Abstract
Many studies provide detailed behavioural and neurophysiological information on the ability of crickets to localize a sound source under ideal acoustic conditions, but very little is known about how they perform in real habitats. We investigated directional hearing of crickets in the field using a neurophysiological approach, by recording the activity of the two prominent, bilaterally homologous AN1 neurons simultaneously in a cricket’s habitat. The discharge and latency differences of the pair of neurons in response to conspecific chirps presented at different distances and directions were taken as a measure of directional information. The maximum hearing distance differed between individuals and weather conditions from 1 to 15 m (mean 9.2 m). Although the AN1 activity generally decreased with increasing distance, large fluctuations in the magnitude of responses occurred with distance, indicating that the intensity gradient over distance is often irregular. The directional information provided in the discharge differences of the two neurons also varied with distance. Again, there was no simple directional gradient on the transmission channel; rather, with decreasing distance to the source there were receiver locations providing suprathreshold responses, but no directional information. The consequences for the ability of field crickets to communicate acoustically close to the ground are discussed.
Keywords: Sound transmission, Cricket, Binaural processing, Directional hearing
Introduction
Studies on the impact of the transmission channel for sound signals have been pioneered by Morton (1975) and Wiley and Richards (1978, 1982). They used bird song to demonstrate how the structure and physical properties of the habitat affect excess attenuation, frequency filtering or the degradation of the species-specific amplitude modulation of sound signals. These studies also demonstrated that patterns of sound attenuation and degradation differ between habitats of birds or other vertebrates and that these physical constraints have implications for the evolution of animal vocalizations (for reviews see Wiley and Richards 1982; Michelsen and Larsen 1983; Embleton 1996). As pointed out by Gerhardt (1982), the signal perceived by receivers in the field at some distance from the source may differ considerably from the broadcast signal, with consequences for its ability for signal discrimination in nature compared to the soundproof room.
Effects of the transmission channel on sound signals are even more pronounced when considering insect acoustic communication. Basically, this is due to the small size of insects and the fact that most species produce sound in the high sonic or ultrasonic frequency range (for the physics of sound production see Bennet-Clark 1970; Michelsen and Nocke 1974). Since the mechanisms resulting in excess attenuation are strongly frequency dependent, one would thus expect a stronger attenuation of insect sound signalscompared to those of anurans, birds and most other terrestrial vertebrates. In fact, a quantitative study of frequency dependent attenuation for carrier frequencies from 5 to 40 kHz revealed attenuation in excess to geometric spreading of more than 30 dB over a distance of 10 m for 40 kHz, compared to only 2 dB for 5 kHz over the same distance, as measured in the natural habitat of a katydid (Römer and Lewald 1992; Schul and Patterson 2003).
Field crickets, however, are exceptions to the rule. They produce low-frequency sound with carriers in the range from 2 kHz to about 9 kHz (but see Robillard and Desutter-Grandcolas (2004) for crickets calling at ultrasonic frequencies). Thus, they appear to be well adapted with their carrier frequencies to the low-frequency filter properties of the natural habitat (Keuper and Kühne 1983). However, whereas they avoid strong excess attenuation in dense vegetation, they face another complicated problem of sound transmission. Since field crickets live and communicate on the ground, the sound waves of a male calling song are produced at a grazing angle with the ground, which is almost zero, and travel almost parallel to the ground. This can create complicated patterns of interactions between the direct wave and the one reflected from the ground, so that the propagation parallel to the ground is sometimes referred to as the “forbidden mode of propagation” (see Embleton 1996 for review). Moreover, all relevant studies consider a distance of 1–2 m as “close to the ground” (Ingard 1951; Daigle et al. 1983; Attenborough 1988). This is still far from crickets with their ears elevated only 5–10 mm from the ground. Empirical data for two cricket species in rather different habitats would indicate that the maximum hearing distances vary substantially from about 1–2 m (Mhatre and Balakrishnan 2008) to 10–15 m (Popov et al. 1972).
The task for a listening receiver is even more complicated, because it also needs to localize the sound source for a correct phonotactic approach. Whereas many studies provided useful information about sound localization cues available for this task (see Michelsen 1998a), little is known about how well such cues are preserved after transmission of the signal in natural habitats (Rheinlaender and Römer 1986; Michelsen and Rohrseitz 1997; Gilbert and Elsner 2000). To study the influences of the transmission channel on directional hearing in crickets, we used a modified “biological microphone” approach (Rheinlaender and Römer 1986), by analysing the bilateral responses of the pair of AN1 neurons in the field cricket Gryllus campestris in its own habitat.
Materials and methods
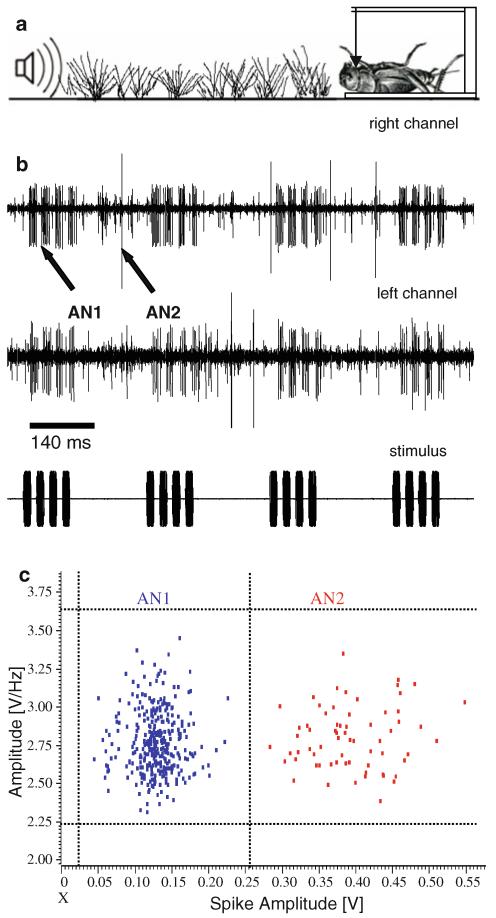
Crickets (G. campestris) were collected in the natural habitat in the vicinity of Graz in Styria (Austria). Prior to experiments, they were kept isolated in plastic boxes on a 12/12-h light/dark cycle and fed ad libitum with apple, lettuce and fish food. Experiments in the field were performed during the summer from May to July in 2005 and 2006. No experiments were performed on rainy days, when wind speed exceeded 5 m/s, or under conditions of high humidity of the vegetation. The experimental location was a gardening area surrounded by meadows and forest. The actual stretch of habitat where the transmission and recording experiments were performed was an area of flat grassland (length 19 m; width about 5 m) surrounded by some trees and bushes (47°06′10″N; 15°27′26″E). The same linear transect was used in both years. The height of grassland vegetation was kept at nearly 15 cm above the ground. Starting from the position of the speaker at one end of the stretch, small markers were placed in increments of 1 m for the later placement of the neurophysiological preparation. If not otherwise stated, both the height of the speaker and the preparation was adjusted at about 1 cm above ground level, i.e. the natural height of a listening field cricket.
