Abstract
Study Objectives:
To investigate the effects of post-learning sleep and sleep architecture on false memory in healthy older adults.
Design:
Balanced, crossover design. False memory was induced using the Deese-Roediger-McDermott (DRM) paradigm and assessed following nocturnal sleep and following a period of daytime wakefulness. Post-learning sleep structure was evaluated using polysomnography (PSG).
Setting:
Sleep research laboratory.
Participants:
Fourteen healthy older adults from the Singapore-Longitudinal Aging Brain Study (mean age ± standard deviation = 66.6 ± 4.1 y; 7 males).
Measurements and Results:
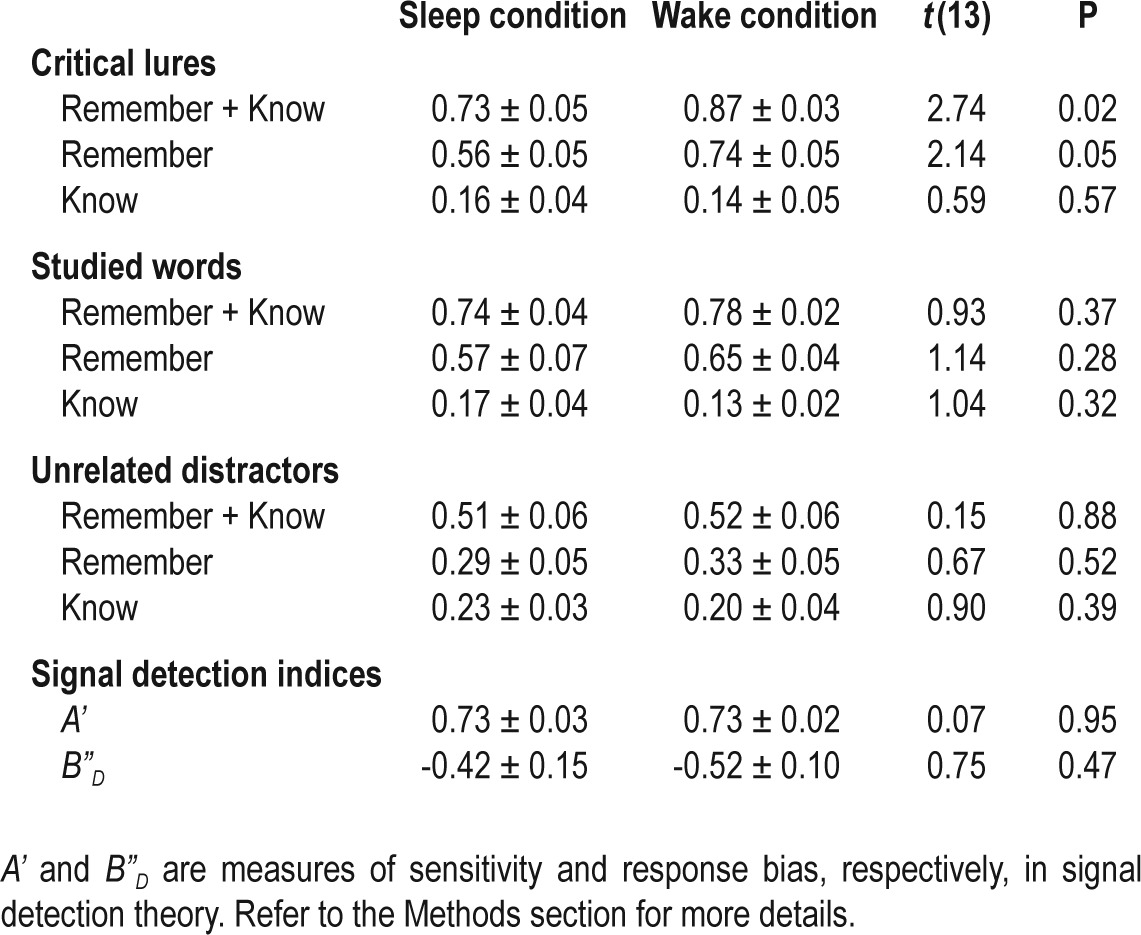
At encoding, participants studied lists of words that were semantically related to non-presented critical lures. At retrieval, they made “remember”/“know” and “new” judgments. Compared to wakefulness, post-learning sleep was associated with reduced “remember” responses, but not “know” responses to critical lures. In contrast, there were no significant differences in the veridical recognition of studied words, false recognition of unrelated distractors, discriminability, or response bias between the sleep and the wake conditions. More post-learning slow wave sleep was associated with greater reduction in false memory.
Conclusions:
In healthy older adults, sleep facilitates the reduction in false memory without affecting veridical memory. This benefit correlates with the amount of slow wave sleep in the post-learning sleep episode.
Citation:
Lo JC; Sim SK; Chee MW. Sleep reduces false memory in healthy older adults. SLEEP 2014;37(4):665-671.
