Fullard JH, Dawson JW, Jacobs DS. Auditory encoding during the last moment of a moth’s life. J. Exp. Biol. 2003;206:281–294. doi: 10.1242/jeb.00085. [DOI] [PubMed] [Google Scholar]
- Gerhardt HC. Sound pattern recognition in some north american treefrogs (Anura: Hylidae): implications for mate choice. Am. Zool. 1982;22:581–595. [Google Scholar]
- Gerhardt HC, Huber F. Acoustic Communication in Insects and Anurans: Common Problems Diverse Solutions. The University of Chicago Press; Chicago, London: 2002. [Google Scholar]
- Gerhardt HC, Klump GM. Phonotactic responses and selectivity of barking treefrogs (Hyla gratiosa) to chorus sounds. J. Comp. Physiol. A. 1988;163:795–802. [Google Scholar]
- Givois V, Pollack GS. Sensory habituation of auditory receptor neurons: implications for sound localization. J. Exp. Biol. 2000;203:2529–2537. doi: 10.1242/jeb.203.17.2529. [DOI] [PubMed] [Google Scholar]
- Gollisch T, Herz AVM. Input-driven components of spike-frequency adaptation can be unmasked in vivo. J. Neurosci. 2004;24:7435–7444. doi: 10.1523/JNEUROSCI.0398-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield MD. Signalers and Receivers: Mechanisms and Evolution of Arthropod Communication. Oxford University Press; New York: 2002. [Google Scholar]
- Hartbauer M, Kratzer S, Steiner K, Römer H. Mechanisms for synchrony and alternation in song interactions of the bushcricket Mecopoda elongata (Tettigoniidae: Orthoptera) J. Comp. Physiol. A. 2005;191:175–188. doi: 10.1007/s00359-004-0586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartbauer M, Kratzer S, Römer H. Chirp rate is independent of male condition in a synchronising bushcricket. J. Insect Physiol. 2006;52:221–230. doi: 10.1016/j.jinsphys.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Herz AVM, Benda J, Gollisch T, Machens CK, Schaette R, Schütze H, Stemmler MB. Auditory processing of acoustic communication signals: sensory biophysics, neural coding, and discrimination of conspecific songs. In: Christensen AT, editor. Methods in Insect Sensory Neuroscience. CRC Press; Boca Raton: 2005. pp. 129–156. [Google Scholar]
- Holderied MW, von Helversen O. Echolocation range and wingbeat period match in aerial-hawking bats. Proc. Biol. Sci. 2003;270:2293–2299. doi: 10.1098/rspb.2003.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy RR. The evolution of hearing in insects as an adaptation of predation from bats. In: Webster DB, Fay RR, Popper AN, editors. The Evolutionary Biology of Hearing. Springer-Verlag; New York: 1992. pp. 115–129. [Google Scholar]
- Hoy RR, Robert D. Tympanal hearing in insects. Annu. Rev. Neurosci. 1996;41:433–450. doi: 10.1146/annurev.en.41.010196.002245. [DOI] [PubMed] [Google Scholar]
- Johnstone RA. Honest signalling, perceptual error and the evolution of ‘all-or-nothing’ displays. Proc Biol. Sci. 1994;256:169–175. [Google Scholar]
- Johnstone RA. Efficacy and honesty in communication between relatives. Am. Nat. 1998;152:45–58. doi: 10.1086/286148. [DOI] [PubMed] [Google Scholar]
- Johnstone RA, Earn DJD. Imperfect female choice and male mating skew on leks of different sizes. Behav. Ecol. Sociobiol. 1999;45:277–281. [Google Scholar]
- Jung K, Kalko EKV, Helversen OV. Echolocation calls in Central American emballonurid bats: signal design and call frequency alternation. J. Zool. 2007;272:125–137. [Google Scholar]
- Kick SA, Simmons JA. Automatic gain control in the bat’s sonar receiver and the neuroethology of echolocation. J. Neurosci. 1984;4:2725–2737. doi: 10.1523/JNEUROSCI.04-11-02725.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AB, Teppner I, Hartbauer M, Römer H. Predation and noise in communication networks of neotropical katydids. In: McGregor PK, editor. Animal Communication Networks. Cambridge University Press; Cambridge: 2005. pp. 152–169. [Google Scholar]