Acoustic stimulation
The standard model song represented the male calling songs of G. campestris. It consisted of four syllables per chirp with a syllable duration of 21 ms and an interpulse interval of 16 ms. The interval between chirps was 220 ms so that the overall stimulus rate was close to the mean chirp rate of males at higher temperatures (own measurements). Sound stimuli were generated with Cool Edit software and broadcast by a small speaker (isophon, SKK 10; 25 mm). The standard song was emitted with a constant frequency of 4.7 kHz according to the mean value of the carrier frequency of calling songs in the male population (Kostarakos et al. 2008). Each chirp represented one stimulus, and for each distance the mean value of 10 responses/chirp was used. Signals were amplified (power amplifier NAD 214), and the sound pressure level (SPL) at a distance of 0.5 m was adjusted to 85 dB SPL (rel. 20 μPa) using an attenuator (837 attenuator, KAY Elemetrics corp.) and sound level meter (Rion NL-21) with integrated microphone (UC-52). The distance of the preparation relative to the speaker was varied, thus mimicking different distances of a female.
Neurophysiology
Extracellular recordings of the discharges of a prominent interneuron in the prothoracic ganglion, the AN1 neuron, were used to examine the representation of the conspecific stimulus over distance. The pair of AN1 neurons forwards auditory information to the brain. Manipulation of the discharge difference in this pair of neurons changes lateral steering during phonotactic behaviour (Schildberger and Hörner 1988). Further evidence for the importance of this neuron stems from strong correlations between AN1 discharges and the choice of females in behavioural trials (Kostarakos et al. 2008). We therefore developed a portable device for simultaneously recording the extracellular activity of both AN1 neurons in a bilateral intact system (for details of the methods see Stabel et al. 1989; Kostarakos et al. 2008). Discharges of both AN1 neurons were recorded with electrolytically sharpened tungsten hook electrodes, amplified via separated channels of a custom-made amplifier and recorded by a powerlab 4/25 (Model: ML845). In experiments during summer 2006, a sound level meter (Rion NL-21) with an integrated microphone (UC-52) recorded the sound signal at the position of the preparation on a separate channel. Recordings were processed in “spike 2” (Cambridge Electronic Design; England) for oZine analysis. A clustering algorithm allowed a reliable separation between the discharges of the AN1 neuron, those of the AN2-neuron with much higher amplitude and other neuronal activity with small amplitude spikes (Fig. 1). To determine the maximum hearing distance and to establish distance–response functions, the preparation was placed at increasing distances to the speaker at an angular position of 90°, so that the ipsilateral ear was directed to the speaker. Since the representation of the species-specific sound pattern in neuronal responses is crucial for eliciting phonotactic behaviour (Stabel et al.1989; Weber and Thorson 1989; Poulet and Hedwig 2005), the maximal hearing distance was defined as the maximum distance where the sound pattern was still represented in AN1 activity in three out of five stimulations. AN1 activity represented the sound pattern when all four syllables of a chirp elicited at least one AP in AN1 or three syllables elicited more than one AP. Occasionally, the AN1 neurons also responded to the stimulus at further distances, albeit less reliably. To study directional hearing, for each distance the orientation of the preparation with respect to the longitudinal body axis relative to the speaker was varied from 30° left to 30° right. These frontal angular positions of ±30° are rather typical positions during the zig-zag phonotactic walk of a female (Weber and Thorson 1989) and thus represent a useful measure of the directional gradient in the frontal position of the insect. Since binaural differences in response strength and/or response latency may serve as the code for localization (Pollack 2000; Mörchen et al. 1978; Ronacher and Krahe 1998), we calculated both the latency differences and spike count differences for a given stimulation angle and distance.
Fig. 1.
a Schematic sketch of a transect with the loudspeaker: preparation with bilateral recording of both AN1 neurons and the intervening vegetation. Note that the loudspeaker, preparation and transect dimensions are not at scale. b Example of a simultaneous bilateral recording of both AN1 neurons; larger spikes are from AN2. c Spike clustering analysis was performed on spike 2 (Cambridge Electronic Design; England) using only two parameters: spike amplitude (mV) and the first amplitude maximum of the spectrogram (waveform of spikes after FFT conversion) given in V/Hz
For the two positions at a distance of 1 and 5 m, we also studied the bilateral discharges of both neurons at angular positions from 90° right to 90° left, thus covering the whole frontal hemicircle. For each distance, the mean value of the number of spikes/chirp and response latency was evaluated for ten chirps. Since the latency between the stimulus and AN1 response increased with increasing distance due to increasing travelling time for sound and attenuation, the time window used for spike counting was adjusted depending on distance, varying between 180 and 250 ms.
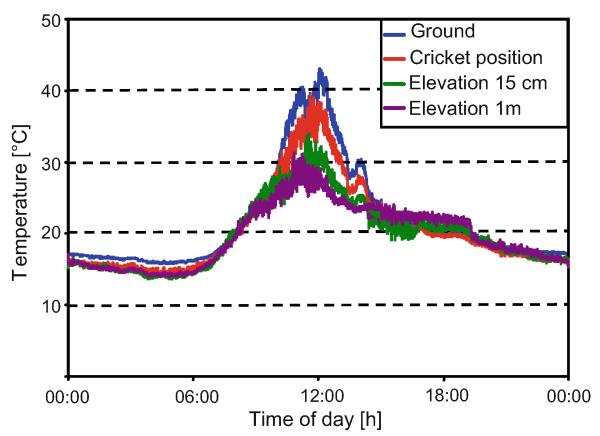
Ambient temperature at the transmission channel was measured at different elevations to analyse the influence of temperature and temperature gradients on sound transmission and neural responses. Four thermosensors (nickel–chrome elements; Omega Engineering Inc.; Deckenpfronn, Germany) were placed on the ground, at the cricket position, at a height of 15 cm and one at 1 m, and temperature was recorded using an Almemo 2690-8 recording device. Measurements were taken every 10 s.
Results
Maximum hearing distance
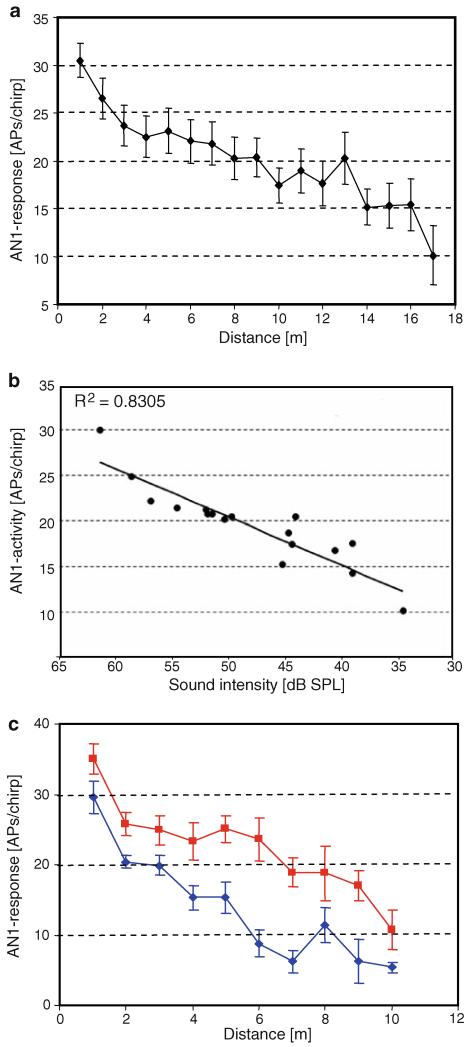
The maximal hearing distance was determined for a total of 21 preparations. Figure 2a illustrates the averaged AN1 activity at different distances. The decrease of AN1 activity with increasing distance is not linear. Starting with the maximum response of about 30 APs/chirp at a distance of 1 m, the decrease of AN1 activity is stronger in the first 4 m and then decreases at greater distances almost linearly. This would indicate that, on average, the range over which there is stimulus-related activity is in the order of 10 m (but see below). The maximal hearing distances of single crickets varied from 1 up to 15 m, with a mean of 9.2 m, depending on the sensitivity of the individuals and weather conditions. AN1 activity appears to reflect the average sound amplitude at the position of the receiver, as there is a high correlation between AN1 discharges and the recorded sound amplitude at different distances (Fig. 2b). However, at greater distances the AN1 neuron still responded to the chirps even when the microphone recordings revealed no signal.