Keywords: Declarative memory, false memory, memory consolidation, sleep, slow wave sleep
INTRODUCTION
Most of us have at one time or another experienced vivid recollection of things or events that were related but did not actually occur. Of interest, such false memories increase with age,1 and appear to be more robust in older than younger adults.2,3 Sleep is known to facilitate the offline processing of memory4,5 and has been reported to reduce false memory.6
In the laboratory, the Deese-Roediger-McDermott (DRM) paradigm7 has been widely used to induce false memory. Participants study semantically related words (e.g. “nose,” “breathe,” and “sniff”) that share a common theme word (e.g., “smell”) that is never presented. The retrieval of these unstudied critical lures is used to measure false memory.7,8 Remarkably, unstudied lures can be more resistant to forgetting than studied words even after long intervals.9–11 Further, when “remember” / “know” judgments12 are added to the experimental procedure, participants often report being able to relive contextual details present at encoding, for example, whether the lures were “presented” in auditory or visual form.13,14
A recent meta-analysis of veridical and false recognition found that false recognition increases with age, and that aging affects false remembering more than false knowing.1 Strikingly, significant age differences in false recognition remain detectable 3 days after learning,15 and in older adults, erroneous memoranda are less effectively suppressed by manipulations that are effective in young adults.2,3
Growing interest in the role of sleep in the offline processing of memory4,5 has motivated studies on how it affects false recognition. Relative to wakefulness during the day6 or sleep deprivation,16 sleep has been shown to reduce false recognition of the critical lures without affecting veridical recognition of the studied words. This pair of findings has been taken as evidence for an active role of sleep in the strengthening of contextual and sensory details related to studied words. This could improve the discriminability between studied words and critical lures. An alternative explanation for the same findings is that sleep merely plays a passive role in memory consolidation by protecting item-specific information from interference and forgetting. Extant studies have not examined the contribution of post-learning sleep, and specifically, each sleep stage, to sleep-dependent reduction of false recognition in older persons.
Slow wave sleep (SWS)17,18 is thought to facilitate declarative memory consolidation through the transfer and integration of newly acquired memories temporarily stored in the hippocampus to neocortical networks for long-term storage.19 Of particular relevance, SWS decreases by 2% per decade and is the sleep stage most affected by aging.20 Decrease in SWS has been linked to diminished offline processing of declarative memory with age.21
Although sleep continues to aid the consolidation of declarative memory in older adults,22–24 it is currently undetermined if it concurrently reduces false recognition. Here, we hypothesized that sleep would reduce the false recognition rate of critical lures in healthy older adults and that the observed benefit would be dependent on the quantity of post-learning SWS.
METHODS
Participants
Nineteen participants (mean age ± standard deviation [SD] = 66.5 ± 4.3 y; 10 males) who were native English speakers were recruited from the Singapore-Longitudinal Aging Brain Study25 for the current study. Participants were persons aged 60 years or older with no known active medical conditions other than treated, uncomplicated hypertension (n = 5) or diabetes mellitus (n = 2). Persons with any of the following were excluded: (1) history of significant vascular events (i.e., myocardial infarction, stroke, or peripheral vascular disease); (2) history of malignant neoplasia of any form; (3) history of cardiac, lung, liver, or kidney failure; (4) active or inadequately treated thyroid disease; (5) active neurological or psychiatric conditions; (6) a history of head trauma with loss of consciousness; (7) a Mini-Mental State Examination26 score < 26; or (8) a 15-item Geriatric Depression Scale27 score > 5. However, during the PSG night (refer to Experimental Design section for more details), two of the participants were found to have sleep apnea, whereas one had low SaO2 levels (< 95%); hence, their data were excluded from analysis. Additionally, one participant failed to understand the instructions of the memory task and equipment failure excluded a further participant. The final sample size was 14 (mean age ± SD = 66.6 ± 4.1 y; 7 males). Three persons were mildly hypertensive and one had well-controlled diabetes mellitus. Participants received an average of 13.7 y of formal education (SD = 3.0 years).
Experimental Design
We used a balanced, crossover design. Each participant was evaluated in both the sleep and the wake conditions. The order in which the conditions were presented was counterbalanced across participants. The two conditions were separated by at least 5 days.
To assess habitual sleep timing and duration, participants wore a wrist actigraph (Actiwatch 2, Philips Respironics, Murrysville, PA) for 1 week. In the sleep condition, actigraphy was also used to verify adherence to habitual sleep-wake schedules the week before participants were admitted to the laboratory. They arrived at the laboratory in the evening and were prepared for PSG recording. The learning session started 30 min before their habitual bedtime, whereas the recognition session started 90 min after their habitual wake time. This was to minimize any effect of sleep inertia on memory retrieval. The mean retention interval was 8.5 h (SD = 1.0 h).
In the wake condition, participants came to the laboratory for the learning session in the morning. Afterward, they were discharged and allowed to engage in their daily routine. Napping was not permitted and this was verified with actigraphy. After the retention interval (habitual sleep duration + 120 min), the participants returned for the recognition session. The mean retention interval was 8.3 h (SD = 1.1 h).
Memory Task
The DRM7 paradigm was used to induce false memory. In each condition, a new set of 10 DRM word lists8 was presented through headphones. Each list consisted of 15 words read aloud by an unfamiliar female voice at 2-sec intervals. The words were ordered in decreasing strength of association with the critical lure. To mark the beginning and the end of a list, a beep was presented 2 sec before the first word and again after the last word of each list. The beeps between successive lists were separated by 6 sec. Stimulus presentation was controlled using Matlab (R2012a, The MathWorks Inc., Natick, MA.). Participants were instructed to memorize as many words as possible for a memory task that would be administered several hours later.
The two sets of word lists used were equivalent in their effectiveness in creating false recognition of the critical lures (mean ± standard error derived from the normative data8: 66.7 ± 4.7% versus 67.7 ± 4.2%, t(18) = 0.16, P = 0.88). The critical lures of the word lists in set 1 were “window,” “soft,” “anger,” “high,” “thief,” “music,” “bread,” “spider,” “pen,” and “lion,” and those in set 2 were “smell,” “cup,” “sweet,” “smoke,” “doctor,” “needle,” “rubber,” “girl,” “shirt,” and “car.”