- Libersat F, Hoy RR. Ultrasonic startle behavior in bushcrickets (Orthoptera; Tettigoniidae) J. Comp. Physiol. A. 1991;169:507–514. doi: 10.1007/BF00197663. [DOI] [PubMed] [Google Scholar]
- Lohrey AK, Clark DL, Gordon SD, Uetz GW. Antipredator responses of wolf spiders (Araneae: Lycosidae) to sensory cues representing an avian predator. Anim. Behav. 2009;77:813–821. [Google Scholar]
- Machens CK, Stemmler MB, Prinz P, Ronacher B, Herz AVM. Representation of acoustic communication signals by insect auditory receptor neurons. J. Neurosci. 2001;21:3215–3227. doi: 10.1523/JNEUROSCI.21-09-03215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márquez R, Bosch J. Female preference in complex acoustical environments in the midwife toads Alytes obstetricans and Alytes cisternasii. Behav. Ecol. 1997;8:588–594. [Google Scholar]
- Marsat G, Pollack GS. A behavioral role for feature detection by sensory bursts. J. Neurosci. 2006;26:10542–10547. doi: 10.1523/JNEUROSCI.2221-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LE, Surlykke A. How some insects detect and avoid being eaten by bats: tactics and countertactics of prey and predator. BioScience. 2001;51:570–581. [Google Scholar]
- Nolen TG, Hoy RH. Phonotaxis in flying crickets. J. Comp. Physiol. A. 1986;159:423–439. doi: 10.1007/BF00604163. [DOI] [PubMed] [Google Scholar]
- Poesel A, Dabelsteen T, Pedersen S. Implications of conspecific background noise for features of blue tit, Cyanistes caeruleus, communication networks at dawn. J. Ornithol. 2007;148:123–128. [Google Scholar]
- Pollack GS. Ultrasound-sensitive neurons descending in the thoracic nervous system of the cricket Teleogryllus oceanicus. Can. J. Zool. 1984;62:555–562. [Google Scholar]
- Pollack GS. Selective attention in an insect auditory neuron. J. Neurosci. 1988;8:2635–2639. doi: 10.1523/JNEUROSCI.08-07-02635.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinlaender J, Römer H. Insect hearing in the field. I. The use of identified nerve cells as ‘biological microphones’. J. Comp. Physiol. A. 1986;158:647–651. [Google Scholar]
- Riede K. Bioacoustic monitoring of insect communities in a Bornean rainforest canopy. In: Stork NE, Adis J, Didham PK, editors. Canopy Arthropods. Chapman and Hall; London: 1997. pp. 442–452. [Google Scholar]
- Roeder KD. Turning tendency of moths exposed to ultrasound while in stationary flight. J. Insect Physiol. 1967;13:873–888. [Google Scholar]
- Römer H. Anatomical representation of frequency and intensity in the auditory system of orthoptera. In: Kalmring K, Elsner N, editors. Acoustic and Vibrational Communication in Insects. Paul Parey; Hamburg: 1985. pp. 25–32. [Google Scholar]
- Römer H, Krusch M. A gain-control mechanism for processing of chorus sounds in the afferent auditory pathway of the bushcricket Tettigonia viridissima (Orthoptera; Tettigoniidae) J. Comp. Physiol. A. 2000;186:181–191. doi: 10.1007/s003590050018. [DOI] [PubMed] [Google Scholar]
- Römer H, Lewald J. High-frequency sound transmission in natural habitats: Implications for the evolution of insect acoustic communication. Behav. Ecol. Sociobiol. 1992;157:631–642. [Google Scholar]
- Römer H, Bailey W, Dadour I. Insect hearing in the field. III. Masking by noise. J. Comp. Physiol. A. 1989;164:609–620. [Google Scholar]
- Ronacher B, Hennig RM. Neuronal adaptation improves the recognition of temporal patterns in a grasshopper. J. Comp. Physiol. A. 2004;190:311–319. doi: 10.1007/s00359-004-0498-3. [DOI] [PubMed] [Google Scholar]