Fig. 2.
a Distance–response relationship of AN1 responses in monaural preparations (mean ± SE; N = 21). b Correlation of the strength of AN1 response and variation in SPL, both recorded at the same position (N = 14). c AN1 discharges in a single cricket preparation, placed either on the ground (blue) or at an elevation of 15 cm (red) (mean of 10 responses per stimulus ± SD)
To demonstrate the special constraint of sound transmission very close to the ground, we studied in some preparations distance–response functions with the receiver elevated by only 15 cm, i.e. at the top of the grass layer. Figure 2c gives one example demonstrating that even when the speaker remained at ground level, for each distance the discharge in the elevated situation was significantly stronger compared to the ground situation. In this condition, the maximum hearing distance also increased by about 4 m.
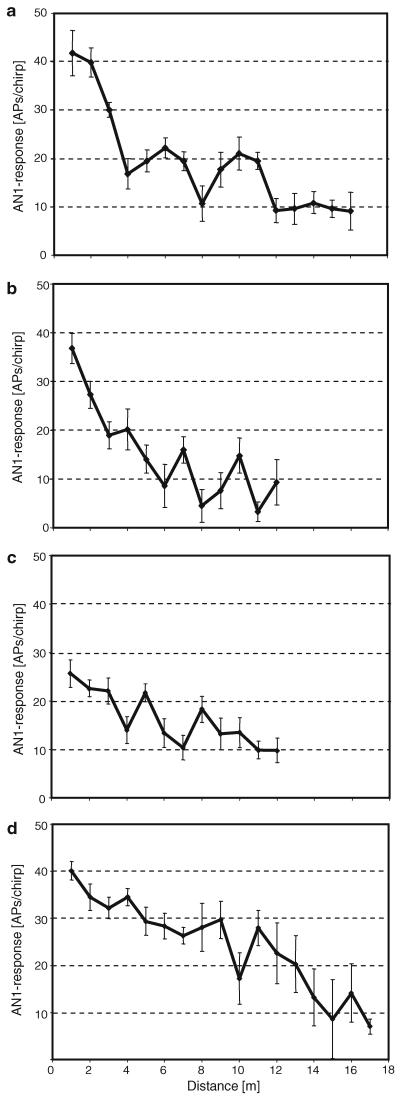
The mean distance–response function of 21 individuals (Fig. 2a) would indicate an almost linear, at least monotonically decreasing response function with distance. The activity of 11 individual AN1 preparations, however, demonstrates a more complicated pattern in response strength over distance. Figure 3 illustrates the AN1 distance–response function for four different preparations. One common property in all examples is the occurrence of strong irregularities in activity. For example, the preparation shown in Fig. 3a declined strongly from more than 40 APs/chirp to less than 20 APs/chirp within the first 4 m, but then showed two maxima in activity at 6 and 10 m, interspersed by a clear minimum of 10 APs/chirp at a distance of 8 m. Similar minima and maxima in activity are characteristic of the other response patterns in Fig. 3 as well.
Fig. 3.
a–d Distance–response functions of four cricket preparations (mean of 10 responses per stimulus ± SD). Note the discontinuous pattern with decreasing distance from the source, including the existence of local maxima and minima
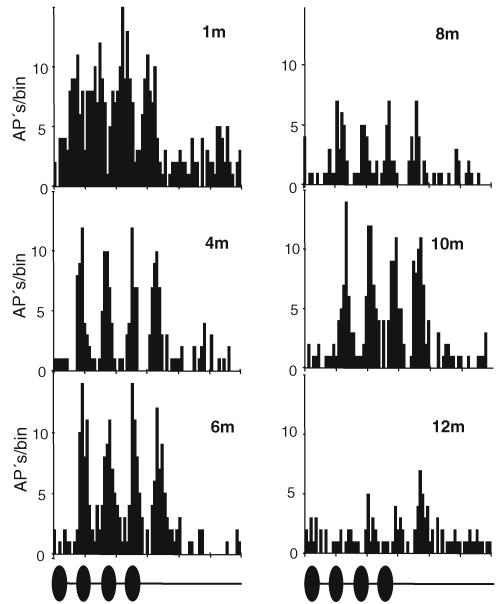
The existence of such local minima and maxima in response strength of AN1 is further illustrated by the PST-histogram calculated at various distances for one preparation in Fig. 4 (same preparation as in Fig. 3a). In particular, the local minimum at 8 m compared to the maximum at 6 and 10 m reveals a barely detectable temporal pattern of the chirp in the spike discharges, in contrast to PSTHs at 6 and 10 m. Such non-linearities with differences of more than 5 APs/chirp between local maxima and minima occurred in 11 of the 21 preparations. Such local minima did not always occur at the same position on the transmission channel in different preparations. Data for Fig. 3a and b were collected during the summer of 2005 and those for Fig. 3c and d during the summer of 2006. Nevertheless, the local minima and maxima differed between individuals also within the same season.
Fig. 4.
Peri-stimulus time histograms (PSTHs) of AN1 responses in a single cricket preparation at different distances from the source. Averaged responses over ten stimuli (chirp duration 140 ms)
Directional hearing: variation with location and time
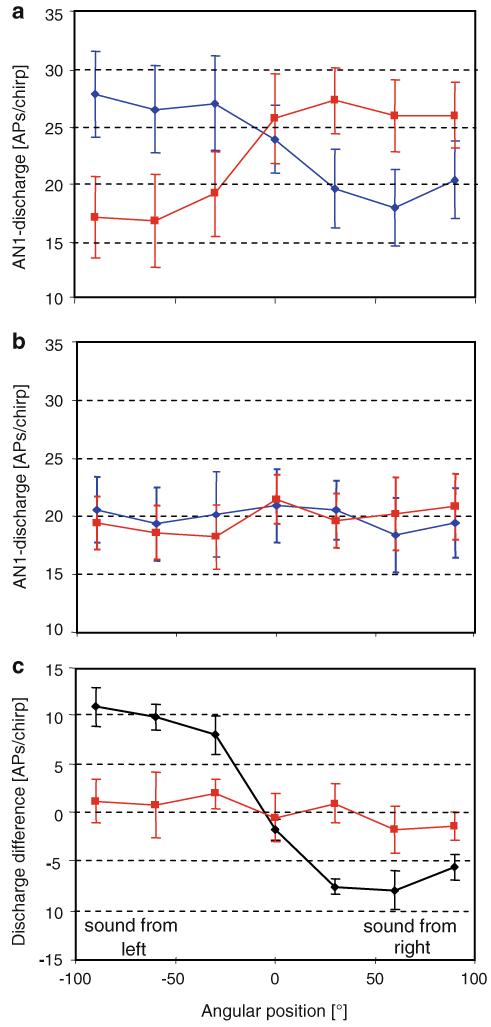
Directional hearing under conditions of a natural transmission channel was analysed for 25 preparations. As a standard measure for the directionality of the crickets’ auditory system in the field, we used the bilateral discharge differences of the pair of AN1 neurons, when the preparation was stimulated at frontal angles of either 30° right or left (see below). In control experiments performed at a distance of 1 and 5 m, we also tested the directionality in the complete frontal hemicircle, at angular positions from 90° right to 90° left (Fig. 5a, b). At a distance of 1 m from the speaker, the right neuron fired more strongly compared to the left one at angular positions from 90° right to 30° right, and the opposite was true with angular positions from the other side. The longitudinal body axis was close to the symmetry axis of discharges at 0°. Thus, if the bilateral discharge differences are important information for the female’s phonotaxis, these differences are clearly available at stimulus angles of 30° and higher at a distance of 1 m (Fig. 5c). However, the same analysis performed at a distance of 5 m revealed no differences, even for the largest stimulus angles tested on both sides (Fig. 5b, c). This is remarkable since it demonstrates that although the cricket can detect the calling song, the directional cues are no longer available for the receiver.