In the recognition task, participants were shown a total of 60 words in random order. These stimuli included 30 studied words (words at serial positions 1, 8, and 10 of each studied list), the 10 critical lures, and 20 unrelated distractors (the critical lure and the list words at serial positions 1, 8, and 10 in five different DRM lists: “rough,” “trash,” “mountain,” “man,” and “fruit” in set 1, and “cold,” “chair,” “slow,” “foot,” and “black” in set 2). Participants were required to indicate whether each word was “new” (“This word did not appear in the study lists”), “old – remember” (“This word appeared in the study lists, and I can mentally relive the experience”), or “old – know” (“I am confident that the item appeared on the list but unable to re-experience (i.e. remember) its occurrence”).
We calculated the proportion of “old” responses to (1) the critical lures – overall false recognition rate of critical lures, (2) the studied words – overall veridical recognition rate, and (3) unrelated distractors – overall false recognition rate of unrelated distractors. For false recognition of critical lures, we also derived the proportions of “remember” responses (false remembering rate) and “know” responses (false knowing rate). Similarly, we derived the proportion of “remember” and “know” responses to the studied words and the unrelated distractors.
As false recognition rates of the critical lures in the sleep and the wake conditions differed significantly (see Results section), we additionally computed a “corrected false recognition rate” for the “remember” and the “know” responses together (corrected overall rate) and separately (corrected false remembering rate and corrected false knowing rate). These three indices were derived by subtracting the false recognition rate in the wake condition from the corresponding rate in the sleep condition. With these corrected indices, we could quantify for each participant the effects of sleep on false recognition. The more negative the values, the greater the effect sleep had on reducing false recognition.
Moreover, we computed the nonparametric measures of sensitivity (A') and response bias (B”D) as used in the signal detection theory with the following formula28–30:
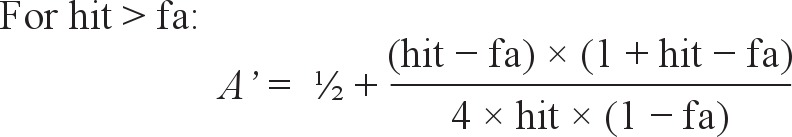 |
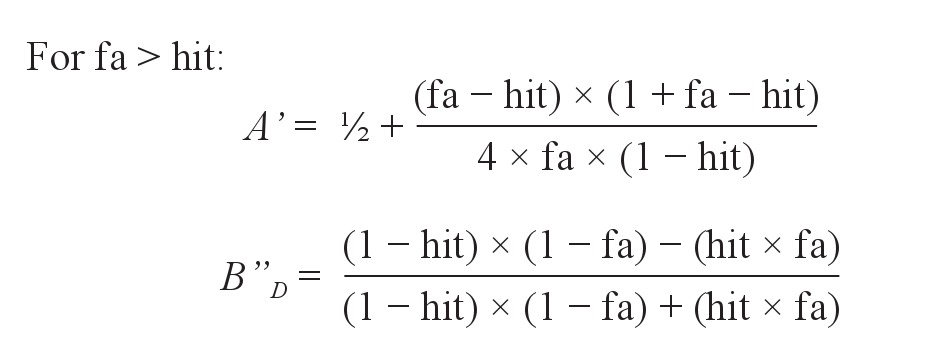 |
where hit = hit rate or proportion of “old” responses to the studied words; and fa = false alarm rate or proportion of “old” responses to the unrelated distractors.
A' is a nonparametric analogue of the more widely used d' and values generated range from 0 to 1, with 0.5 suggesting chance performance. Its corresponding bias measure, B”D, indicates the extent to which participants were liberal at selecting “old” responses (B”D < 0), conservative (B”D > 0), or neutral in their responses (B”D = 0).
Upon completing the entire experiment, participants were asked to report anything special they noticed about the memory task. No one noticed that the task was designed to induce false memory.
Polysomnography
Electroencephalographic (EEG) signals during the PSG night were recorded using a six-channel EEG montage (F3-A2, F4-A1, C3-A2, C4-A1, O1-A2, and O2-A1) according to the 10-20 system. Eye movement and muscle tone were recorded through left and right electrooculographic (EOG) and submental electro-myographic (EMG) electrodes that were respectively referenced to A2 and A1. The ground and common reference electrodes were placed at Cz and FPz, respectively. Participants also wore an abdominal band, a nasal airflow sensor, and a pulse oximeter to monitor for sleep-related breathing disorders.
EEG, EOG, and EMG signals were recorded using a Comet Portable EEG system from Grass Technologies (Astro-Med, Inc., West Warwick, RI). The sampling rate and the storage rate were 800 Hz and 200 Hz, respectively. The low-pass and high-pass filters were set at 35 Hz and 0.3 Hz for the EEG signals and 70 Hz and 10 Hz for the EMG signals. Electrode impedance was kept below 5 kΩ. Sleep staging was performed according to the revised American Academy of Sleep Medicine criteria.31
Statistical Analyses
SPSS 20.0 (IBM, Chicago, USA) was used for all the analyses. Paired-samples t tests were used to investigate whether false recognition rates of the critical lures (overall, “remember,” and “know”), veridical recognition rates of the studied words, false recognition rates of the unrelated distractors, A', and B”D differed between the sleep and the wake conditions. Spearman rank correlational analyses were performed to determine if any of these behavioral indices were associated with total sleep time (TST), the duration and the percentage of each sleep stage in the sleep condition, as well as the duration of retention interval in the wake condition.
As we adopted a within-subject design, DRM performance might improve across sessions as a result of practice. To determine whether the order effects on veridical and false recognition as well as the signal detection indices were significant, we used independent-samples t tests to contrast performance in participants performing the task for the first time and for the second time. This was separately evaluated for the sleep and the wake conditions. Furthermore, to examine whether sequence (sleep first versus wake first) significantly interacted with condition (sleep vs. wake), we performed 2 × 2 repeated-measures ANOVA.