- Ronacher B, Franz A, Wohlgemuth S, Hennig RM. Variability of spike trains and the processing of temporal patterns of acoustic signals – problems, constraints, and solutions. J. Comp. Physiol. A. 2004;190:257–277. doi: 10.1007/s00359-004-0494-7. [DOI] [PubMed] [Google Scholar]
- Schul J, Matt F, von Helversen O. Listening for bats: the hearing range of the bushcricket Phaneroptera falcata for bat echolocation calls measured in the field. Proc. R. Soc. Lond. B. Biol. Sci. 2000;267:1711–1715. doi: 10.1098/rspb.2000.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze W, Schul J. Ultrasound avoidance behaviour in the bushcricket Tettigonia viridissima (Orthoptera: Tettigoniidae) J. Exp. Biol. 2001;204:733–740. doi: 10.1242/jeb.204.4.733. [DOI] [PubMed] [Google Scholar]
- Sobel EC, Tank DW. In vivo Ca2+ dynamics in a cricket auditory neuron: An example of chemical computation. Science. 1994;263:823–826. doi: 10.1126/science.263.5148.823. [DOI] [PubMed] [Google Scholar]
- Stumpner A, Molina J. Diversity of intersegmental auditory neurons in a bush cricket. J. Comp. Physiol. A. 2006;192:1359–1376. doi: 10.1007/s00359-006-0164-z. [DOI] [PubMed] [Google Scholar]
- Stumpner A, von Helversen D. Evolution and function of auditory systems in insects. Naturwissenschaften. 2001;88:159–170. doi: 10.1007/s001140100223. [DOI] [PubMed] [Google Scholar]
- Surlykke A, Kalko EKV. Echolocating bats cry out loud to detect their prey. PLoS ONE. 2008;3:e2036. doi: 10.1371/journal.pone.0002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swets JA, Green DM, Getty DJ, Swets JB. Signal detection and identification at successive stages of observation. Percept Psychophys. 1978;23:275–289. doi: 10.3758/bf03199711. [DOI] [PubMed] [Google Scholar]
- Telford SR, Dyson ML, Passmore NI. Mate choice occurs only in small choruses of painted reed frogs Hyperolius marmoratus. Bioacoustics. 1989;2:47–53. [Google Scholar]
- Triblehorn JD, Yager DD. Broad versus narrow auditory tuning and corresponding bat-evasive flight behaviour in praying mantids. J. Zool. Lond. 2001;254:27–40. [Google Scholar]
- Waters DA. The peripheral auditory characteristics of noctuid moths: information encoding and endogenous noise. J. Exp. Biol. 1996;199:857–868. doi: 10.1242/jeb.199.4.857. [DOI] [PubMed] [Google Scholar]
- Wiley RH. Errors, exaggeration, and deception in animal communication. In: Real LR, editor. Behavioral Mechanisms in Evolutionary Ecology. University of Chicago Press; Chicago: 1994. pp. 157–189. [Google Scholar]
- Wiley RH. Sexual selection and mate choice: trade-offs for males and females. In: Apollonio M, Festa-Bianchet M, Mainardi D, editors. Vertebrate Mating Systems. World Scientific Publishing; USA: 2000. [Google Scholar]
- Wollermann L, Wiley RH. Possibilities for error during communication by neotropical frogs in a complex acoustic environment. Behav. Ecol. Sociobiol. 2002;52:465–473. [Google Scholar]
- Wysocki L, Ladich F. Hearing in fishes under noise conditions. J. Assoc. Res. Otolaryngol. 2005;6:28–36. doi: 10.1007/s10162-004-4043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyttenbach RA, May ML, Hoy RR. Categorical perception of sound frequency by crickets. Science. 1996;273:1542–1544. doi: 10.1126/science.273.5281.1542. [DOI] [PubMed] [Google Scholar]
- Yager DD. Structure, development, and evolution of insect auditory systems. Microsc. Res. Techn. 1999;47:380–400. doi: 10.1002/(SICI)1097-0029(19991215)47:6<380::AID-JEMT3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Zhantiev RD, Korsunovskaja OS. Structure and functions of two auditory neurons in the bush cricket Tettigonia cantans Fuess. (Orthoptera, Tettigoniidae) Ent. Obozr. 1983;62:462–469. [Google Scholar]