Fig. 5.
a, b Directional responses of both AN1 neurons (blue left neuron, red right neuron) when sound was broadcast from different angular positions (from 90° right to 90° left) at a distance of 1 and 5 m (mean ± SE; N = 7 and 8, respectively). c Calculated bilateral discharge differences between both neurons for the situation shown in a and b, at a distance of 1 m (black) and 5 m (red mean ± SE; N = 7 and 8, respectively)
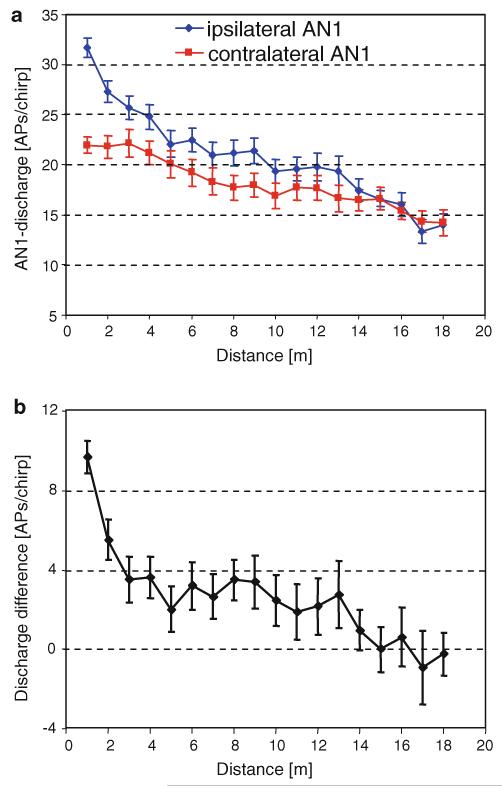
For a total of 25 cricket preparations, the angular positions 30° right and left were tested more systematically at increasing distances from the source. The average result for the discharge of the ipsi- and contralateral neuron, and the calculated discharge differences, is shown in Fig. 6a and b, respectively. The discharge difference dropped strongly from about 10 to 3.5 APs/chirp when the distance was increased to 3 m. For higher distances up to 13 m these differences remain between 2 and 4 APs/chirp, and for even larger distances no discharge differences were available, although both neurons still responded to the stimulus.
Fig. 6.
a AN1 discharges of the respective ipsi- and contralateral AN1 neuron for angular positions of 30° right and 30° left at different distances (mean ± SE; N = 25). b Corresponding bilateral discharge differences between both neurons (mean ± SE; N = 25). Negative values indicate stronger responses for the contralateral AN1 neuron
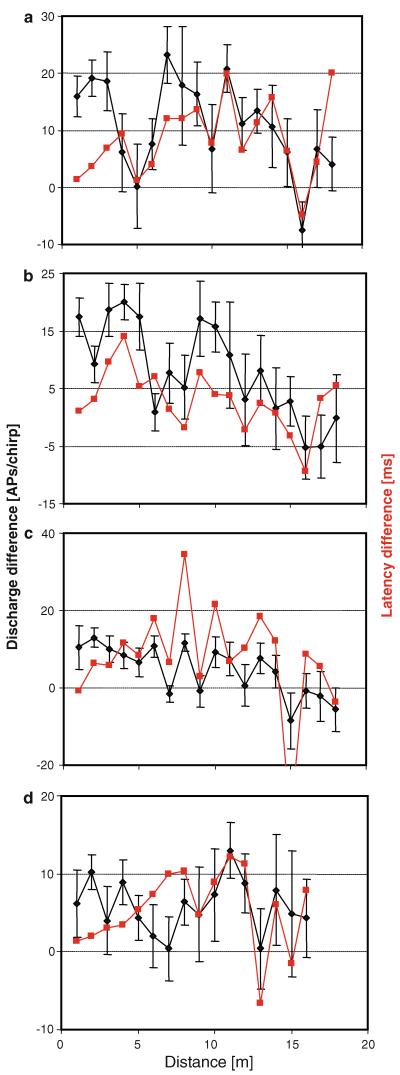
However, whereas the mean discharge differences suggest that there is some non-linear monotonic decline in this neuronal cue for sound localization, the same analysis for individual preparations indicate a highly irregular pattern of discharge differences over distance (Fig. 7a–d). For example, the preparation in Fig. 7a exhibits excellent directionality up to a distance of about 14 m, with the ipsilateral AN1 discharge 10–20 APs higher than the contralateral one. For the distance 4–6 m, however, the mean discharge differences decreased to zero. Similar locations with no directional cue available were found in other preparations (Fig. 7b–d), but did not occur at the same distance. In addition to binaural discharge differences, we also analysed binaural latency differences as potential cues for directional information for the same four preparations. In the experiments of Fig. 7a and b, the latency differences closely correlate with the maxima and minima of discharge differences, except at distances close to the source (1–3 m; see for example the minimum at 5 and 10 m, and the maximum at 11 m in Fig. 7a). In the preparation in Fig. 7d, latency differences are large at 8 m, whereas discharge differences are almost zero.