RESULTS
Selective Effects of Sleep on False Remembering of Critical Lures
In the sleep condition, the false recognition rate of critical lures was significantly lower than in the wake condition (Figure 1A). This was not merely a consequence of reduced “old” responses after sleep, because the sleep and wake conditions neither differed in the veridical recognition of studied words (Figure 1B) nor the false recognition of unrelated distractors (Figure 1C). Participants were also similarly liberal in making “old” judgments in both conditions (B”D in Table 1). Furthermore, their ability to distinguish between studied and unstudied words was above chance and did not differ significantly between the two conditions (A' in Table 1).
Figure 1.
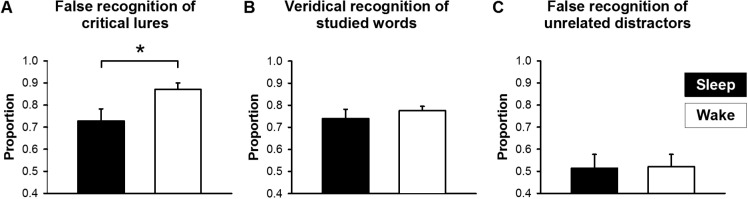
Effects of sleep on performance in the Deese-Roediger-McDermott paradigm. (A) False recognition rate of the critical lures was lower in the sleep condition (filled bars) than in the wake condition (open bars). (B) Veridical recognition of the studied words and (C) false recognition of the unrelated distractors were similar after sleep and after wakefulness. *P < 0.05.
Table 1.
Mean and standard error of the proportion of “remember” and “know” responses as well as signal detection indices after sleep and after wakefulness
The effect of sleep on false recognition was constrained to “remember” responses to the critical lures (Figure 2A). There was no significant difference in the false knowing rate between the sleep and the wake conditions (Figure 2B). “Remember” and “know” responses to both studied words and unrelated distractors also did not differ between the two conditions (Table 1).
Figure 2.
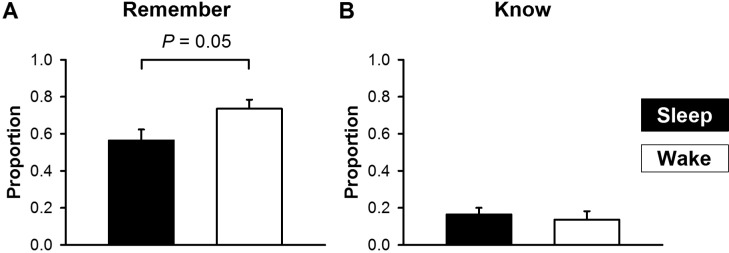
Effects of sleep on “remember” and “know” responses to critical lures. (A) Fewer “remember” responses to critical lures were observed in the sleep (filled bars) than in the wake conditions (open bars). (B) Proportion of “know” responses to critical lures did not differ between the two conditions.
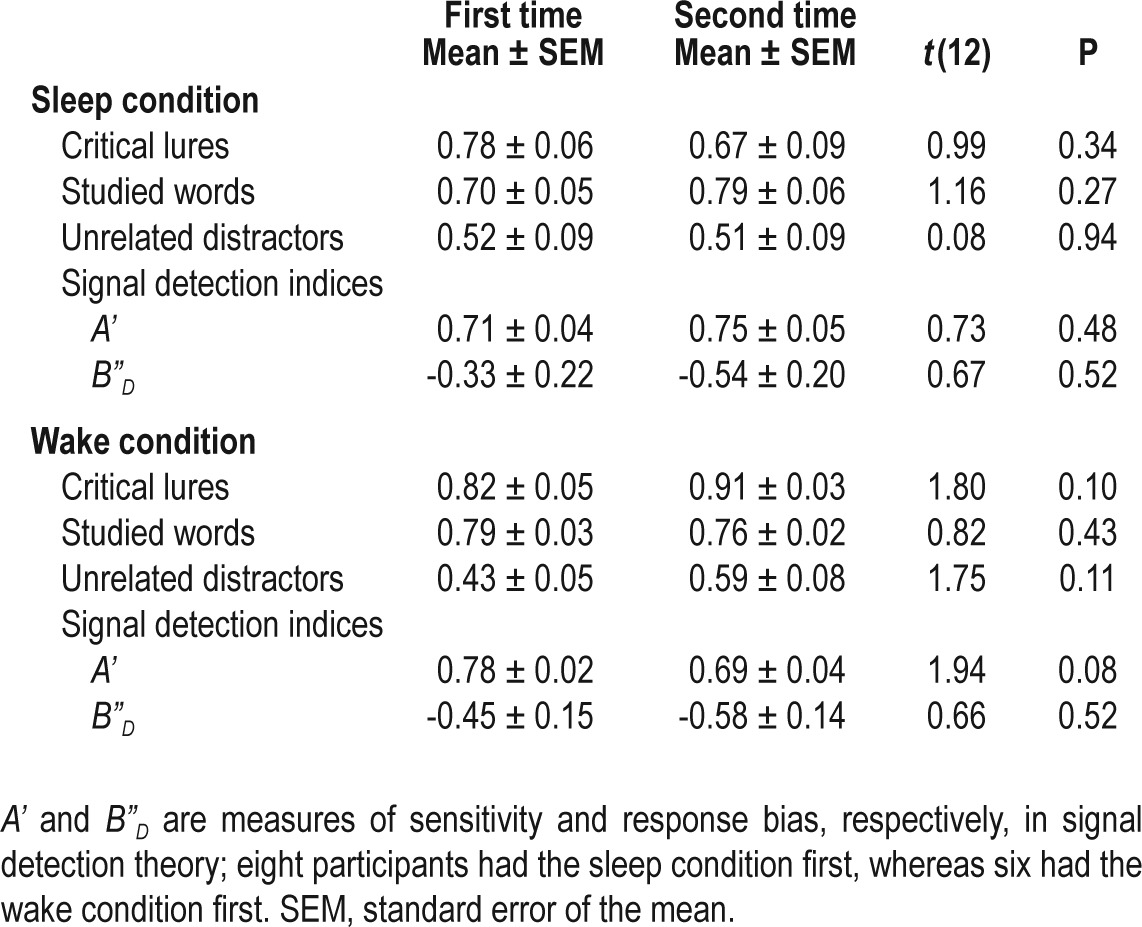
We did not find a significant order effect on DRM performance in either the sleep or the wake conditions. This was true for false recognition of critical lures and unrelated distractors, veridical recognition of studied words, A', and B”D (Table 2). In addition, performance was independent of sequence, i.e., whether the participants had the sleep or the wake condition first (F(1,12) = 0.03-2.39, P = 0.15-0.88), and its interaction with condition (F(1,12) = 0.01-3.35, P = 0.09-0.91).