Fig. 7.
a–d Bilateral AN1 discharge differences (black) and binaural latency differences (red) in four preparations at different distances (mean of 10 responses per stimulus ± SD; SD of latency differences were very large and are not demonstrated for clarity). Negative values indicate either stronger responses or responses with shorter latencies for the contralateral AN1 neuron. Note the existence of local minima and maxima, where directional information is either low or high
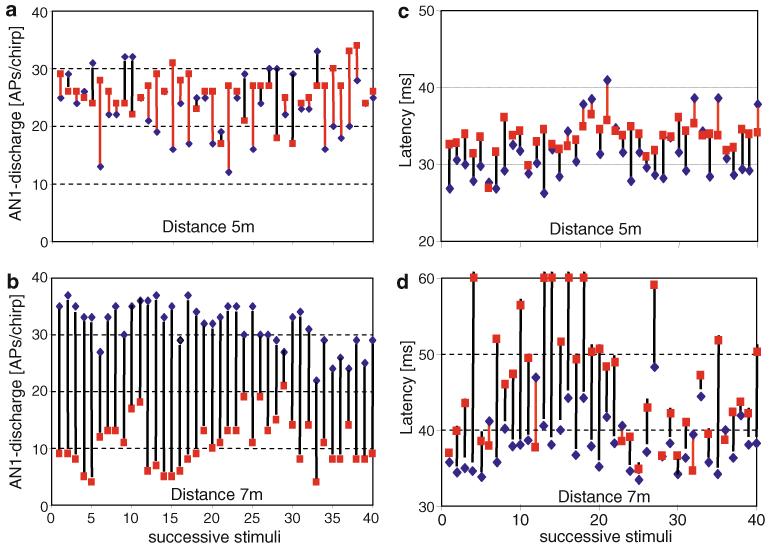
We also analysed the consistency of the directional cues over time. An analysis of more than 40 consecutive chirp responses (Fig. 8; same preparation as in Fig. 7a) indicated that such a general pattern of high and low directionality at locations only 2 m apart is rather consistent, but still large variations in the amount of directionality can occur within a time window of a minute. At a distance of 7 m, where the preparation showed large binaural discharge differences on average, in all 40 consecutive responses the ipsilateral AN1 fired more strongly compared to its counterpart (Fig. 8b), but the overall discharge differences varied from 6 to 30 APs over time. At a distance of 5 m, however, the mean discharge difference was close to zero (Fig. 8a), and the differences varied over time from −15 to 10 APs around this mean value. Remarkably, the contralateral neuron responded more strongly (>5 APs/chirp) in 13 of 40 stimuli compared to only 7 cases for the ipsilateral one. At the same time, directionality based on bilateral latency differences varied even more strongly at 7 m from 0 to more than 35 ms, and 5 of 40 responses showed incorrect latency differences (0 or even negative values, i.e. contralateral neuron leading in time). At 5 m, latency differences were small, and 12 of 40 responses revealed an incorrect directionality.
Fig. 8.
a, b Variation of bilateral AN1 discharge differences over 40 successive stimuli, recorded in one preparation at a distance of 5 and 7 m (same preparation as shown in Fig. 7a). Blue diamonds and red squares indicate the discharge of the respective ipsilateral and contralateral AN1 neuron. Black bars indicate correct directional information (ipsilateral neuron fired more strongly, or with shorter latency compared to contralateral neuron); red bars indicate wrong directional information. Note the variation in the amount of directional information at the same location over time
Influence of ambient temperature and temperature gradient on hearing in the field
Figure 9 illustrates the typical ambient temperature profile measured at four different elevations on the transmission channel over the course of 24 h. During noon, the highest temperature occurs on the ground, with decreasing values at increasing elevations. After 3.00 p.m., the temperature gradient is reversed with highest temperatures now at an elevation of 1 m until the evening. However, no correlation was found between the maximal hearing distance and the temperature gradient (the difference between temperature on the ground and at an elevation of 1 m).
Fig. 9.
Ambient temperature on the transmission channel at four different elevations for a time period of 1 day (N = 3; from 12.00 to 15.00, N = 21)
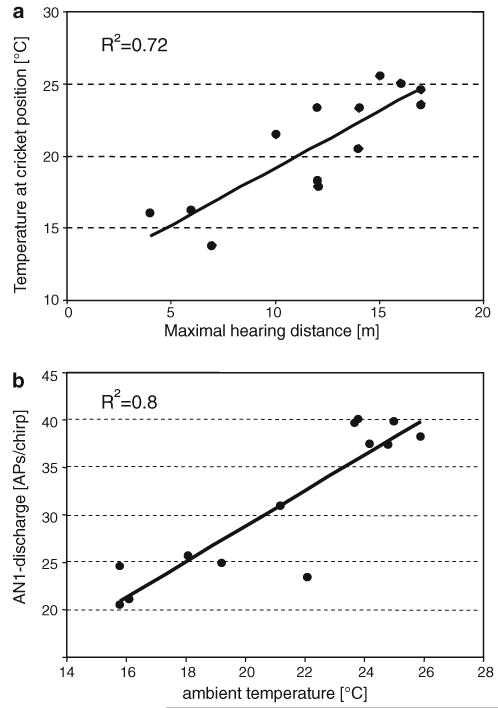
In addition to the possible effect of a temperature gradient for the propagation of sound (Larom et al. 1997; van Staaden and Römer 1997), the ambient temperature is of great importance for any poikilothermic animal. Indeed, there was a strong correlation between the maximal hearing distance and the absolute temperature at the position of the cricket (Fig. 10a). This indicates that temperature exerts a much stronger influence on the sensitivity of the sensory system than on signal transmission. This correlation is due to the fact that the AN1 discharge is strongly dependent on temperature (Fig. 10b): for an increase in ambient temperature of 10° the AN1 discharge was doubled.
Fig. 10.
a Correlation between AN1 discharges at a distance of 1 m and ambient temperature at the position of the cricket (N = 13). b Positive correlation between ambient temperature and the maximal hearing distance (N = 13)
Discussion
The aim of the present paper is to provide experimental data on two problems associated with the ground-living lifestyle of field crickets: the first problem is the complicated sound transmission parallel to the ground, and the second deals with directional cues available for receivers at this position using ears based on the pressure difference principle. Although these two problems are interconnected, we will first discuss them separately.
Sound transmission parallel to the ground: the intensity gradient over distance
The earth layer of a meadow forms the habitat for many indigenous insects, some of which utilize acoustic communication in the context of mate finding and aggressive interactions with rivals. The acoustical properties that are directly found at the ground layer (i.e. when the height of the signaller and receiver is about 1–2 cm) are still mostly unknown. This holds especially true for frequencies above 1 kHz. Furthermore, the idealized assumptions for classical models of sound propagation that come from reflection of spherical waves at an infinite plane are not given any more. The propagation of sounds near boundaries such as the soil of a field cricket’s meadow appears to be very complicated, and can in theory be described by several types of waves that can simultaneously propagate sounds (Bradbury and Vehrencamp 1998; Dusenberry 1992): a direct wave that travels in a straight line between the sender and receiver, a reflected wave that strikes the surface and rebounds towards the receiver, and a boundary wave travelling along the surface of the boundary. Boundary waves are created when some of the incident sound energy is absorbed by the second medium, which in turn can have two effects: one is the horizontal propagation of the signal through the second medium and its eventual re-radiation back into the first medium (ground wave propagation). Alternatively, complex movements of the first medium into and out of pores in the surface of the second can arise from interactions between the incident sound field and induced vibrations in the second medium, generating a surface wave that propagates just above the boundary.