Table 2.
Order effects on the proportion of “old” responses and signal detection indices in the sleep and the wake conditions
Correlations Between Sleep Architecture and Recognition Performance
Participants slept an average of 346.0 min (SD = 62.9 min) with a sleep efficiency of 85.0% (SD = 7.8%). The average amount of SWS was 19.3 min (SD = 25.0 min; Table 3).
Table 3.
Mean and standard deviation of sleep architecture and Spearman rank correlations with performance in the Deese-Roediger-McDermott paradigm
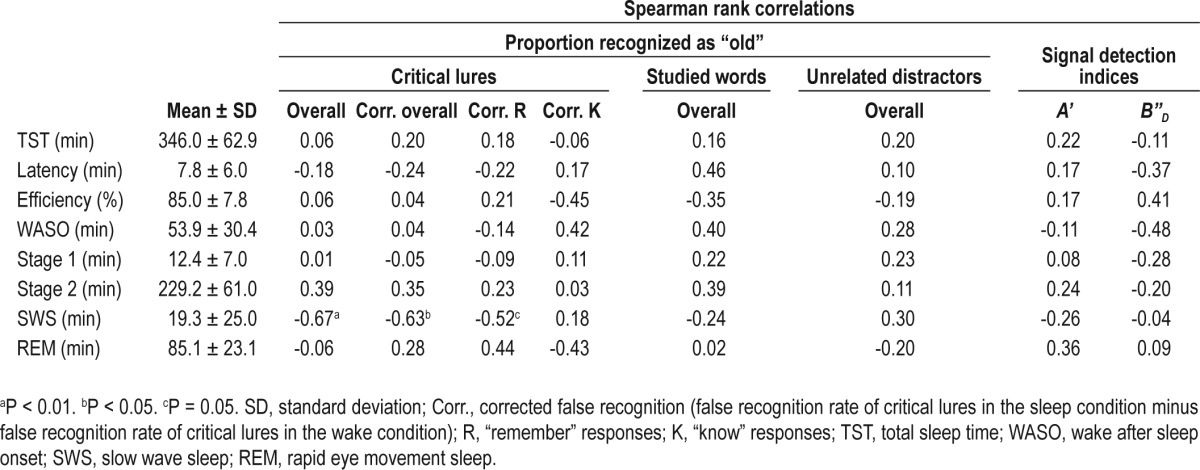
Longer duration of SWS was significantly correlated with lower overall false recognition of critical lures (ρ(12) = -0.67, P = 0.008). This correlation remained significant after controlling for the false recognition rate in the wake condition (corrected overall false recognition rate: ρ(12) = -0.63, P = 0.02; Figure 3A). Association with SWS duration was present only for “remember” responses to critical lures (corrected false remembering rate: ρ(12) = -0.52, P = 0.05; Figure 3B) and not with “know” responses (corrected false knowing rate: ρ(12) = 0.18, P = 0.54). In contrast, there were no significant associations between sleep parameters and veridical recognition of the studied words, false recognition of the unrelated distractors, A', or B”D (ρ(12) = -0.48-0.46, P > 0.08; Table 3). TST was not correlated to any of the behavioral measures studied (ρ(12) = -0.11-0.22, P > 0.45; Table 3).
Figure 3.
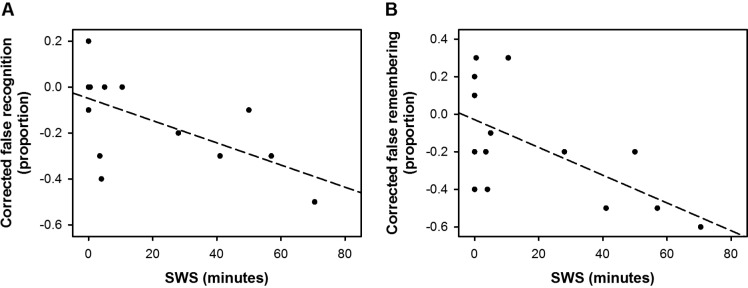
Correlations between slow wave sleep (SWS) and false recognition of the critical lures. More SWS in the post-learning sleep episode was associated with (A) a greater reduction in false recognition in the sleep condition relative to the wake condition, specifically for (B) “remember” responses.
To control for the interindividual differences in TST, Spearman correlations were also computed between percentage of SWS and the corrected false recognition rates of critical lures. SWS remained correlated with the corrected overall false recognition rate (ρ(12) = -0.63, P = 0.02) as well as the corrected false remembering rate (ρ(12) = -0.52, P = 0.05). SWS was not correlated with false “know” responses to critical lures (ρ(12) = 0.18, P = 0.54).