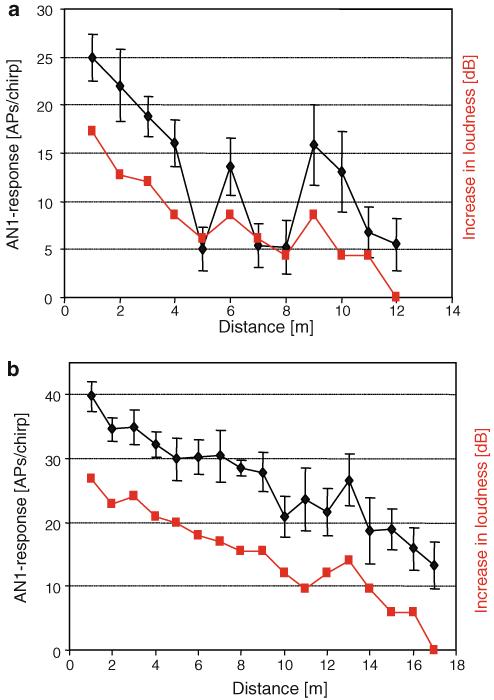
In the present paper, we made no attempts to measure or calculate the physical parameters that affect these waves, such as the acoustic impedances of the two media; rather, we used an acoustic interneuron most sensitive to the calling song CF (AN1 neuron) as an indicator for the representation of the signal within the auditory pathway of the receiver (the “biological microphone” approach; Rheinlaender and Römer 1986; Römer and Lewald 1992; Gilbert and Elsner 2000). From the receivers’ point of view, it is commonly assumed that once it has detected the sound signal of a calling male at some threshold value (the maximal hearing distance), the phonotactic path is guided by a gradient of intensity. By moving upstream in the gradient, it finally reaches the position of the signaller. Indeed, if we assume that the amplitude of a male chirp is reflected in the spike discharge of the AN1 neuron at the receivers’ position, then the mean distance response curve of all preparations would indicate a rather monotonic decrease in the chirp representation from about 30 APs/chirp (i.e. 7.5 APs/syllable) to about 15 APs/chirp (Fig. 2a). However, the individual distance response curves of more than 50% of the preparations indicate a rather discontinuous gradient in intensity, with respect to its representation in the CNS of the receiver. A comparison of the data based on microphone recordings with neuronal data at the same location demonstrates that in general the strength in AN1 activity correlated with the increase and decrease in the SPL of the stimulus (Fig. 2b). Some individual preparations also demonstrate this correlation (Fig. 11b), whereas in other cases variation of only 2–3 dB resulted in large fluctuation in AN1 discharges, with local maxima at 6 and 9 m (Fig. 11a). However, even in these experiments, the location of the ears of the insect and the microphone was different; the microphone had to be placed about 5 cm next and posterior to the insect in order not to disturb the directionality of the sound field. In addition, it is possible that factors other than differences in sound amplitude might be responsible for the observed strong variations in AN1 discharge and binaural discharge differences. Reflections that occur at specific locations might have altered the balance of stimulation of both ears, which in turn alters the amount of excitation and inhibition onto both AN1 neurons (the latter mediated by the pair of omega-neurons).
Fig. 11.
a, b Correlation of the strength of AN1 response (black) and variation in SPL (red), both recorded at the same position, for two different preparations (mean of 10 responses per stimulus ± SD; SD of SPL values was less than 1 dB)
Thus, whatever the reason for these discontinuities, a female approaching a calling male will eventually experience “silent spots” where the male song is barely detectable, as also evidenced in the PSTH analysed for such a “silent spot” (Fig. 6). This situation has some analogy to the complicated problem of orientation in a pheromone plume, which is to a much larger extent irregular (Dusenberry 1992). As the individual distance response functions would suggest, the best strategy of the approaching female would simply be to move forward roughly into the same direction as before, since this would bring her into a position out of the “silent spot”. The finding by Poulet and Hedwig (2005) that female crickets continue to track the previous acoustic target for some seconds even after cessation of the stimulus would indicate that crickets would be able to cope with such “silent spots”. The extent to which such discontinuities in the intensity gradient affect the phonotaxis of females can ultimately only be shown in a detailed quantitative analysis of the movement of females in the field. To our knowledge, the studies by Mhatre and Balakrishnan (2006, 2008) are the only field studies providing quantitative data on female phonotaxis. They have first shown that the SPL of the male call decreases monotonically with distance, with an estimated active range of 1.3 m. Further, females in fact oriented in a complex, real-world environment with multiple sound sources and approached the speaker broadcasting the most intense call. However, in their example phonotactic paths were limited to distances of less than 1 m, and females approached the source over flat soil without any plant structure. This may explain in part the difference from our results, which indicate strong discontinuities in sound transmission over distance for more than half of the preparations. Clearly, more outdoor studies with crickets orienting on the ground are needed.
Theoretically, such “silent spots” might have been expected from sound propagation close to the ground, when there is an interaction of a direct wave and one reflected from the ground, and when they are out of phase (see Embleton 1996 for review). The data on individual distance–response functions (Fig. 3) indicate that these “silent spots” do not always occur at the same position on the transmission channel, which would be the case if small structural inhomogeneities on the channel would have caused the interaction. On the other hand, air temperature (see below), wind speed and/or direction or air turbulence was not identical on the different recording days, and these differences could well be responsible for the different locations of the observed “silent spots”.
Directional hearing
For a quantitative analysis of directional cues in the field, we chose a standard test for bilateral discharge differences in the pair of AN1 neurons at a stimulus angle of 30° right or left of the longitudinal body axis. The rationale behind choosing this stimulus angle was that both behavioural and physiological information demonstrates that under ideal conditions in the laboratory or on walking compensators, (1) females can easily track sound sources at this stimulus angle, (2) IIDs in the order of 7–25 dB (depending on species and carrier frequency of the song) are available (Kostarakos et al. 2009), and (3) females show meandering with turning angles of about ±30° in their phonotactic approach (Weber and Thorson 1989). Our measurement at 1 m (Fig. 5a) also demonstrated that at stimulus angles of 30° discharge differences were large and reliable and did not increase substantially with larger stimulus angles.
On average (1) the directional information drops rapidly with distance within the first 3–4 m (Fig. 6), and (2) analogous to the “silent spots”, neighbouring positions on the transmission channel may provide either high or low directional information (Fig. 7). Such a dramatic loss of directional cues over relatively short distances is most surprising since in G. campestris the largest IIDs were demonstrated in a comparison of four species of field cricket (Kostarakos et al. 2009). In some individuals, IIDs of up to 25 dB were found (average 17.4 dB compared to only 7.7 in G. bimaculatus). Thus, despite large IIDs under ideal conditions, the field situation reduces directional cues to such an extent that no reliable binaural differences are available. In addition, even at locations where binaural cues were large, there were remarkable variations over time at the same location, in both binaural discharge and latency differences (Fig. 8).
Again, from the viewpoint of a receiver performing phonotaxis, it will experience locations on the transmission channel where the detection of the signal is possible, but directional information is lost. This situation has been reported in two similar approaches, both using a modified “biological microphone approach”. Gilbert and Elsner (2000) developed a portable preparation allowing recordings from afferent tympanal receptor Wbres of an Acridid grasshopper. They studied the degradation of directional cues over various types of habitat vegetation, in comparison to the undistorted directionality in the free sound field of the laboratory. On gravel and in sparse vegetation, the overall patterns of directionality were similar to those in the free sound field. However, the binaural differences were smaller compared to the laboratory, and the directional patterns were greatly affected in dense vegetation. Moreover, a minimum in nerve activity was not always reached on the contralateral side, as is typical for the free sound field situation. The loss of directional information, but not of signal detection, was also reported for hearing of katydids in the field, when the receiver was close to the ground in dense vegetation (Rheinlaender and Römer 1986). In this case, two factors may have influenced the loss of directional cues: for high frequencies, sound arrives at the receiver from almost any direction due to multiple scattering from objects on the transmission channel (the extreme of a diffuse sound field). Furthermore, frequency-dependent excess attenuation in dense vegetation reduces the amplitudes of high audio and ultrasonic frequencies more strongly. These are the frequencies that provide the best directionality for the ears (Rheinlaender and Römer 1986; Römer and Lewald 1992).