Correlations Between Wake Duration and Recognition Performance
The retention interval in the wake condition ranged from 6.5 h to 10.7 h. We did not find any significant correlation between the duration of the retention interval in the wake condition and any of the behavioral metrics evaluated, including false recognition of critical lures (ρ(12) = -0.21, P = 0.48), veridical recognition of studied words (ρ(12) = -0.15, P = 0.61), false recognition of unrelated distractors (ρ(12) = -0.06, P = 0.83), discriminability (ρ(12) = -0.11, P = 0.72), and response bias (ρ(12) = 0.12, P = 0.68).
DISCUSSION
We found that in healthy older adults, sleep reduced false recognition of critical lures that were semantically related to the studied information. This effect was not accompanied by changes in veridical memory, a shift in criterion toward greater conservatism, or an increase in sensitivity to the discrimination between studied and unstudied materials. Sleep reduced “remember” responses, i.e., false memory recollected with contextual details, but not “know” responses. Post-learning sleep related reductions in false memory were correlated with the duration of SWS.
Extending previous results concerning the benefit of post-learning sleep on declarative memory22,24 as well as work on the importance of sleep for good cognitive functioning of older adults,32–34 we found that SWS lowers false recognition.
The absence of a significant effect of sleep on veridical recognition may appear to contradict evidence for superior declarative memory after sleep compared to performance after wakefulness. However, most of the previous studies used either free or cued recall tests.35,36 When recognition testing was performed, significant forgetting was not observed 1 day after learning.37 In general, less forgetting is detected when memory is probed with recognition as opposed to recall,38 even with older adults.39 As a result, the relatively short average retention interval of 8.3-8.5 h used here may have been insufficient to elicit significant post-sleep gains in veridical memory.
In the current experiment, the memory of older adults was tested using recognition. In contrast, when memory was probed using recall, sleep was shown to promote false memory.40–42 These divergent findings might have arisen from how evaluating false memory with recall and recognition highlights the different benefits of sleep on offline memory processing.40
On one hand, sleep might facilitate memory consolidation through the extraction of the common gist linking semantically related studied words. This would serve to enhance the retrieval of the unstudied critical lures, when memory is assessed by recall, resulting in an increase in false memory. On the other hand, because sleep can enhance the contextual details of studied words, it will reduce false memory in recognition tests when learned and novel stimuli are encountered. The co-occurrence of increased false recall and decreased false recognition remains to be formally tested in an experiment evaluating both recall and recognition performance in the same participants.
Sleep May Play an Active Role in Reducing False Memory
In earlier studies, post-learning sleep was proposed to have a passive effect on memory. Specifically, it was argued that recently formed memories would be protected from interference arising from incoming information encountered during wakefulness.35 Accordingly, less interference and better memory performance would be expected with longer post-learning sleep. Conversely, a longer duration of wakefulness following learning might be expected to incur greater interference, and consequently more impairment of offline memory processing. However, we did not find any significant correlation between TST and recognition performance. We also did not observe greater forgetting of veridical memory or increase in false recognition with longer wakefulness. Nevertheless, in the current study, the shortest wakefulness retention interval was 6.5 h, precluding focus on the first hour after learning – the period when interference has the greatest impact on newly acquired memory.43,44
In contrast, the association between more SWS and lower false recognition rates suggests that SWS might actively reduce subsequent false recognition of unstudied materials. We would caution that as in previous work involving relatively small study samples,42 high correlations (ρ(12) = -0.52 to -0.67) should not be taken as evidence for strong association until replicated in large-sample studies.
The association of SWS with false memory reported here, together with previous reports on SWS and veridical memory,42 indicate that SWS contributes to the offline processing of both types of memory, although each may be supported by different neural mechanisms.45
Mechanisms Underlying Sleep-Dependent Reduction in False Recognition
Post-sleep reduction in false recognition, without an associated benefit to veridical memory, has been reported in young adults.6 This combination of findings could indicate that sleep strengthens the contextual details associated with studied words and hence, improves source monitoring.6 Alternatively, sleep might weaken the links between contextual details and false memory of critical lures. The observed reduction in “remember” responses to critical lures following sleep supports this notion. Nevertheless, either mechanism (or both in tandem) would increase the distinctiveness of representations associated with studied and unstudied items.
Our findings are also congruent with predictions arising from the synaptic homeostasis hypothesis, whereby synaptic strengths that increase with learning while we are awake down-scale during subsequent SWS46 (but see 47–49). This results in an improvement of the signal-to-noise ratio of memory through the elimination of weak synapses that store noisy information. Of specific interest, critical lures have fewer sensory and contextual details than veridical memory of studied words.50 One might expect synaptic connections for contextual details to be weaker for false than for veridical memories, resulting in their being pruned during SWS. Conversely, because of their stronger synaptic connections, contextual and sensory information related to veridical memories are less likely to be removed during synaptic downscaling. Consequently, no noticeable difference was found in the “remember” responses to the studied words after sleep and after wakefulness.
Our results may appear inconsistent with the higher false recognition rate after sleep compared to after sleep deprivation reported in a recent study.51 However, there are differences in experimental designs, such as the control condition used (daytime versus nocturnal wakefulness) and the administration of a secondary task between successive DRM lists.
Limitations
The critical conditions in this study differed not only in the physiological state (sleep versus wakefulness) during the retention interval but also the time of day of the learning and the testing sessions. False recognition of critical lures may have been lower in the morning after sleep because morning is the optimal time for cognitive testing of older adults.52,53 This finding may be particularly applicable when inhibition needs to be engaged,54 for example, when inhibiting an “old” response to the unstudied words.
Importantly, in our older adult cohort, there was no association between morning preference and better DRM performance in the morning, or between evening preference and better performance in the evening (Table S1). Also, previous studies in young adults have not found significant time-of-day effects on false memory.6,41,42 Additionally, time of day is unlikely to account for the selective benefit of sleep we observed on the false recognition of critical lures without affecting false recognition of unrelated distractors, or veridical recognition. Finally, inhibition and time-of-day effects would be expected to elicit greater inhibition and more conservative response bias in the morning than in the evening, again against what we report here.
CONCLUSION
In healthy older adults, sleep reduced false recognition without affecting veridical memory. This benefit correlated with the amount of SWS and may arise from the role sleep plays in increasing the distinction between studied and unstudied information.
DISCLOSURE STATEMENT
This was not an industry supported study. Financial support was provided by the National Medical Research Council, Singapore (STaR/0004/2008). This work was approved by the Institutional Review Board of the National University of Singapore (08-332). All participants provided written, informed consent. The authors have indicated no financial conflicts of interest.
SUPPLEMENTAL MATERIAL
Table S1.
Pearson correlations between morningness-eveningness preference and the proportion of “remember” and “know” responses as well as signal detection indices in the sleep and the wake conditions
REFERENCES
- 1.McCabe DP, Roediger HL, 3rd, McDaniel MA, Balota DA. Aging reduces veridical remembering but increases false remembering: Neuropsychological test correlates of remember-know judgments. Neuropsychologia. 2009;47:2164–73. doi: 10.1016/j.neuropsychologia.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kensinger EA, Schacter DL. When true memories suppress false memories: Effects of ageing. Cognitive Neuropsych. 1999;16:399–415. [Google Scholar]
- 3.Tun PA, Wingfield A, Rosen MJ, Blanchard L. Response latencies for false memories: Gist-based processes in normal aging. Psychol Aging. 1998;13:230–41. doi: 10.1037//0882-7974.13.2.230. [DOI] [PubMed] [Google Scholar]
- 4.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 5.Stickgold R, Walker MP. Sleep-dependent memory triage: Evolving generalization through selective processing. Nat Neurosci. 2013;16:139–45. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenn KM, Gallo DA, Margoliash D, Roediger HL., 3rd Nusbaum HC. Reduced false memory after sleep. Learn Mem. 2009;16:509–13. doi: 10.1101/lm.1500808. [DOI] [PubMed] [Google Scholar]
- 7.Roediger HL, McDermott KB. Creating false memories: Remembering words not presented in lists. J Exp Psychol Learn Mem Cogn. 1995;21:803–14. [Google Scholar]
- 8.Stadler MA, Roediger HL, 3rd, McDermott KB. Norms for word lists that create false memories. Mem Cognit. 1999;27:494–500. doi: 10.3758/bf03211543. [DOI] [PubMed] [Google Scholar]
- 9.Payne DG, Elie CJ, Blackwell JM, Neuschatz JS. Memory illusions: Recalling, recognizing, and recollecting events that never occurred. J Mem Lang. 1996;35:261–85. [Google Scholar]
- 10.Seamon JG, Luo CR, Kopecky JJ, et al. Are false memories more difficult to forget than accurate memories? The effect of retention interval on recall and recognition. Mem Cognit. 2002;30:1054–64. doi: 10.3758/bf03194323. [DOI] [PubMed] [Google Scholar]
- 11.Thapar A, McDermott KB. False recall and false recognition induced by presentation of associated words: Effects of retention interval and level of processing. Mem Cognit. 2001;29:424–32. doi: 10.3758/bf03196393. [DOI] [PubMed] [Google Scholar]
- 12.Tulving E. Memory and consciousness. Can Psychol. 1985;26:1–12. [Google Scholar]
- 13.Gallo DA, McDermott KB, Percer JM, Roediger HL., 3rd Modality effects in false recall and false recognition. J Exp Psychol Learn Mem Cogn. 2001;27:339–53. doi: 10.1037/0278-7393.27.2.339. [DOI] [PubMed] [Google Scholar]
- 14.Gallo DA, Roediger HL., 3rd The effects of associations and aging on illusory recollection. Mem Cognit. 2003;31:1036–44. doi: 10.3758/bf03196124. [DOI] [PubMed] [Google Scholar]
- 15.Koutstaal W, Schacter DL. Gist-based false recognition of pictures in older and younger adults. J Mem Lang. 1997;37:555–83. [Google Scholar]
- 16.Diekelmann S, Landolt HP, Lahl O, Born J, Wagner U. Sleep loss produces false memories. PLoS One. 2008;3:e3512. doi: 10.1371/journal.pone.0003512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peigneux P, Laureys S, Fuchs S, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–45. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Rasch B, Buchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–9. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 19.Gais S, Born J. Low acetylcholine during slow-wave sleep is critical for declarative memory consolidation. Proc Natl Acad Sci U S A. 2004;101:2140–4. doi: 10.1073/pnas.0305404101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 21.Backhaus J, Born J, Hoeckesfeld R, Fokuhl S, Hohagen F, Junghanns K. Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learn Mem. 2007;14:336–41. doi: 10.1101/lm.470507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aly M, Moscovitch M. The effects of sleep on episodic memory in older and younger adults. Memory. 2010;18:327–34. doi: 10.1080/09658211003601548. [DOI] [PubMed] [Google Scholar]
- 23.Seeck-Hirschner M, Baier PC, Weinhold SL, et al. Declarative memory performance is associated with the number of sleep spindles in elderly women. Am J Geriatr Psychiatry. 2012;20:782–8. doi: 10.1097/JGP.0b013e31823033da. [DOI] [PubMed] [Google Scholar]