In contrast to katydids with their pressure receiver type ears, those of field crickets are based on the pressure difference principle. It has been argued by Michelsen and Rohrseitz (1997) and Michelsen (1998b) that directional hearing in crickets should be more resistant against degradation of directional cues compared to katydids, because even when the amplitude part of the signal is strongly degraded over distance, the phase relationships are not. In fact, by adding the phase information to the (distorted) amplitude information in a theoretical approach, Michelsen (1998b) postulated that female crickets could correctly identify the side of the stimulus starting with stimulus angles of 30°. This is an attractive hypothesis because it would suggest that the evolution of pressure difference ears has solved two major problems at the same time, since it represents an adaptation to the small body size relative to the large wavelength of the sound used for communication, and also to the distorting directional properties of the habitat.
The interpretation of the discrepancy between these theoretical approaches and our own empirical data obtained in the field is not easy, because our study did not measure phase relationships at the two ears of the receivers. However, whatever the physical reason for the increasingly distorted directional cue over distance might have been, by using the insect’s own hearing system as a reliable indicator for the neuronal cue of directionality, it is evident that females are constrained in their phonotactic approach by irregularities in both the intensity gradient and directional cues of the calling song. Evidently, behavioural studies with phonotactically responding females outdoors are badly needed to demonstrate how the distorted directional information provided by diminishing (or strongly varying) binaural discharge differences translate into quantitative parameters of phonotaxis, such as meandering angles or turns to the correct or wrong side.
It is tempting to speculate about the possible source(s) of variation evident in individual preparations with respect to both the intensity gradient and directionality. One major source for this variation appears to be the inter-individual variation in the “matched filters” for sensitivity and directionality (Kostarakos et al. 2008, 2009). In our experiments, we used a CF of 4.7 kHz for the standard stimulus, which is the mean of the male CF in the population of G. campestris. However, individual female receivers can vary in their best frequency of AN1 tuning from 4.3 to 5.1 kHz. Although the width of the tuning curves is the largest of all tested cricket species, this variation can have some effect on the absolute sensitivity of the preparations in response to the standard stimulus. However, variation in receivers more strongly affects the available directional cues. With a CF of 4.7 kHz, the available IIDs in some preparations reached values of more than 20 dB, whereas in others with their directionality tuned to either 3.6 or 5.7 kHz the resulting IIDs were only 6 or 8 dB, respectively (Kostarakos et al. 2009). Thus, for some of the preparations in this study, the available IIDs might have been high or low, despite the same transmission channel and all other conditions being equal.
Temperature effects and ambient noise
Any poikilothermic animal is strongly dependent on temperature, and the variation in temperature in a typical habitat of the field cricket G. campestris can be more than 10°C over a height of only 30 cm (Römer 2001). Variation in temperature can affect cricket communication in two rather different ways. One effect comes about as a result of the general dependence of nervous systems on temperature, which produced a twofold increase in firing with an increase in ambient temperature by 10° (Fig. 10a). Although we performed the experiments in the summer periods, we avoided experiments on days with extreme temperature. Nevertheless, the temperature variation recorded was from 16° to about 26°, and this variation produced a positive correlation between temperature and the maximal hearing distance (Fig. 10b), since receivers at higher temperatures fired more strongly in response to the stimulus. Whether the increase in maximal hearing distance also resulted from a temperature effect on the threshold of hearing could not be controlled in our experiments.
We also considered the possibility that temperature gradients might have developed on the transmission channel, which could have modified both the maximum hearing distances and directional hearing. Temperature inversions after sunset have been shown to strongly enhance hearing distances for elephants in the African savannah (Garstang et al. 1995; Larom et al. 1997) and for bladder grasshoppers (van Staaden and Römer 1997). Since even larger gradients can develop over the height of grass in the habitat of a cricket, Seither (1998) therefore compared the sound transmission characteristics of such a meadow under a variety of meteorological conditions and demonstrated that a steep temperature gradient indeed favoured the transmission of sound signals in the frequency range of 300–6,000 Hz (thus including the calling song frequencies of field crickets). However, the enhancing effect of the temperature gradient was only prominent at larger distances of 20–30 m, and thus at distances well beyond the hearing distances found in our study. Consequently, we found no correlation between maximum hearing distances and the formation of temperature gradients in our test habitat.
The outdoor experiments gave also some hints as to the potential of masking interference by background noise, since we always had a control microphone recording at the position of the preparation and thus could also determine whether some neuronal activity was due to background noise. In general, the AN1 neuron with its rather narrow frequency tuning to the pure-tone calling song (Kostarakos et al. 2008) is less affected by background noise, because less energy of masking sound is picked up by the narrow filter when compared with other insects like katydids (Lang et al. 2005). However, there was still the potential of masking by bird song and/or other sound caused by insects. In the sound recordings next to the preparations, occasionally the AN1 fired bursts of APs in response to some unidentified background sound. However, such neural activity did not interfere with the detection of conspecific calling songs, as long as the two did not coincide in time and the amplitude temporal pattern was different.
In conclusion, our findings demonstrate some of the problems associated with sound detection and localization for cricket receivers when performing phonotaxis on the ground in real habitats. When approaching a signaller, receivers may face situations where the neuronal representation of the calling song decreases, rather than increases with decreasing distance. Similarly, the available directional information does not increase with decreasing distance and may vary even at the same location over time. Future behavioural studies under such outdoor conditions are therefore badly needed.
Acknowledgments
Funding was provided by the Austrian Science Foundation (FWF), project P17986-B06 to HR. We thank Josefa Machold and the “Ökohof” for the use of facilities to perform the outdoor experiments. We also thank Anton Stabentheiner, Institute of Zoology Graz for providing the equipment for recordings of ambient temperature in the field.
Abbreviations
- SPL
Sound pressure level
- CF
Carrier frequency
- AN1
Ascending neuron 1
- AP
Action potential
Contributor Information
Konstantinos Kostarakos, Department of Zoology, University of Cambridge, Downing Street, Cambridge CB2 3EJ, UK.
Heiner Römer, Department of Zoology, Karl-Franzens-University, 8010 Graz, Austria.
References
- Attenborough K. Review of ground effects on outdoor sound propagation from continous broadband sources. Appl Acoust. 1988;24:289–319. [Google Scholar]
- Bennet-Clark HC. The mechanism and efficiency of sound production in mole crickets. J Exp Biol. 1970;52:619–652. [Google Scholar]
- Bradbury JB, Vehrencamp SL. Principles of animal communication. Sinauer associates. Inc; Sunderland: 1998. [Google Scholar]
- Daigle GA, Pieccy JE, Embleton TFW. Line-of-sight propagation through atmospheric turbulence near the ground. J Acoust Soc Am. 1983;74:1505–1513. [Google Scholar]
- Dusenberry DB. Sensory ecology. WH Freeman and Company; New York: 1992. [Google Scholar]
- Embleton TFW. Tutorial on sound propagation outdoors. J Acoust Soc Am. 1996;100:31–48. [Google Scholar]
- Garstang M, Larom D, Raspet R, Lindeque M. Atmospheric controls on elephant communication. J Exp Biol. 1995;198:939–951. doi: 10.1242/jeb.198.4.939. [DOI] [PubMed] [Google Scholar]
- Gerhardt HC. Sound pattern recognition in some North American treefrogs (Anura: Hylidae): implications for mate choice. Am Zool. 1982;22:581–595. [Google Scholar]
- Gilbert F, Elsner N. Directional hearing of a grasshopper in the field. J Exp Biol. 2000;203:983–993. doi: 10.1242/jeb.203.6.983. [DOI] [PubMed] [Google Scholar]
- Ingard U. On the reflection of a spherical sound wave from an infinite plane. J Acoust Soc Am. 1951;23:329–335. [Google Scholar]
- Keuper A, Kühne K. The acoustic behaviour of the bushcricket, Tettigonia cantans. II. Transmission of airborne sound and vibration signals in the biotope. Behav Process. 1983;8:125–146. doi: 10.1016/0376-6357(83)90002-5. [DOI] [PubMed] [Google Scholar]
- Kostarakos K, Hartbauer M, Römer H. Matched Wlters, mate choice and the evolution of sexually selected traits. PLoS ONE. 2008;3:e3005. doi: 10.1371/journal.pone.0003005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostarakos K, Hennig M, Römer H. Two matched filters and the evolution of mating signals in four species of cricket. Front Zool. 2009;6:22. doi: 10.1186/1742-9994-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AB, Kalko EKV, Römer H, Bockholdt C, Dechmann DKN. Activity levels of bats and katydids in relation to the lunar cycle. Oecologia. 2005;146:650–666. doi: 10.1007/s00442-005-0131-3. [DOI] [PubMed] [Google Scholar]
- Larom D, Garstang M, Payne K, Raspet R, Lindeque M. The influence of surface atmospheric conditions on the range and area reached by animal vocalizations. J Exp Biol. 1997;200:421–431. doi: 10.1242/jeb.200.3.421. [DOI] [PubMed] [Google Scholar]
- Mhatre N, Balakrishnan R. Male spacing behaviour and acoustic interactions in a field cricket: implications for female mate choice. Anim Behav. 2006;72:1045–1058. [Google Scholar]
- Mhatre N, Balakrishnan R. Predicting acoustic orientation in complex real-world environments. J Exp Biol. 2008;211:2779–2785. doi: 10.1242/jeb.017756. [DOI] [PubMed] [Google Scholar]
- Michelsen A. Biophysical basis of sound localization in insects. In: Hoy RR, Popper AN, Fay RR, editors. Comparative hearing: insects. Springer; New York: 1998a. pp. 18–22. [Google Scholar]
- Michelsen A. The tuned cricket. News Physiol Sci. 1998b;13:32–38. doi: 10.1152/physiologyonline.1998.13.1.32. [DOI] [PubMed] [Google Scholar]
- Michelsen A, Larsen ON. Strategies for acoustic communication in complex environments. In: Huber F, Markl H, editors. Neuroethology and behavioural physiology. Springer; Berlin: 1983. pp. 321–331. [Google Scholar]
- Michelsen A, Nocke H. Biophysical aspects of sound communication in insects. Adv Insect Physiol. 1974;10:247–296. [Google Scholar]
- Michelsen A, Rohrseitz K. Sound localisation in a habitat: an analytical approach to quantifying the degradation of directional cues. Bioacoustics. 1997;7:291–313. [Google Scholar]
- Mörchen A, Rheinlaender J, Schwartzkopff J. Latency shift in insect auditory nerve fibers. Naturwiss. 1978;65:656–657. [Google Scholar]
- Morton E. Ecological sources of selection on avian sounds. Am Nat. 1975;107:17–34. [Google Scholar]
- Pollack G. Who, what, where? Recognition and localization of acoustic signals by insects. Curr Opin Neurobiol. 2000;10:763–767. doi: 10.1016/s0959-4388(00)00161-6. [DOI] [PubMed] [Google Scholar]
- Popov AV, Shuvalov VF, Svetlogorskaya ID, Markovich AM. Acoustic behavior and auditory system of insects. Rhein-Westf Akad Wiss. 1972;53:281–306. [Google Scholar]
- Poulet JFA, Hedwig B. Auditory orientation in crickets: pattern recognition controls reactive steering. PNAS. 2005;102(43):15665–15669. doi: 10.1073/pnas.0505282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinlaender J, Römer H. Insect hearing in the field. I. The use of identified nerve cells as ‘biological microphones’. J Comp Physiol A. 1986;158:647–651. [Google Scholar]
- Robillard T, Desutter-Grandcolas L. Evolution of acoustic communication in crickets: phylogeny of Eneopterinae reveals an adaptive radiation involving high-frequency calling (Orthoptera, Grylloidea, Eneopteridae) An Acad Bras Cienc. 2004;76(2):297–300. doi: 10.1590/s0001-37652004000200018. [DOI] [PubMed] [Google Scholar]
- Römer H. Ecological constraints for sound communication: from grasshoppers to elephants. In: Barth FG, Schmidt A, editors. Ecology of sensing. Springer; Berlin: 2001. pp. 59–77. [Google Scholar]
- Römer H, Lewald J. High-frequency sound transmission in natural habitats: implications for the evolution of insect acoustic communication. Behav Ecol Sociobiol. 1992;29:437–444. [Google Scholar]
- Ronacher B, Krahe R. Song recognition in the grasshopper Chorthippus biguttulus is not impaired by shortening song signals: implications for neuronal encoding. J Comp Physiol A. 1998;183:729–735. [Google Scholar]
- Schildberger K, Hörner M. The function of auditory neurons in cricket phonotaxis. I. Influence of hyperpolarization of identified neurons on sound localization. J Comp Physiol A. 1988;163:621–631. [Google Scholar]
- Schul J, Patterson AC. What determines the tuning of hearing organs and the frequency of calls? A comparative study in the katydid genus Neoconocephalus (Orthoptera, Tettigoniidae) J Exp Biol. 2003;206:141–152. doi: 10.1242/jeb.00070. [DOI] [PubMed] [Google Scholar]
- Seither S. Schallausbreitung am Boden einer Wiese [Master thesis] Technical University of Graz; 1998. [Google Scholar]
- Stabel J, Wendler G, Scharstein H. Cricket phonotaxis: localization depends on recognition of the calling song pattern. J Comp Physiol A. 1989;165:165–177. [Google Scholar]
- van Staaden MJ, Römer H. Sexual signalling in bladder grasshoppers: tactical design for maximizing calling range. J Exp Biol. 1997;200:2597–2608. doi: 10.1242/jeb.200.20.2597. [DOI] [PubMed] [Google Scholar]
- Weber T, Thorson J. Phonotactic behaviour of walking crickets. In: Huber F, Moore TE, Loher W, editors. Cricket behaviour and neurobiology. Cornell University Press; Ithaca: 1989. pp. 310–339. [Google Scholar]
- Wiley RH, Richards DG. Physical constraints on acoustic communication in the atmosphere: implications for the evolution of animal vocalizations. Behav Ecol Sociobiol. 1978;3:69–94. [Google Scholar]
- Wiley RH, Richards DG. Adaptations for acoustic communication in birds: sound transmission and signal detection. In: Kroodsma DE, Miller EH, Quellet H, editors. Acoustic communication in birds. Academic Press; New York: 1982. pp. 131–181. [Google Scholar]