- 24.Wilson JK, Baran B, Pace-Schott EF, Ivry RB, Spencer RM. Sleep modulates word-pair learning but not motor sequence learning in healthy older adults. Neurobiol Aging. 2012;33:991–1000. doi: 10.1016/j.neurobiolaging.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chee MW, Chen KH, Zheng H, et al. Cognitive function and brain structure correlations in healthy elderly East Asians. Neuroimage. 2009;46:257–69. doi: 10.1016/j.neuroimage.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. In: Brink TL, editor. Clinical gerontology: A guide to assessment and intervention. New York: The Haworth Press; 1986. pp. 165–73. [Google Scholar]
- 28.Pollack L, Norman DA. A non-parametric analysis of recognition experiments. Psychon Sci. 1964;1:125–6. [Google Scholar]
- 29.Macmillan NA, Creelman CD. Detection theory: A user's guide. New York: Cambridge University Press; 1991. [Google Scholar]
- 30.Macmillan NA, Creelman CD. Triangles in ROC space: History and theory of “nonparametric” measures of sensitivity and response bias. Psychon Bull Rev. 1996;3:164–70. doi: 10.3758/BF03212415. [DOI] [PubMed] [Google Scholar]
- 31.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 32.Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimaki M, Singh-Manoux A. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep. 2011;34:565–73. doi: 10.1093/sleep/34.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jonelis MB, Drummond SP, Salamat JS, McKenna BS, Ancoli-Israel S, Bondi MW. Age-related influences of prior sleep on brain activation during verbal encoding. Front Neurol. 2012;3:49. doi: 10.3389/fneur.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu L, Jiang CQ, Lam TH, et al. Short or long sleep duration is associated with memory impairment in older Chinese: The Guangzhou Biobank Cohort Study. Sleep. 2011;34:575–80. doi: 10.1093/sleep/34.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenkins JG, Dallenbach KM. Oblivescence during sleep and waking. Am J Psychol. 1924;35:605–12. [Google Scholar]
- 36.Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9:534–47. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 37.Takashima A, Petersson KM, Rutters F, et al. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci U S A. 2006;103:756–61. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruce D, Cofer CN. A comparison of recognition and recall in short-term-memory. Am Psychol. 1965;20:583–4. [Google Scholar]
- 39.Davis HP, Small SA, Stern Y, Mayeux R, Feldstein SN, Keller FR. Acquisition, recall, and forgetting of verbal information in long-term memory by young, middle-aged, and elderly individuals. Cortex. 2003;39:1063–91. doi: 10.1016/s0010-9452(08)70878-5. [DOI] [PubMed] [Google Scholar]
- 40.Diekelmann S, Born J, Wagner U. Sleep enhances false memories depending on general memory performance. Behav Brain Res. 2010;208:425–9. doi: 10.1016/j.bbr.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 41.McKeon S, Pace-Schott EF, Spencer RM. Interaction of sleep and emotional content on the production of false memories. PLoS One. 2012;7:e49353. doi: 10.1371/journal.pone.0049353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Payne JD, Schacter DL, Propper RE, et al. The role of sleep in false memory formation. Neurobiol Learn Mem. 2009;92:327–34. doi: 10.1016/j.nlm.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lechner HA, Squire LR, Byrne JH. 100 years of consolidation--remembering Muller and Pilzecker. Learn Mem. 1999;6:77–87. [PubMed] [Google Scholar]
- 44.Muller GE, Pilzecker A. Experimentelle Beitrage zur Lehre vom Gedachtnis [Experimental contributions to the science of memory] Z Psychol Erganzungsband. 1900;1:1–300. [Google Scholar]
- 45.Schacter DL, Slotnick SD. The cognitive neuroscience of memory distortion. Neuron. 2004;44:149–60. doi: 10.1016/j.neuron.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 46.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Born J, Feld GB. Sleep to upscale, sleep to downscale: balancing homeostasis and plasticity. Neuron. 2012;75:933–5. doi: 10.1016/j.neuron.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Chauvette S, Seigneur J, Timofeev I. Sleep oscillations in the thalamocortical system induce long-term neuronal plasticity. Neuron. 2012;75:1105–13. doi: 10.1016/j.neuron.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grosmark AD, Mizuseki K, Pastalkova E, Diba K, Buzsaki G. REM sleep reorganizes hippocampal excitability. Neuron. 2012;75:1001–7. doi: 10.1016/j.neuron.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Norman KA, Schacter DL. False recognition in younger and older adults: Exploring the characteristics of illusory memories. Mem Cognit. 1997;25:838–48. doi: 10.3758/bf03211328. [DOI] [PubMed] [Google Scholar]
- 51.Darsaud A, Dehon H, Lahl O, et al. Does sleep promote false memories? J Cogn Neurosci. 2011;23:26–40. doi: 10.1162/jocn.2010.21448. [DOI] [PubMed] [Google Scholar]
- 52.Intons-Peterson MJ, Rocchi P, West T, McLellan K, Hackney A. Age, testing at preferred or nonpreferred times (testing optimality), and false memory. J Exp Psychol Learn Mem Cogn. 1999;25:23–40. doi: 10.1037//0278-7393.25.1.23. [DOI] [PubMed] [Google Scholar]
- 53.May CP, Hasher L, Stoltzfus ER. Optimal time of day and the magnitude of age differences in memory. Psychol Sci. 1993;4:326–30. [Google Scholar]
- 54.May CP, Hasher L. Synchrony effects in inhibitory control over thought and action. J Exp Psychol Hum Percept Perform. 1998;24:363–79. doi: 10.1037//0096-1523.24.2.363. [DOI] [PubMed] [Google Scholar]